Abstract
The optimal configuration of Thin-Layer Photobioreactors (TLP) for the production of microalgae is analyzed. For that, a TLP of 40 m long, 1.5 m wide, and a slope of 1% was used, with both Computational Fluid Dynamics (CFD) and experimental measurements being used as data sources. First, the influence of culture inlet flows on the thickness of the fluid sheet and liquid velocity was studied, and a laminar flow was observed. Next, the light gradients at which the cells are exposed inside the cultures were calculated by considering both the light attenuation and movement of the cells along the reactor. A low frequency of light exposure was found. Combining the light regime to which the cells are exposed and different photosynthesis models the expected oxygen production rate was calculated. Although dynamic models are more precise, the use of static models is also suitable because of the low frequency of light exposition. The overall model of the reactor integrating fluid-dynamic and photosynthesis rates allows the optimization of the operation conditions on the photobioreactor. Results show that the optimal biomass concentration is 4 g L−1, at which the frequency of L/D cycles, oxygen production, and dissolved oxygen saturation is maintained at adequate values. Whatever the operating conditions the desorption of oxygen in the bubble column has been identified as essential for optimal operation. In conclusion, major phenomena taking place in this type of photobioreactors are determined by the thickness of the culture depth which is a function of the culture flow rate provided to the channel, otherwise, the liquid flow determines the energy consumption on the reactor. Thus, the optimization of the overall configuration of this type of photobioreactor still is a challenge for its further industrial development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are photosynthetic microorganisms naturally present in seawater and freshwater environments. These microorganisms have great potential to produce biomass that can be used in agriculture, aquaculture, feed and food production, among others (Trivedi et al. 2015; Lafarga 2020). Microalgae can be produced in open and closed bioreactors; however, the choice of each depends on the strain of microalgae to be used and the quality of the biomass to be obtained. Generally, open bioreactors such as raceway ponds (RW) are commonly used for the production of microalgae such as Dunaliella, Scenedesmus, and Arthrospira/Spirulina. This type of photobioreactor is well known for its simplicity, versatility, and relatively low construction and operating costs (Wang et al. 2012; Mendoza et al. 2013a, b). Meanwhile, closed reactors such as tubular photobioreactors (TPB) are the most widely used systems to produce high value-added biomass (Molina Grima et al. 1996), which counteracts the high construction and operating costs of this type of bioreactors. As an alternative, the use of thin-layer photobioreactors (TLPs) has been proposed, these reactors allow the production of high-value biomass such as tubular photobioreactors but at production cost close to open raceways (Akhtar et al. 2020). Studies of TLPs started in the 1960s in Czechoslovakia and were quickly implemented worldwide for their low cost and promising productivity but the design and scaling of these reactors is still challenging (Masojídek et al. 2015).
Although TLPs have been proposed for a long time, still the design of this type of photobioreactor is not completely solved. Evidence of the inadequacy of the current design includes the existence of large gradients of properties along the reactor such as pH and dissolved oxygen, the existence of carbon limitation and excess of dissolved oxygen concentration, the increase of temperature over the daylight period, etc. (Doucha and Lívanský 2006; Barceló-Villalobos et al. 2019b; Masojídek et al. 2021). However, despite these difficulties, this type of reactor provides the highest values in term of biomass productivity per land surface, with values up to 50 g m−2 day−1 being reported (Masojídek and Prášil 2010). Thus, the challenge is to optimize the design and operation of these reactors to allow to maximize the performance of the microalgae cultures and to scale up this technology to the industrial scale. For that detailed analysis of the fluid-dynamic and photosynthesis performance of this type of reactor is required.
Concerning fluid-dynamics issues, in TLP both the culture depth and liquid velocity are mainly a function of the culture flow being recirculated, which determines the energy consumption of the reactor. The culture is pumped at the entrance of the channel, then flows by gravity to the end depending on the slope and rugosity of the surface. The culture depth influences the light gradients in the culture as a function of biomass concentration and light attenuation by the biomass. The liquid velocity influences the hydrodynamic mixing in the culture, especially the vertical movement of the cells, in addition to the circulation time along the channel on which the cells perform photosynthesis. In the case of open raceways, the water depth and liquid velocities are about 0.2 m and 0.2 m s−1, thus the flow pattern is laminar and frequencies of light exposure is low, in the range of 0.01 Hz (Barceló-Villalobos et al. 2019a). In this type of photobioreactor, the solar radiation is excessive for the microalgae cells close to the surface of the culture causing photoinhibition, whereas it is insufficient for most of the microalgal cells occupying most of the culture volume, including large volumes in completely dark (Chiarini and Quadrio 2021). To reduce this effect a reduction of water depth is required, with values below 10 mm being proposed (Grivalský et al. 2019). Thus, thin-layer photobioreactors are characterized by having a low depth (0.5 to 5.0 cm), the culture being recirculated at similar velocities than in raceway reactors (0.1–0.4 m s−1) for surfaces with a slope of 0.1 to 6.0% (Masojídek and Prášil 2010). It is assumed that at these conditions the vertical mixing is enough to allow full integration of the light by the microalgae cells, for which a frequency of light exposure greater than 1 Hz is required (Brindley et al. 2016). However, the light regime at which the cells are exposed to the light in these reactors is not so high, ranging from 0.1 to 0.3 Hz (Chiarini and Quadrio 2021). To solve this problem different approaches have been proposed such as generating toroidal vortices or creating waves in the bottom of the reactor (Moroni et al. 2019; Chiarini and Quadrio 2021).
Concerning photosynthesis, the use of models for the prediction of photosynthesis rate as a function of culture conditions to which the cells are exposed is a valuable tool for the optimization of the design and operation of photobioreactors for the production of microalgae. In general, static models considering the average values for culture parameters such as irradiance, temperature, pH and dissolved oxygen are utilized (Molina Grima et al. 1996; Costache et al. 2013). The use of these models is adequate if the characteristic time of the phenomena is shorter than the variation of the culture conditions, which is reasonable for some variables such as temperature, pH and dissolved oxygen that have characteristics times of the order of minutes/hours. However, the irradiance at which the cells are exposed in the cultures varies faster, in terms of milliseconds, thus influencing the photosynthesis rate. To consider this fast response the use of dynamic models is required (Rubio et al. 2003; Fernández-Sevilla et al. 2018).
In previous works where the fluid dynamic of thin-layer reactors has been studied a laminar flow pattern was usually found (Severin et al. 2018, 2019). Moreover, the mixing and light regime on these reactors has been also studied, with results demonstrating that it is possible to achieve full mixing and high frequencies of light exposure hogher than 1 Hz at certain conditions (Akhtar et al. 2020; Chiarini and Quadrio 2021). However, to understand better the influence of these factors in the final performance of the cultures, it is necessary to also integrate the use of the light by the microalgae cells through adequate photosynthesis models. Thus, for the development of models considering the overall design and operation of photobioreactors, the influence of fluid dynamics on the variation of photosynthesis rate must be included. For that numerical methods such as the Discrete Ordering Method (DOM) and the Finite Volume Method (FVM) can be used (Ben Salah et al. 2004). The use of FVM integrates the light on the control volume with the fluid dynamic of the culture (Duran et al. 2010) unlike DOM which only does so on the control volume thus not allowing an optimal coupling with fluid dynamics (Huang et al. 2011). Therefore, the use of FVM allows the exploration of the influence of design and operational parameters on the performance of the reactor including the light regime at which the cells are exposed and based on that the conversion of CO2 to biomass and the production of oxygen. The excessive accumulation of O2 largely reduces the productivity of microalgae cultures, with dissolved oxygen concentrations higher than 250%Sat. causing inhibition (Barceló-Villalobos et al. 2019a, b; Rubio et al. 1999).
This work aims to elucidate the most relevant parameters for the optimization of the design and operation of TLP. For that, a detailed analysis of both the fluid dynamics and photosynthesis rate in a thin-layer photobioreactor is performed. CFD tools allow for the characterisation of the variations of water depth and liquid velocity, in addition, to estimating the movement of the microalgae cells in time. Models of photosynthesis rate allow estimating the oxygen production rate and then the biomass productivity at different scenarios. Analysis of results allows to determine the optimal biomass concentration and length of the channel for a fixed configuration of the reactor: Moreover, the developed model is a useful tool for the optimization of the design of this type of photobioreactor or for defining the optimal operation conditions for those already in operation, thus it is a valuable tool for the industrial development of this technology.
Materials and methods
Description of the thin-layer photobioreactor
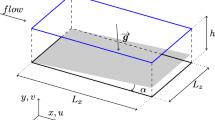
A TLP located in the Research Center IFAPA (Almería, Spain) was used. It consists of an inclined culture plate with a slope of 1%, a total length of 40 m and width of 1.5 m, area of 60 m2 directly exposed to solar radiation (Fig. 1). The inclined plate is built in fibreglass with smooth surfaces guaranteeing the suspension of microalgae throughout the width of the channel. The entrance of the culture is made using a PVC pipe with 2 rectangular grooves 0.2 m wide and 0.01 m high. In addition, a tank of 3 m3 capacity and bubble column of 2 m height and 0.4 m diameter are also installed. The culture is recirculated from the tank to the culture plate by passing previously by the bubble column for CO2 supply or O2 removal. The CO2 is provided on demand for the control of pH and to avoid carbon limitation, whereas aeration is provided also on-demand to avoid dissolved oxygen levels higher than 200%Sat. The culture volume and depth are functions of the inlet culture flow, which is modified on demand by regulating the inverter connected to the pump impulsing the liquid. Numerical analysis was performed to determine the depth of the culture along the channel and the liquid velocity. Based on these results, a more detailed analysis was performed on a section of the reactor to analyze the pattern flow and oxygen production using a numerical model. Experimental measurements of both water depth and liquid velocity, in addition to the photosynthesis rate were performed in parallel.
Analysis of the system by computational fluid dynamics
For the analysis of fluid dynamics in the entire photobioreactor, a structured hexahedral mesh was created using the ANSYS Meshing 2020R2. The mesh is composed of a linear order of elements with an average orthogonal quality of 100% and a standard deviation of 1.2 × 10–7, it is composed of 1.5 × 106 cells. The ANSYS Fluent software was used to solve the constant multiphase flow equations (pseudo transient time) using the Volume Of Fluid (VOF) method for both phases (water–air). The coupled algorithm was used to solve the simulation of all cases. The turbulence model used was \(k-\epsilon\), it being adequate to capture the effect of turbulent flow conditions, It is a low computational cost model to carry out analysis of large sizes with an acceptable precision of free turbulence. This model belongs to the Reynolds-averaged Navier–Stokes (RANS) models, where all the effects of turbulence are modelled. The boundary conditions were defined at four different inlet flows rates of culture to the plate of 1.15, 2.20, 3.00 and 3.60 L s−1. The system was simulated considering atmospheric pressure and roughness of the walls of 0.0015 mm. Based on the Courant-Friedrichs-Lewy (CFL) condition, which assures the convergence of the numerical solution of partial differential equations, the time step was set to \(\Delta t\) = 0.01 s (Courant et al. 1928).
Due to the large size of the photobioreactor, to perform a detailed analysis of particle tracking in reasonable time it was necessary to define a section of the reactor with a lower volume. Thus, a small domain of the global system was discretized (Fig. 2b), on which a structured hexahedral mesh was used with a linear element of mean orthogonal quality of 100%, composed of 2.6 × 106 cells. This domain has been discretized for the evaluation of the movement of small particles represented as microalgae cells. The mesh was designed using the criterion of the Law of the Wall, where the distance of the cellular centroid from the wall is given by \({y}_{p}\) (Eq. 1), where \({y}^{+}\) = 0.9 (< 1 for \(\mathrm{LES Turbulent Model}\)), μ is the dynamic viscosity, \({u}_{\tau }\) is the friction velocity and \(\rho\) is the density of the fluid (Severin 2017).
Multiphase flow equations using the Volume of Fluid (VOF) were utilized. Now, the Large Eddy Simulation (LES) turbulence model was used, which solves a wide range of eddies sizes. The smaller scales were solved by Kinetic-Energy Transport (Sub-grid Scale Model). It has been solved in transient mode with the PISO algorithm (Moroni et al. 2019) with a time step of 0.001 s calculated using CFL condition. This time the simulation was performed with the submodels of the open channel boundary condition and the open channel wave boundary condition involving a free surface between the flowing fluid and the fluid on the free surface, which is air at atmospheric pressure and the open channel wave boundary condition. This allows simulation of the propagation of the wave that will influence the trajectory of the particles contained in the liquids as shown in Fig. 2c.
Particle tracking
The trajectory of the Lagrange phase was predicted by integrating the balance of forces in the particles, this balance of forces equals the inertia of the particles with the forces acting on the particles, it can be written as follows:\(\frac{d{u}_{p}}{dt}={F}_{D}\left(u-{u}_{p}\right)+\frac{g\left({\rho }_{p}-\rho \right)}{{\rho }_{p}}+{F}_{p}\),\({F}_{D}=\frac{18\mu }{{\rho }_{p}{d}_{p}^{2}}\frac{{C}_{D}Re}{24}\), where \(u\) is the velocity of the fluid or culture in m s−1, \({u}_{p}\) is the velocity of the particle in m s−1, \(\mu\) = 0.001 Pa s is the fluid viscosity, \(\rho\) = 1000 kg m−3 is the water density, the particle density has been considered \({\rho }_{p}\) = 864 kg m−3 and particle diameter \({d}_{p}=7 \mu m\) (Ali et al. 2015; Akhtar et al. 2020; Chiarini and Quadrio 2021),\({F}_{D}\): drag force per unit mass,\({C}_{D}\): spherical drag coefficient,\({F}_{p}\): additional acceleration terms (force/unit mass of the particle), the number of particles (N) used was 60.
Photosynthesis rate
To determine the photosynthesis rate in the culture two different approaches were considered. The static model considers that the photosynthesis rate is a direct function of culture conditions at different vertical positions. Applying the Lambert law it is possible to determine the light availability at different water depths, and using the hyperbolic model (Fernández Sevilla et al. 1998) to calculate the oxygen production rate at each one, by integrating these values the overall oxygen production rate is calculated. The dynamic model use considers the trajectories of the cells to estimate the oxygen production rate by suspended microalgae cells at different positions (Brindley et al. 2011; Fernández del Olmo et al. 2021). This can be analyzed using the dynamic models proposed by Eilers and Peeters, (1988) and Rubio et al. (2003). The Camacho-Rubio model was used for this experiment assuming that the deactivation rate (\(r\)) is not proportional to the concentration of excited Photosynthetic Centers (PSFs) but is controlled by reaction measured by enzymes that can become saturated, \(r\) it can be written as follows:
where \({r}_{m}^{*}\) is the product of the limiting enzyme concentration, its velocity constant and this can be expressed as the energy consumed per cell in unit time, \({K}_{s}^{*}\) represent the concentration of photosynthetic units (PSUs) that produces a rate of photosynthesis equal to half the maximum rate, \(a\) is the total concentration of mole PSU per cell, \({a}^{\circ }\) is the molar power supply per cell in the resting state and \({a}^{*}\) is the molar power supply per cell in the activated state. The balance of activated centres can be represented by the Camacho–Rubio model:
where \({k}_{a}\)= is the absorption coefficient in m2 g−1 and \(I\) is the irradiance in µmol photons m−2 s−1 and \(t\) in seconds. Rubio et al. (2003) proposed a dimensionless model dividing each parameter by the total concentration of photosynthetic centres. Thus, the concentration of activated centres (a*) becomes a fraction (\({x}^{*}\), the fraction of activated centers). In the original work, the authors conveniently organized the parameters of the model into the following groups (Fernández del Olmo et al. 2021):
Mass transfer analysis
The mass transfer across the gas–liquid interface can be expressed using the mass transfer coefficients on both sides, in the liquid phase is \({k}_{l}=\) 6.2 × 10−5 m s−1 and the gaseous phase is \({k}_{g}=\) 5.2 × 10−4 m s−1, both previously studied by Petera et al. (2021). Its combination will give the overall mass transfer coefficient is \(\frac{1}{{K}_{\mathrm{L}}}=\frac{1}{{k}_{l}}+\frac{1}{{k}_{g}H}\), where Henry's constant under equilibrium conditions (Schuhfried et al. 2011, 2016) is \({H}_{{O}_{2}}=\frac{\left[{O}_{2}^{*}\right]}{{P}_{{O}_{2}}}\) = 8.8 × 10−8 kg m−3 Pa−1 where \({P}_{{O}_{2}}\)= 101,303 Pa is the partial pressure of oxygen in the gas phase and \(\left[{O}_{2}^{*}\right]\) = 9 mgO2 L−1 is oxygen dissolved in air–water equilibrium. The interfacial area (\(a\)) represents the specific surface of the interfacial area which can be derived from the ratio of differential element surface dA and its volume dV, therefore, \(a=\frac{1}{h},\) where h is the average height of the culture. Hence, the volume coefficient of mass transfer (\({K}_{L}a\)) in \({\mathrm{s}}^{-1}\) can be calculated by the following equation:
Results and discussion
Fluid dynamics analysis of a TLP
Recently TLP photobioreactors have been thoroughly studied to standardize their design variables. The challenge is to achieve circulation velocities in the range of 0.2 m s−1 as a standardized optimum for open channels and higher than 0.10 m s−1 as a critical rate with sedimentation risk (Chisti 2016; Inostroza et al. 2021). The relationship between the velocity of circulation and the height of the fluid sheet is a function of the slope and the friction work, the lower the height of the fluid sheet the friction work is increased decreasing the circulation velocity to almost critical levels. To avoid this effect it is proposed to increase the slope of the channel. Some authors such as Akhtar et al. (2020), Apel et al. (2017), Moroni et al. (2019) and Severin et al. 2018 have reported velocities over 0.20 m s−1 and even up to 1 m s−1 using higher slopes. It has been reported that for low depths (< 10 mm) the usual velocities of 0.40–0.50 m s−1 have better performance in productivity (Grivalský et al. 2019). Mixing is improved directly by increasing the slope or higher inlet flow rate, suggesting that an optimal configuration can be compared with a waterfall with high flows and steep slopes that can keep in operation reactors with increasing biomass concentration. The mixing intensity is directly related to turbulence, therefore, sedimentation is decreased (Severin et al. 2018). Moreover, the mixing is not directly related to the conventional Reynolds Number, for this reason, it is advisable to do a Lagrangian study for more accurate analysis by particles.
To study the fluid dynamics of the thin-film reactor considered, numerical simulations have been carried out using CFD software, these results being compared with those obtained experimentally. The modified variable has been the culture flow rate contributed to the reactor inlet and the measured variables included height and velocity fluid in addition to the circulation regime by the turbulent Reynolds number. As shown in Fig. 3a, the fluid sheet at flows of 3.00 and 3.60 L s−1 is ≥ 10 mm for much of the channel, 2.20 and 1.15 L s−1 a recommended fluid layer thickness of 10 mm (Grivalský et al. 2019). Akhtar et al. (2020), Apel et al. (2017), and Severin et al. (2018) have reported depth < 10 mm with fluid dynamic results in terms of optimal circulation velocity, but increasing the slope of operation. In this case it is a constant of 1%. As observed in the Fig. 3b, the predominant circulation velocities for flows of 1.15, 2.20 and 1.15 L s−1 are located between 0.10 and 0.20 m s−1 simulated and experimentally along the channel, being fluid velocities not recommended due to the low mixing intensity generated and high risk of sedimentation, in this experiment it is observed for flows than for the flow of 3.60 L s−1 the velocities were ≥ 0.20 m s−1 which is a safe velocity of circulation. These results indicate the importance of increasing the inlet flow to obtain better fluid-dynamic results, it has been standardized that at least inlet flow per meter of reactor width is 2.4 L s−1 to get a velocity of 0.2 m s−1.
The light regime at which the cells are exposed
The TLP is a channelled photobioreactor that maintains a defined direction of the fluid and low turbulence determined by the slope and gravity. A Lagrangian analysis was carried out and 60 particles with vertical resolution were chosen to represent the behaviour of the entire culture. The volumetric fluid dynamics of the reactor is the factor that influenced the movement of the cells contained in the culture which moves in the form of a wave in time and space. The greatest variation is achieved at a greater layer thickness where the resistance to movement is zero, and it decreased as the depth increases due to the resistance caused by the roughness of the lower wall in direct contact with the fluid. The distribution of particles is very similar to what was achieved by Severin et al. (2018) where the particles have slight vertical variations not exceeding 1 mm in the deepest zone and no more than 5 mm in the surface area. These results are similar to those of this study because their operational parameters are similar to the inlet flow and slope. As a consequence the linear velocity of the particles is high in the upper zone, varying between 0.21 to 0.3 m s−1. Particles that are travelling in the area at medium depth and particles closer to that to the lower plate do so at the safe velocity limit of 0.2 m s−1. The vertical velocity of the particles is a consequence of the vertical position and its linear velocity and is directly related. The greater the variation of the vertical position and its linear velocity, the greater its vertical velocity, and the same relationship for the particles that travel at greater depth.
TLPs have several fluid-dynamic advantages due to their low depth, circulation velocity and slope used. Having a sheet of fluid of lower depth is a smaller viscous area on its walls. Another advantage over its low depth in scale of mm makes it very attractive equipment for its high photosynthetic conversion, as they can maintain a fully illuminated culture up to high biomass concentrations. With the data of vertical position and velocities, an individual study of the particles that travel at different depths has been carried out to evaluate the attenuation of the light to which they have been subjected to evaluate their photosynthetic activity.
Microalgae respond to the amount of light available by varying photosynthetic activity, the available light (µmolphotons m−2 s−1) can be calculated using the Lambert–Beer Law, expressed as follows:
where \({I}_{0}\)= 1000 µmolphotons m−2 s−1 is the incident irradiance, \({k}_{a}\) = 0.1 m2 g−1, \({C}_{b}\) = 1, 2, 3, 4, 5 and 6 g L−1 and \(L\) is the depth of the culture. A compensation irradiance has been established \(({I}_{c}\)) ≤ 40 µmolphotons m−2 s−1, where it is considered that the cells are in a dark volume (Barceló-Villalobos et al. 2019a). Below this irradiance there is not enough light to carry out photosynthesis and productivity is 0. The saturated zone by the saturation coefficient is α = 222 µmolphotons m−2 s−1 obtained from Brindley et al. 2016 and the limited zone is located between α and \({I}_{c}\). Fig. 4, shows as up to a concentration of 3 g L−1 is the 100% of illuminated cells, at concentrations of 4, 5 and 6 g L−1 the percentage of light cells is 95, 84 and 56% respectively, having the most cells in darkness at 6 g L−1. At higher concentrations of 12.5 g L−1 with light/dark cycles it is advisable to decrease the thickness of the fluid layer up to 6 mm (Grivalský et al. 2019). At a higher concentration, there is slower cell multiplication but not a total stop of cell growth. Therefore, it is essential to know what the optimal biomass concentration of operation is. In the reactor of this study, the importance of a thin layer of culture has been considered (11 mm) to have the 100% of the illuminated volume in such a way as to maintain a high absorption of photons generating a fast and non-enzymatic step to activate the Photosynthetic Units (PSU).
As the cells are continuously travelling between the different zones means that they are exposed to different irradiances facilitating the light integration and photosynthesis rate (Ali et al. 2015). A high level of mixing is directly related to the integration of light (Γ) which is a function of the frequency or cycle L/D (ν), and the illuminated fraction or duty cycle (\(\phi\)), this last parameter being a function of the concentration of biomass (Cb). The vertical movement of the cells is one of the major factors that affect the performance of TLP photobioreactors. The photosynthetic response of each cell will depend on the light intensity of each zone through which it moves, especially the saturated zone and the dark zone where the transition is between the minimum and the maximum. The numeric parameter is represented by frequency or cycle L/D (ν) and illuminated fraction or duty cycle (\(\phi\)). Due to the high number of particles (N) that have been used, it must be calculated by a statistical characterization using the arithmetic mean using the following formula:
where \(n\) is the number of total cycles in the 0.2 m of the subdomain of each particle, is the \({t}_{f}\) light time of the particles in seconds when their irradiance is \({>I}_{c}\) and \({t}_{d}\) is the time of darkness of the particle when its irradiance is \({\le I}_{c}\). The results can be seen in Fig. 5. When performing an analysis of the set of particles up to a biomass concentration of 3 g·L−1 leads to a calculated mean indicating that on average the frequency is \({\nu }_{av}\) = 0 Hz because the culture is maintained all the time in saturated and limited illuminated zones without travelling through the dark zone, as can also be seen in the measure of the duty cycle that is maintained \({\phi }_{av}\) = 1. This means that 100% of the cells are fully illuminated, by staying all the time with light generates low use by the microalgae of this resource. At 4 g L−1 a slight increas in the dark area begins, therefore, appears the cycles L/D being \({\nu }_{av}\) = 1.18 Hz having the maximum integration of the light of 60%. At 5 g L−1 frequency decreases at 1 Hz having 55% integration of light. From this concentration, the cells begin to suffer a deficit of light because the dark zone increases and fewer cells can enter and leave this area because the dark fraction increases to 49%. With 6 g L−1 the frequency decreases to \({\nu }_{av}\) = 0.9 Hz having an integration of the light of the 50% because the dark zone increases more and fewer cells can enter and leave this area because the dark fraction continues to increase to 62%. The light integration data can be seen in Fig. 5 in Fernández del Olmo et al. (2021). All of this phenomenon can be seen in conjunction with Fig. 4.
Photosynthetic activity
In the subsequent steps enzymatic reactions are activated that provide energy for oxygen production. The dynamic model has been used to perform an analysis of each particle (Rubio et al. 2003). The parameters of Eq. 5 when replaced in Eq. 4 make it easier to calculate because it transforms the concentration of PSU to a fraction, following the following equation:
where \({I}_{p}\) is the irradiance of the attenuated particles concerning depth and time \(\mathrm{x}(t)\) for each particle, \(\beta =5\) Hz is a characteristic frequency of the system and represents the specific photosynthetic maximum rate, κ = 0.1 is a saturation constant for the velocity control enzymatic reactions.
Oxygen generation (\({RO}_{2,DIN}\), mgO2 gbiomass−1 h−1) for the dynamic model is calculated according to the next equation.
where \({RO}_{2, max}\) = 88 mgO2 gbiomass−1 h−1 (Costache et al. 2013), representative measurement under optimal temperature conditions at 25 °C and pH 8. (Barceló-Villalobos et al. 2019b). The results of the rate of photosynthesis per particle can be seen in Fig. 6 at concentrations 1 and 2 g L−1 the culture is saturated, that is, all the particles are producing oxygen regardless of the depth they are located. The attenuation of light is minimal compared to the photosynthetic velocity of the cells. Between 3—4 g L−1 is an ideal concentration because the particles have decreased their saturation level and few cells are not performing photosynthesis ≤ 5%. Between 5—6 g L−1 it is observed that the cells that do not perform oxygen increase, initiating the process of loss of productivity up to 16% and 44% to 6 g L−1 respectively. These conditions affect the optimal development of the culture because long periods in darkness deactivate the PSUs, the reactivation depending on the conditions of cultures can be slow affecting the photosynthetic efficiency.
Comparison of photosynthesis models
To carry out an objective study, a comparison was made between the dynamic method and static methods with light integration and without light integration. Due to the high number of particles (N) that are used, it must be calculated by a statistical characterization using the arithmetic mean using the following formula:
The static model with light integration (Γ) assumes that the entire volume of the reactor is mixed, it is indifferent to the location of the particles and time. They assume that all particles are exposed to an average irradiance (\({I}_{av}\)), Therefore, to determine this parameter the simplified equation proposed by Molina Grima et al. (1994) is used.
where L = 11 mm is the total depth or thickness of the reactor fluid layer.
Oxygen generation (RO2, mgO2 gbiomass−1 h−1) for the static model with light integration is calculated according to the equation:
where \({I}_{k}\) = 120 µmolphotons m−2 s−1 and \(n\) = 2 (Grima et al. 1994; Barceló-Villalobos et al. 2019b).
RO2 is directly proportional to biomass production (Pb) in g m−2 day−1, considering 10 productive hours per day and 11 L per m2. Productivity (Pb) is an effect observed longer than instantaneous photosynthesis, for the study analysis has been carried out projected on the same graph. Considering a stoichiometric value of \({Y}_{b}\) in the analysis of Fig. 7 is studied biomass concentration as a limiting factor of photosynthesis.
When analyzing the behaviour of the dynamic method compared to the static method of Fig. 7 more details are observed with the dynamic method if it is analyzed together with the trajectory of the particles through the different illuminated areas of Fig. 4. The frequency and an illuminated fraction of Fig. 5 and the rate of photosynthesis of the particles of Fig. 6. It is conclusive that the cells keep saturated and slightly limited of light their behaviour in terms of oxygen production is linear to 3 g L−1. Between > 3—4 g L−1 The production of oxygen is accelerated because the 3 photosynthetic zones are present (saturated, limited and dark) increasing the symmetry of illuminated areas up to 4 g L−1 where the maximum production occurs, the maximum frequency cycles L/D being this fundamental parameter that confirms that it is the instant of maximum integration of light and 2/3 of illuminated volume and only 5% of cells that at no time can come out of the darkness. Between 5—6 g L−1, oxygen production decreases at a low rate because an acceptable high frequency and illuminated fraction are maintained, having 16% and 44% of cells that at no time can come out of the darkness respectively.
Using the dynamic model which has greater accuracy in its results, the maximum PO2 is 310 mgO2 L−1 h−1 has been reached to 4 g L−1 where maximum light integration is achieved, as concentration increases the rate of photosynthesis decreases linearly to 280 mgO2 L−1 h−1 to the 6 g L−1, as the concentration increases, the particles change from saturation to non-saturation state to a dark state without achieving photosynthesis, as has been observed in Fig. 6, the accuracy of the observed results is due to the individual analysis of the particles which directly relates the vertical position and its velocities, it is observed that the higher the vertical velocity, the greater its efficiency of light integration. The static model with light integration assumes that the entire volume is exposed to an average irradiance, such as microalgae located near the surface where the irradiance is greater as particles located in the zone of greater depth where it is a dark volume at high concentrations, it can be observed that the maximum PO2 is 366 mgO2 L−1 h−1 has been reached to 6 g L−1 Because it is the maximum concentration studied, it is observed that the maximum is greater than 6 g L−1 where the stationary zone appears, as it is volumetric method averaged for this analysis is less reliable because it is not possible to identify the particles that are in an irradiance to \({\le I}_{c}\).
Regarding biomass productivity, the surface exposed to light was 60 m2 and the maximum productivity achieved was 39 g m−2 day−1 at 4 g L−1 decreasing to 34 g m−2 day−1 at 6 g L−1 according to the dynamic method, through the static method with light integration the maximum productivity achieved was 46 g m−2 d−1 at 6 g L−1. Doucha and Lívanský (2009) have reported much higher productivity than 38 g m−2 day−1 in a reactor with 224 m2 of surface exposed to light, biomass concentration and productivity is a highly discussed topic due to high experimentation and over the years, cultivation techniques, layer thickness (productivity is very different in 5 and 11 mm) and the area is highly important, for example, as reported Tomáš Grivalský et al. (2019) in Třeboň (Czech Republic) when the second generation of TLP was started from 1963 to 1970 productivity in 900 m2 has increased from 7 to 12 g m−2 day−1 at the best time of year. Since the year 2000 to 2017 the study of the third generation of TLP in 650 m2 was conducted where an increase in the productivity of 14 to 18 g m−2 day−1 was observed. This analysis does not integrate the generation of turbulence and the results obtained have improved considerably with the other experiments carried out, different from Masojídek et al. (2011) who have reported up to 55 g m−2 day−1 in a reactor built with a base formed by series of plates that perform the function of cascades to increase turbulence and that in the path of the microalgae can experience light/dark cycles. This study demonstrates the importance that the plate where the culture is travelling has disturbances to favour vertical mixing. As has been observed, the dynamic model and the static model without a concentration of light show the same trend, both concluding that the optimal concentration of work is 4 g L−1, the static model with light integration assumes a perfect mix therefore the results obtained are overrated.
Mass transfer and oxygen saturation in the reactor
The accumulation of DO in TLP reactors is a serious problem to be solved due to the inhibition of the growth of microalgae, and can be calculated using the following formulas:
where \({K}_{\mathrm{L}}a\) is measured by Eq. 6, \(\overline{{\mu }_{p}}\) is the specific rate of growth in h−1 according to the model of Rubio et al. (2003), \({\mu }_{max}\)= 0.045 h−1 is the maximum growth rate, where it has been assumed that the motion of the particles in the small domain of 0.2 m length is maintained up to 40 m length, \(\frac{d\left[{O}_{2}\right]}{dt}\) has been implemented according to Mendoza et al. (2013b).
Oxygen saturation is directly related to the performance of microalgae cells thus it is a relevant factor in the design and operation of photobioreactors. Accumulation of dissolved oxygen at concentrations higher than 200%Sat and 300%Sat provokes a reduction of productivity of 17% and 25%, respectively (Park et al. 2011). In this sense, the production of oxygen due to photosynthetic metabolism is very fast and, depending on the culture conditions, it can achieve oxygen production rates of 380 mgO2 L−1 h−1 (Barceló-Villalobos et al. 2019b). Because the removal of oxygen is usually poor in the channel of thin-layer reactors there is a natural phenomenon of accumulation of dissolved oxygen along the channel. Results obtained using the dynamic model confirm this behaviour, with data showing that excess dissolved oxygen concentration reduces the photosynthesis rate. To avoid this problem it is necessary to install a desorption unit or bubble column system (see Fig. 1) capable of removing the excess oxygen accumulated. The longer the culture travels exposed to light, the greater the accumulation of oxygen and for this reason for correct reactor design it is essential to evaluate the optimal length of the channelling. The results of the modelling can be studied in Fig. 8. A similar analysis has been previously reported. Petera et al. (2021) performed a first analysis using an Eulerian Model to establish the OD saturation along a reactor, where it established a maximum of 225% DO, indicating that a maximum length of 40 m is safe before growth inhibition is generated. Barceló-Villalobos et al. (2019b) in their study established a safe limit of 250% DO and in their experimental study results range from 141 and 197% DO, recommended levels for most strains, and the diffusion of oxygen into the air is deficient so methods should be sought to avoid this toxicity parameter. Sforza et al. (2020) set by photorespiration found the limit of 200% DO as a safe limit before the productivity falls abruptly, especially at low concentrations but may be more tolerant as culture concentration increases. The experimental analysis by Barceló-Villalobos et al. (2019b) showed that the concentration of DO remains relatively stable along the photosynthetic channel, as seen in Fig. 8, using a dynamic model of photosynthesis, which corroborates this hypothesis. The study carried out by Petera et al. (2021) using a static model assuming the integration of light also supports this finding. To avoid excess DO in the photosynthetic channel, other parameters such as layer height must be considered, as shown in Rearte et al. (2021) who studied a TLP with 6 mm layer thickness and 20 m length. Their results ranged from 135 to 398% OD. Therefore, it is mandatory to have a system of elimination of DO and to demonstrate that the depth layer of cultivation is not beneficial, especially during times of maximum solar radiation. To decrease this inhibitory effect, much higher concentrations are required to increase photo saturation.
Controlling the biomass concentration is essential to avoid photosaturation, and it is also important to achieve a light regime as close to light integration as possible. This issue has received little attention when designing this type of reactor. Our simulations show that for cultures at 1 and 2 g L−1, no DO saturation problems occur. However, for biomass concentrations of 3 g L−1 and above, the O2 concentration becomes too high. If oxygen transfer to the environment of the photosynthetic channel is taken into account, it is possible to operate at higher biomass concentrations as the O2 concentration can be kept below the safe limit of 250%, with 4 g L−1 being the ideal concentration. Beyond 4 g L−1, saturation decreases due to the increase in photolimitation and photorespiration, especially in the dark zone as shown in Fig. 4. In terms of the light regime, increasing the frequency of L/D cycles (\(\nu\)) decreases the saturated zone, which is essential for the good photosynthetic health of microalgae, since those photosynthesizing in the limited zone take the best advantage of the light. Beyond 6 g L−1 (44% of cells in darkness), it is less advisable to operate, as photorespiration increases, but due to the high concentration, acceptable productivity and saturation close to the limit of 225% can still be maintained. Therefore, it is only advisable to operate at 3 g L−1, and it is necessary to force oxygen diffusion. In practice, cases, where the diffusion to the environment is almost zero, are more common.
Conclusion
The design of TLP encompasses a wide range of factors, from fluid dynamics to the photosynthetic capacity of the microalgae culture. This study highlights the crucial role of culture concentration in affecting light regime and photosynthesis, which are important design parameters in preventing the accumulation of dissolved oxygen, a known inhibitor of the system's productivity. Results confirm that the optimal biomass concentration in thin-layer reactors is in the range of 4 g L−1 for optimal light utilization whereas the overall design of this type of reactor must face the problem to maintain the dissolved oxygen concentration below 225%Sat to allow the maximal performance of the photosynthesis process. CFD software has been demonstrated to be a powerful tool for the optimization of the design and operation of this type of photobioreactor for microalgae production.
Data availability
The data used is confidential.
References
Akhtar S, Ali H, Park CW (2020) Complete evaluation of cell mixing and hydrodynamic performance of thin-layer cascade reactor. Appl Sci 10:746
Ali H, Cheema TA, Yoon HS, Do Y, Park CW (2015) Numerical prediction of algae cell mixing feature in raceway ponds using particle tracing methods. Biotechnol Bioeng 112:297–307
Apel AC, Pfaffinger CE, Basedahl N, Mittwollen N, Göbel J, Sauter J, Brück T, Weuster-Botz D (2017) Open thin-layer cascade reactors for saline microalgae production evaluated in a physically simulated Mediterranean summer climate. Algal Res 25:381–390
Barceló-Villalobos M, Fernández-Del Olmo P, Guzmán JL, Fernández-Sevilla JM, Acién Fernández FG (2019a) Evaluation of photosynthetic light integration by microalgae in a pilot-scale raceway reactor. Bioresour Technol 280:404–411
Barceló-Villalobos M, Serrano CG, Zurano AS, García LA, Maldonado SE, Peña J, Fernández FGA (2019b) Variations of culture parameters in a pilot-scale thin-layer reactor and their influence on the performance of Scenedesmus almeriensis culture. Bioresour Technol Rep 6:190–197
Ben Salah M, Askri F, Slimi K, Ben Nasrallah S (2004) Numerical resolution of the radiative transfer equation in a cylindrical enclosure with the finite-volume method. Int J Heat Mass Transf 47:2501–2509
Brindley C, Acién Fernández FG, Fernández-Sevilla JM (2011) Analysis of light regime in continuous light distributions in photobioreactors. Bioresour Technol 102:3138–3148
Brindley C, Jiménez-Ruíz N, Acién FG, Fernández-Sevilla JM (2016) Light regime optimization in photobioreactors using a dynamic photosynthesis model. Algal Res 16:399–408
Chiarini A, Quadrio M (2021) The light/dark cycle of microalgae in a thin-layer photobioreactor. J Appl Phycol 33:183–195
Chisti Y (2016) Large-Scale production of algal biomass: Raceway ponds. In: Box F, Chisty Y (eds) Algae Biotechnology. Products and processes. Springer, Cham pp 21–40
Costache TA, Acién Fernández FG, Morales MM, Fernández-Sevilla JM, Stamatin I, Molina E (2013) Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Appl Microbiol Biotechnol 97:7627–7637
Courant R, Friedrichs K, Lewy H (1928) Über die partiellen Differenzengleichungen der mathematischen Physik. Math Ann 100:32–74
Doucha J, Lívanský K (2006) Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. J Appl Phycol 18:811–826
Doucha J, Lívanský K (2009) Outdoor open thin-layer microalgal photobioreactor: Potential productivity. J Appl Phycol 21:111–117
Duran JE, Taghipour F, Mohseni M (2010) Irradiance modeling in annular photoreactors using the finite-volume method. J Photochem Photobiol A 215:81–89
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215
Fernández-Sevilla JM, Brindley C, Jiménez-Ruíz N, Acién FG (2018) A simple equation to quantify the effect of frequency of light/dark cycles on the photosynthetic response of microalgae under intermittent light. Algal Res 35:479–487
Fernández del Olmo P, Acién FG, Fernández-Sevilla JM (2021) Analysis of productivity in raceway photobioreactor using computational fluid dynamics particle tracking coupled to a dynamic photosynthesis model. Bioresour Technol 334:125226
Fernández Sevilla JM, Molina Grima E, García Camacho F, Acién Fernández FG, Sánchez Pérez JA (1998) Photolimitation and photoinhibition as factors determining optimal dilution rate to produce eicosapentaenoic acid from cultures of the microalga Isochrysis galbana. Appl Microbiol Biotechnol 50:199–205
Grima EM, Camacho FG, Pérez JAS, Sevilla JMF, Fernández FGA, Gómez AC (1994) A mathematical model of microalgal growth in light-limited chemostat culture. J Chem Technol Biotechnol 61:167–173
Grivalský T, Ranglová K, da Câmara Manoel JA, Lakatos GE, Lhotský R, Masojídek J (2019) Development of thin-layer cascades for microalgae cultivation: milestones (review). Folia Microbiol (Praha) 64:603–614
Huang Q, Liu T, Yang J, Yao L, Gao L (2011) Evaluation of radiative transfer using the finite volume method in cylindrical photoreactors. Chem Eng Sci 66:3930–3940
Inostroza C, Solimeno A, García J, Fernández-Sevilla JM, Acién FG (2021) Improvement of real-scale raceway bioreactors for microalgae production using Computational Fluid Dynamics (CFD). Algal Res 54
Lafarga T (2020) Cultured microalgae and compounds derived thereof for food applications: Strain selection and cultivation, drying, and processing strategies. Food Rev Int 36:559–583
Masojídek J, Kopecký J, Giannelli L, Torzillo G (2011) Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J Ind Microbiol Biotechnol 38:307–317
Masojídek J, Malapascua JR, Kopecký J, Sergejevová M (2015) Thin-layer systems for mass cultivation of microalgae: Flat panels and sloping cascades. In: Prokop A, Bajpai RK, Zappi ME (eds) Algal Biorefineries, vol 2. Products and Refinery Design. Springer, Cham, pp 237–261
Masojídek J, Prášil O (2010) The development of microalgal biotechnology in the Czech Republic. J Indust Microbiol Biotechnol 37:1307–1317
Masojídek J, Ranglová K, Lakatos GE, Silva Benavides AM, Torzillo G (2021) Variables governing photosynthesis and growth in microalgae mass cultures. Processes 9:820
Mendoza JL, Granados MR, de Godos I, Acien FG, Molina E, Banks C, Heaven S (2013a) Fluid-dynamic characterization of real-scale raceway reactors for microalgae production. Biomass Bioenergy 54:267–275
Mendoza JL, Granados MR, de Godos I, Acién FG, Molina E, Heaven S, Banks CJ (2013b) Oxygen transfer and evolution in microalgal culture in open raceways. Bioresour Technol 137:188–195
Molina Grima E, Fernández Sevilla JM, Sánchez Pérez JA, Garcia Camacho F (1996) A study on simultaneous photolimitation and photoinhibition in dense microalgal cultures taking into account incident and averaged irradiances. J Biotechnol 45:59–69
Moroni M, Lorino S, Cicci A, Bravi M (2019) Design and bench-scale hydrodynamic testing of thin-layer wavy photobioreactors. Water (Switzerland) 11:1521
Park JBK, Craggs RJ, Shilton AN (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol 102:35–42
Petera K, Papáček Š, González CI, Fernández-Sevilla JM, Acién Fernández FG (2021) Advanced computational fluid dynamics study of the dissolved oxygen concentration within a thin-layer cascade reactor for microalgae cultivation. Energies 14:7284
Rearte TA, Celis-Plá PSM, Neori A, Masojídek J, Torzillo G, Gómez-Serrano C, Silva Benavides AM, Álvarez-Gómez F, Abdala-Díaz RT, Ranglová K, Caporgno M, Massocato TF, da Silva JC, Al Mahrouqui H, Atzmüller R, Figueroa FL (2021) Photosynthetic performance of Chlorella vulgaris R117 mass culture is moderated by diurnal oxygen gradients in an outdoor thin layer cascade. Algal Res 54:102176
Rubio FC, Camacho FG, Sevilla JM, Chisti Y, Grima EM (2003) A mechanistic model of photosynthesis in microalgae. Biotechnol Bioeng 81:459–473
Schuhfried E, Biasioli F, Aprea E, Cappellin L, Soukoulis C, Ferrigno A, Märk TD, Gasperi F (2011) PTR-MS measurements and analysis of models for the calculation of Henry’s law constants of monosulfides and disulfides. Chemosphere 83:311–317
Schuhfried E, Romano A, Märk TD, Biasioli F (2016) Proton Transfer Reaction-Mass Spectrometry (PTR-MS) as a tool for the determination of mass transfer coefficients. Chem Eng Sci 141:205–213
Severin TS, Apel AC, Brück T, Weuster-Botz D (2018) Investigation of vertical mixing in thin-layer cascade reactors using computational fluid dynamics. Chem Eng Res Des 132:436–444
Severin TS, Brück T, Weuster-Botz D (2019) Validated numerical fluid simulation of a thin-layer cascade photobioreactor in OpenFOAM. Eng Life Sci 19:97–103
Severin TSS (2017) Computational Fluid Dynamics Assisted Design of Thin-Layer Cascade Photobioreactor Components. Doctoral Thesis, Technical University of Munich
Sforza E, Pastore M, Franke SM, Barbera E (2020) Modeling the oxygen inhibition in microalgae: An experimental approach based on photorespirometry. N Biotechnol 59:26–32
Trivedi J, Aila M, Bangwal DP, Kaul S, Garg MO (2015) Algae based biorefinery—How to make sense? Renew Sustain Energy Rev 47:295–307
Wang B, Lan CQ, Horsman M (2012) Closed photobioreactors for production of microalgal biomasses. Biotechnol Adv 30:904–912
Funding
This work forms part of the SABANA Project of the European Union’s Horizon 2020- Research and Innovation Framework Programme (Grant Agreement 727874), the Horizon Europe Framework Programme for Research and Innovation (2021–2027) under the agreement of grant no. 101060991 REALM, and was founded by the Ministerio de Ciencia e Innovación (grant TED2021-131555B-C21-ALGAHUB). Thanks to the personnel from the IFAPA research centre in Almeria for their support during this research.
Author information
Authors and Affiliations
Contributions
Cristian Inostroza wrote the article and made the simulations and data analysis. Štěpán Papáček worked on the data analysis and writing of the article. José María Fernández Sevilla collaborated in the data analysis and writing of the article. F. Gabriel Acién also participated in the data review, discussion and preparation of the final document.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Inostroza, C., Papáček, Š., Fernández-Sevilla, J.M. et al. Optimization of thin-layer photobioreactors for the production of microalgae by integrating fluid-dynamic and photosynthesis rate aspects. J Appl Phycol 35, 2111–2123 (2023). https://doi.org/10.1007/s10811-023-03050-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03050-8