Abstract
Macrophytes vary in their ability to utilize carbon in the form of HCO3− and/or CO2 for photosynthesis. Some functional groups that solely use CO2 for photosynthesis could receive competitive advantages from the predicted increase in CO2 compared to groups with efficient carbon acquisition strategies of HCO3−. The aim of this study was to identify carbon use strategies in the common macrophytes (macroalgae, charophytes, seagrass, and other angiosperms) that represent a broad range of functional traits to CO2 concentrations in the northeastern Baltic Sea. Mechanistic assessment of the carbon physiology of macrophytes was used to predict productivity and competitive interactions between different functional groups under future climate. Carbon use strategies in macrophytes were determined by analysing the carbon isotopes (δ13C), pH drift experiments, and photosynthesis versus dissolved inorganic carbon. In addition, habitat mapping data was used to interpret the potential implications of the elevated CO2 to this coastal ecosystem. The results suggested that the primary productivity of macrophytes is often limited by carbon availability, and the increasing CO2 concentrations in the brackish Baltic Sea are expected to enhance photosynthetic production. While all species tested showed evidence of carbon concentrating mechanisms (CCMs), differential levels of CCM activity indicate varying levels of competitive fitness in a future high-CO2 environment. Overall, macrophytes which inhabit the shallowest and deepest parts of the vegetated zone are expected to experience physiological benefits under future CO2 conditions, while intermediate communities dominated by the perennial brown alga Fucus vesiculosus may experience loss of fitness. These fitness differences have implications for competitive interaction and species range under future climate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Changing seawater carbon chemistry driven by increasing atmospheric carbon dioxide (CO2) is likely to have broad implications for marine ecosystems and the services that they provide (IPCC 2022). As suggested by the term “Ocean Acidification”, research to date on elevated ocean carbon has predicted predominantly negative consequences for calcifying organisms (e.g. Kuffner et al. 2008; Kroeker et al. 2013; Wittmann and Pörtner 2013; Cornwall et al. 2014; Bednaršek et al. 2019). For non-calcifying macroalgae and seagrasses, an increase in CO2 concentration has often been shown to enhance photosynthesis and boost primary productivity (Koch et al. 2013; Kram et al. 2016). However, the possible impacts of the predicted ~ 200% increase in CO2 concentrations over the next 100 years on the photosynthesis of foundation primary producers have received less attention (IPCC 2022). In addition to elevated carbon concentrations in seawater, organisms will also be affected by a shift in the relative speciation of available inorganic carbon (Koch et al. 2013; Wittmann and Pörtner 2013; van der Loos et al. 2019). Carbon dioxide is the primary substrate for carbon fixation by RUBISCO (Beer et al. 2014) and an increase in this energetically inexpensive form has the potential to enhance primary productivity broadly (Hepburn et al. 2011; Koch et al. 2013).
The majority of submerged macrophytes have CO2 concentrating mechanisms (CCMs) and the ability to utilise the more abundant HCO3− (~ 90% under current pH) as the carbon source for photosynthesis (Giordano et al. 2005). Carbon concentrating mechanisms act mainly via the direct uptake of HCO3− or its conversion into CO2 through the action of internal and/or external carbonic anhydrase (Giordano et al. 2005). There are different types of CCMs present in macrophytes with varying energetic performances (Giordano et al. 2005; Cornwall et al. 2017); this can drive differential organismal responses to elevated carbon. Functional groups include CCM species with a low affinity for DIC (carbon-limited CCM species), CCM species that can downregulate HCO3− use for photosynthesis at high CO2 concentrations, and CCM species with high affinity for CO2 (CCM does not downregulate at high CO2) (Giordano et al. 2005). Macrophytes that possess CCMs and are capable of utilizing additional CO2 will likely benefit from ocean acidification, while species that cannot utilize additional CO2 will not gain a photosynthetic advantage from OA. Understanding the carbon use strategies of key macrophyte species is essential in predicting how marine ecosystems will function under increased CO2 concentrations (e.g. Hepburn et al. 2011). Appropriate management and protection of key habitat-forming species could reduce the impacts of a changing marine environment.
The photosynthesis of primary producers in coastal waters is most often limited by light (Desmond et al. 2015) and nitrogen availability (Elser et al. 2007). The dissolved inorganic carbon (DIC) concentration in the ocean is around 2.4 mM (Cole et al. 2021) which is generally not considered limiting for photosynthesis. Some earlier studies have shown that macroalgal productivity is not saturated at in situ DIC concentrations (Holbrook et al. 1988; Surif and Raven 1989). In fact, high photosynthetic rates, slow diffusion, and limited mixing often result in situations where DIC is depleted at the cell surface, limiting productivity (Wheeler 1980). This is particularly likely during periods with high light and sufficient nutrients combined with restricted water flow. Dense macrophyte beds can create a hydrodynamic environment of slowed flow and reduced mixing (Hurd 2000), leading to nutrient drawdown (e.g. Stephens and Hepburn 2014) and localized carbon limitation within the macrophyte community (France and Holmquist 1997). Additional CO2 delivery from anthropogenic emissions into carbon-limited systems could broadly influence primary productivity and alter established competitive interactions.
In the Baltic Sea dissolved inorganic carbon is spatially and temporally variable and strongly correlated with salinity and alkalinity (Müller et al. 2016). Moreover, the carbonate system in the Baltic Sea region is also affected by the atmosphere–seawater gas exchange, river runoff, sediments, eutrophication, hydrological processes (upwelling) and biological productivity (Kulinski and Pempkowiak 2012). Broader differences in the characteristics of different water masses can also occur (Müller et al. 2016). The Baltic Sea is characterized by a range of productive benthic coastal ecosystems that typically receive high levels of light and are subjected to extended periods of low water motion. This highly productive system can be viewed as the ideal environment to study the carbon limitation of photosynthesis, particularly during periods of high light and low wind typical in the spring and summer.
The Baltic Sea hosts many macrophyte species that provide food and shelter for numerous plant and animal species and are an important spawning substrate for fish (Kautsky et al. 2017). Moreover, submerged macrophytes have shown a potential to mitigate and adapt to climate change through the uptake and storage of carbon (Kennedy et al. 2010; McLeod et al. 2011; Krause-Jensen and Duarte 2016). So far, macrophyte research in the Baltic Sea has primarily focused on how spatial and temporal patterns of salinity, light, temperature, and nutrient load define the patterns of biomass and productivity of these important primary producers (e.g. Martin et al. 2006; Paalme et al. 2011; Jonsson et al. 2018; Barboza et al. 2019).
Charophytes (Chara spp.) are algae with well-developed complex thalli, anchored to sediment by rhizoids, and forming dense beds in shallow sheltered soft-bottom habitats (Schubert and Blindow 2003). Benthic macroalgae dominate in rocky shores habitats in the Baltic Sea (Kautsky et al. 2017). The most important habitat-forming species in shallow-water hard-bottom algal communities in the NE Baltic is bladderwrack Fucus vesiculosus (Torn et al. 2006; Jonsson et al. 2018). Fast-growing filamentous macroalgae (e.g. Cladophora glomerata, Ulva intestinalis, and Pylaiella littoralis) are very common in the whole shallow coastal zone (Eriksson and Bergström 2005). Red macroalgae grow as a rule in deeper water, where attached Furcellaria lumbricalis is the main habitat-forming species (Bučas et al. 2009). In addition to the attached form, the unique unattached loose-lying red algal community formed primarily by F. lumbricalis, in association with Coccotylus truncatus inhabits the soft bottom of Kassari Bay (Kersen 2013). Zostera marina is the only seagrass in the NE Baltic, but as one of the most common macrophytes it is regarded as a keystone species on sandy bottoms (Boström et al. 2014). Submerged angiosperms Myriophyllum spicatum and Stuckenia pectinata are widespread in soft-bottom substrates and often form mixed communities with Z. marina (Gustafsson 2013).
In previous work we found that macroalgae in the northeastern part of the Baltic Sea are expected to respond differentially to elevated carbon (Albert et al. 2020; Pajusalu et al. 2020). The aim of this study was to identify carbon use strategies in the most common macrophytes (macroalgae, charophytes, seagrass, and other angiosperms), representing a broad range of functional traits to CO2 concentrations in the northeastern Baltic Sea. Mechanistic assessment of the carbon physiology of macrophytes was used to predict productivity and competitive interactions between different functional groups under future climate. Carbon use strategies in macrophytes are determined by the natural abundances of carbon isotopes (δ13C) (Raven et al. 2002), pH drift experiments (Maberly 1990), and measurements of photosynthesis versus (vs.) dissolved inorganic carbon (P vs. DIC) (Beardall and Roberts 1999). In addition, extensive habitat mapping data was used to advise potential implications of the increased CO2 concentrations to benthic communities in the northeastern part of the Baltic Sea.
In the current study we predict that DIC limitation would be most severe within sheltered, dense, shallow water charophyte beds (Chara spp.) and that species in these habitats would exhibit strong affinities for HCO3−. On the other hand, DIC limitation in shallow-water hard-bottom algal communities dominated by the slow metabolism brown algal species Fucus vesiculosus is likely to be less severe. Fast-growing filamentous macroalgae would respond positively to elevated CO2, as this would enhance their photosynthesis in dense macroalgal communities with high-CO2 demand during the daytime. We predict that red macroalgae inhabiting deeper water would respond positively to elevated CO2 due to energetic constraints of active carbon uptake in a low-light environment. The responses of angiosperms would be likewise defined by their thallus height and density as well as the exposure of their habitat. The only seagrass in the northeastern Baltic Sea, Z. marina, has shorter leaves, and its habitat is less dense compared to its oceanic conspecifics. As its habitat is primarily found in moderately exposed soft bottom areas, responses of Z. marina to elevated CO2 would be similar to hard bottom macroalgal communities.
Materials & methods
Studied macrophyte species and collection sites
The study focuses on the most dominant macrophyte species in different benthic communities in the northeastern part of the Baltic Sea (Phytobenthos databases of the Estonian Marine Institute, University of Tartu). For this study, we selected six groups of macrophytes that grow in different habitats throughout the Estonian coastal waters (Table 1).
For laboratory experiments, macrophyte species were collected around Saaremaa Island in the West Estonian Archipelago Sea (northern Baltic Sea) in five different bays in July 2018 (Fig. 1; Table 1). All specimens were placed in coolers containing water collected at the site and transported to the lab immediately and cleaned of visible epiphytes prior to the start of the laboratory experiments.
Photosynthesis vs. DIC curves
The photosynthesis of each macrophyte species was quantified at different dissolved inorganic carbon levels to analyse the carbon acquisition of macrophytes across a range of DIC concentrations. For this experiment five macrophyte species were selected, representing the greatest biomass contributors to their benthic community. After harvesting, the individuals were acclimated in seawater chambers with gentle aeration for 24–72 h before experiments. Before experimental use, all seawater was sterilized using an AquaCristal 18W ultraviolet sterilizer and passed through a 1.2 μm pore size glass microfiber filter (GF/F, Ø 47 mm). To maintain the pH throughout each experimental trial and prevent subsequent changes in carbon speciation, seawater was buffered with 15 mM Tris. Since Tris buffer has been known to inhibit the productivity of some macroalgal species, the effect of Tris buffer on each experimental species was tested by measuring the O2 evolution of individuals (Axelsson et al. 2000), and no significant effect at p < 0.05 was found. These tests also indicated that pH within buffered chambers remained stable for the experimental duration.
Inorganic carbon was removed from the Tris-buffered seawater by sparging with nitrogen gas (following the method in Beardall and Roberts 1999). The seawater pH was lowered to 3 with 1 M HCl solution. Acidified seawater was bubbled with nitrogen gas for 2 h and then 1 M NaOH was used to raise pH to the in-situ level of 8.2. Species were sealed in 245 mL cylindrical airtight chambers under a photosynthetically saturating irradiance of 400 μmol photons m−2 s−1. The water temperature in chambers was maintained at 10 °C with a flowing water bath. Oxygen levels within each chamber were monitored using Ocean Opticsʼ NeoFox-TP oxygen probes connected to Ocean Opticsʼ NeoFox Viewer software. A two-point calibration, achieved by bubbling seawater with nitrogen and ambient air, was used to configure the oxygen probes before experimental use. Stir bars within each chamber provided mixing and prevented the formation of that could inhibit photosynthesis.
Before the first addition of the DIC solution, the oxygen level in each chamber was monitored for ~ 15 min to ensure that the internal carbon stores of the species were depleted and that there was no significant carbon remaining in the chamber. Aliquots of 0.3 M NaHCO3¯ solution were injected into the O2 evolution chamber at 8–10 min intervals to increase the DIC concentration (working concentrations: 0.2, 0.6, 1.2, 2, 3, 4.5, 7, 10 mM). Following the conclusion of the trial, samples were dried at 60 °C until a constant weight was achieved. Photosynthetic rates of each species at each DIC concentration were normalized to dry weight (µmol O2 h−1 gDW−1) for further analysis.
pH drift experiment
The pH drift experiment aimed to determine the relative HCO3− affinity of macrophyte species (e.g. Maberly 1990; Murru and Sandgren 2004; Cornwall et al. 2012). Since carbon speciation is coupled with acidity, macrophytes that exclusively fix CO2 (obligate CO2 users) cannot raise the pH of water beyond a certain threshold. At pH levels of 9 in seawater (≈ 35 psu and 2300 µmol L−1 carbonate alkalinity) and above, inorganic carbon in seawater is primarily available as HCO3− and any trace amounts of CO2 still present are too low to support normal photosynthetic activity for obligate CO2 users. Due to differences in salinity and DIC availability between the seawater and the brackish water used in this study, a revised pH compensation point was calculated from data from the sites using the R package seacarb (Gattuso et al. 2022). In this study, the pH compensation point was taken to be 9.2 for brackish water. The pH drift method is a simple and effective way of interrogating macrophyte carbon physiology.
Approximately ~ 0.5–0.6 g wet weight section was cut from each field species and acclimatised for 24 h in tanks of sterile filtered seawater at ambient temperature. Each replicate (n = 3 per species) was sealed into an air-tight 60 mL clear plastic chamber filled with seawater at pH 8.15 and placed onto a shaker table set to 70 rpm. The location of each container on the shaker table was randomized and specimens were maintained under a continuous irradiance of 65 μmol photons m–2 s–1 at 15 °C for the duration of the experiment.
Measurements of pHNBS (National Bureau of Standards scale) in the chambers were recorded at 24, 32, and 48 h. After the final timepoint, species were removed, and containers were left open for 48 h to allow re-equilibration with the atmosphere. At this time, pH was measured again to test for the effect of macrophyte exudates on seawater pH. All pH readings were taken with a Mettler Toledo InLab Expert Pro-ISM-IP67 pH-electrode (accuracy: ± 0.001 and resolution: ± 0.002) connected to a Mettler Toledo model Seven2Go pro S8 pH/Ion meter and was calibrated with NBS buffers.
Carbon isotope (δ13C) analysis
Carbon isotope (δ13C) tissue analysis was conducted on macrophyte species (n = 3 per species) to determine each species’ dependency on diffusive CO2 supply vs. carbon concentrating mechanism (CCM) for carbon acquisition. In general, δ13C values below –30‰ indicate a reliance on diffusive CO2 supply and the absence of a functional CCM. Values of δ13C above –10‰ indicate the presence of an active CCM. Macrophytes with δ13C tissue values that fall between –30 and –10‰ are likely to have a combination of carbon uptake methods at their disposal (Raven et al. 2002).
Macrophyte species were cleaned of visible epiphytes and then dried at 60 °C until a constant weight was achieved. A mortar and pestle were used to grind dried species to a fine powder which was stored in microcentrifuge tubes until analysis. To prevent cross-contamination, mortar and pestle were cleaned with acetone between species. For analysis, 1 mg of sample was weighed into capsules and processed using an elemental analyzer (FlashEA 1112 HT, Thermo Scientific) interfaced through a ConFlo IV dilutor device (Thermo Scientific) with an isotope ratio mass spectrometer (Delta V Plus, Thermo Scientific). The C isotope composition is reported as per mill respective to Vienna Pee Dee Belemnite (V-PDB) and calibrated using international IAEA standards IAEA-CH-3 and IAEA-CH-6. Long-term reproducibility precision and accuracy were ± 0.1‰.
Statistical methods
For the P vs. DIC experiment statistical analysis was conducted using the R statistical software platform (R Core Team, 2017). The Michaelis–Menten curve (Johnson and Goody 2011) was fitted to plots of photosynthetic rate vs. DIC concentration. The Michaelis–Menten equation is P = Pmax / DIC + K0.5, where Pmax is the point at which the maximum photosynthetic rate of the organism is reached, and K0.5 is the concentration of DIC at which the photosynthetic rate of the organism is half of Pmax (Johnson and Goody 2011). A maximum likelihood, non-linear mixed effects modelling approach, using the lme4 package in R (Bates et al. 2015) was used. This approach allowed a single model to be used, whilst accounting for species-level (fixed factor) and individual-level (random factor, replicate incubation id) variability in Pmax and K0.5. Within-species comparisons of Pmax and K0.5 were made using simultaneous t-tests, using the R package multcomp (Hothorn et al. 2008). For the pH drift experiment, Kruskal–Wallis H test was used to analyse differences in pH compensation points (48-h values) among macrophyte species using the Statistica (TIBCO Software Inc.) version 13. Spearman's Rank-Order correlation was conducted to find the relationship between pH compensation points and δ13C values of studied species. For all statistical analyses, a probability of 0.05 was used to determine statistical significance.
Published maps of macrophyte species obtained from an open-source data PlanWise4Blue portal (https://gis.sea.ee/adrienne) allowed us to assess the potential implications of the elevated CO2 environment to the coastal ecosystem of the Baltic Sea.
Results
Carbon physiology
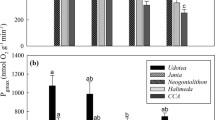
Zostera marina had significantly higher Pmax than C. aspera (post-hoc test; p = 0.040), F. vesiculosus (p = 0.020), and P. littoralis (p = 0.031), but not F. lumbricalis (unattached) (p = 0.057) (Fig. 2; Table 2). Zostera marina had higher K0.5 compared to F. vesiculosus (p = 0.047) and P. littoralis (p = 0.006). No other statistically significant differences in Pmax or K0.5 were observed.
The results from the pH drift experiments showed that all studied species (n = 3 per species) within 6 groups raised pH above 9.2 (Fig. 3), suggesting that they can all use HCO3− for photosynthesis. pH compensation points ranged between 9.31 and 10.51 for the studied species. Red macroalgae did exhibit a significantly lower pH compensation point than filamentous algae (Kruskal Wallis H test, p < 0.001) and Chara (p < 0.001); however, there was no significant difference compared to other studied macrophyte groups (p > 0.05) (Fig. 3). Chara and filamentous algae had higher pH compensation points compared to other studied species. In addition, Zostera and other angiosperms had higher pH compensation points compared to Fucus and red macroalgae. There was a correlation between pH compensation points and δ13C values of the studied macrophyte species (Spearman R = 0.34, p < 0.05).
The carbon isotope (δ13C) values of studied species (n = 3 per species) fall between –30 and –10‰. The only exception was the red macroalgal species Coccotylus truncatus with δ13C value estimated at –36.20 ± 0.11‰ (mean ± SE). The highest mean δ13C value was measured for seagrass Zostera followed by other angiosperms, Fucus and Chara (Fig. 3). Compared to other studied species, filamentous macroalgae had more negative mean δ13C value, and red macroalgae had substantially more negative value with high differences among species (Fig. 3).
Discussion
This study demonstrates that the common submerged macrophytes (macroalgae, charophytes, seagrass, and other angiosperms) in the Baltic Sea likely have carbon concentrating mechanisms (CCMs). All studied species, except for the red macroalga C. truncatus, have the mechanism to take up both carbon forms CO2 and HCO3− for photosynthesis. Our results showed a high level of variability in carbon isotope discrimination in studied species within 6 groups, indicating the use of a different type of CCMs.
Chara
The pH drift experiment showed that the Chara group has the best capability to raise pH (above 10) compared to other macrophytes, although a significant difference was only detected in comparison with red macroalgae. This indicates an effective CCM operation since at pH 9.2 in brackish water the concentration of CO2 is too low to support photosynthetic activity for obligate CO2 users. The high δ13C values of the Chara group also show the use of HCO3− for photosynthesis. It could be suggested that in sheltered dense charophyte beds, energetically inexpensive CO2 is used up quickly, and therefore they have developed an effective CCM. However, the results of the DIC experiment indicated that C. aspera, which is the dominant species of the studied community, might be carbon limited by natural DIC levels. Pajusalu et al. (2015) found that C. aspera in the Estonian coastal waters showed a slightly positive response to elevated pCO2 on a short-term basis. In addition, Chara tomentosa L. from the same study area exhibited a significant increase in net primary production rates when the CO2 concentration was elevated up to 2000 µatm. Therefore, Chara could gain the photosynthetic advantage of elevated CO2 by downregulating the CCM operation e.g., switch from an HCO3− to CO2-based metabolism. This could mean that increased CO2 concentration will have an overall positive impact on sheltered parts of the northeastern Baltic Sea where Chara spp. grow.
Fucus
The perennial brown alga F. vesiculosus had a high δ13C value (close to –10‰) and a final pH value above 10, indicating an efficient use of HCO3−. The results of the DIC experiment showed that the photosynthetic rate of Fucus only slightly increased with increasing DIC concentrations in water (Albert et al. 2020). The low K0.5 value of Fucus suggests a high affinity for DIC, and the carbon saturation is achieved at the same concentrations as naturally occurring in the shallow coastal Baltic Sea. This is consistent with our earlier research, which showed that Fucus, with a slow metabolism, had no response to increased CO2 levels, at least on a short-term basis in the northern Baltic Sea (Pajusalu et al. 2013). Similarly, Graiff et al. (2015) found a weak positive effect of increased pCO2 levels on the growth of F. vesiculosus in the western Baltic Sea.
Filamentous macroalgae
This study focused on the three most dominant fast-growing filamentous macroalgae – U. intestinalis, C. glomerata (greens), and P. littoralis (brown) in the northern Baltic Sea. These filamentous algae had lower δ13C values than other tested groups, with the exception of the red macroalgae. The pH compensation point of filamentous algae was well over pH 10, indicating the use of HCO3−. Larsson et al. (1997) investigated two different carbon acquisition systems for U. intestinalis and showed that species growing in different habitats use different HCO3− acquisition mechanisms for photosynthesis. Pajusalu et al. (2013) showed that increased CO2 levels in seawater favoured the photosynthetic activity of U. intestinalis. Cornwall et al. (2012) found that the proportion of CO2 compared to HCO3− used in photosynthesis increased under short-term CO2 enrichment in the fleshy Ulva spp. It could be suggested that U. intestinalis has efficient use of HCO3− under high pH and low CO2 conditions, and species can also downregulate energetically expensive HCO3− use when there is enough CO2 available. Green macroalgae differ from brown algae in having an inducible mechanism allowing photosynthesis at high pH, probably via HCO3− (Carlberg et al. 1990). The low K0.5 value of P. littoralis suggests that this species is carbon saturated or close to saturation at current DIC concentrations in seawater. Moreover, the results of the DIC experiment showed no difference between the photosynthetic rates of filamentous P. littoralis and perennial alga F. vesiculosus at different DIC concentrations (Albert et al. 2020). Similarly, our research has shown that the nutrient uptake mechanisms of P. littoralis were similar to F. vesiculosus but were significantly different compared to U. intestinalis (authors’ unpublished data). There are likely different types of CCMs present in the studied filamentous macroalgae. Brown filamentous algae had a CCM with a high affinity for DIC, while green algae might downregulate the HCO3− use when there is enough CO2 available (lower CCM activity at high CO2 concentrations).
Red macroalgae
Red macroalgae typically found in deeper waters exhibited the lowest average δ13C value compared to other studied groups, indicating less effective CCM. This was especially true for C. truncatus whose δ13C value was outside of the considered range of any bicarbonate use (< –30%). Similarly, the lowest average pH compensation point was measured for red macroalgae; however, there was a significant difference only with Chara and filamentous algae. However, all studied red macroalgae were able to raise the pH above 9.2, suggesting that they use HCO3− for photosynthesis. Earlier studies have found that many red algal species lack CCMs and use only CO2 for photosynthesis, probably due to energetic constraints of active carbon uptake in a low-light environment (Raven et al. 2005; Hepburn et al. 2011; Kübler and Dudgeon 2015). Based on the DIC experiment F. lumbricalis (unattached) might be carbon limited, due to low affinity for DIC (carbon-limited species), however, the photosynthetic gain from OA is likely lower compared to C. truncatus (Pajusalu et al. 2020) and C. tenuicorne (Albert et al. 2020). Our results suggested that red macroalgae preferentially use CO2 over HCO3−, i.e., they might downregulate HCO3− when there is enough CO2 available and are likely benefit the most out of all groups studied under future elevated CO2 conditions.
Other angiosperms
As expected, based on the δ13C and final pH values, other angiosperms are similar to the seagrass Z. marina. The efficiency of HCO3− uptake mechanisms in submerged angiosperms varies largely between families and species (Hussner et al. 2016). Similarly, earlier research has shown that both species, S. pectinata and M. spicatum can use HCO3− and CO2 for photosynthesis (Madsen and Sand-Jensen 1991; Maberly and Madsen 2002; Hussner et al. 2016). Furthermore, M. spicatum seems to be the most efficient HCO3− user compared to M. heterophyllum and M. aquaticum within this genus. Thus, M. spicatum seems to be the better competitor in dense macrophyte beds with self-induced CO2 depletion during the day in the field, but also M. heterophyllum can withstand and grow under CO2 depletion even at pH > 10 (Hussner et al. 2015).
Zostera
The eelgrass Z. marina δ13C value was close to –10‰, consistent with the common range of values previously presented in the literature (Raven et al. 2002). The pH drift experiment showed that Z. marina could raise pH above 10, indicating the use of HCO3−. Numerous studies have suggested that many species of seagrass, including Z. marina, utilize HCO3− for photosynthesis (e.g. Invers et al. 1999; Raven et al. 2002). For example, Beer and Rehnberg (1997) found that Z. marina uses HCO3− as a major source of inorganic carbon, and bulk CO2 contributed only marginally (less than 20%) for photosynthesis at pH 8.2. Furthermore, Young et al. (2018) found that δ13C signatures of Z. marina decreased significantly when exposed to higher CO2, and isotopic mixing models suggested a switch from primary HCO3− use to CO2 use and potential downregulation of CCM. Our pH drift experiment and δ13C value showed an effective CCM operation. However, our DIC experiment showed that the photosynthetic half-saturation point of Z. marina was achieved at ~ 2.6 mM of DIC concentration. Nevertheless, the DIC concentration was ~ 1.4 mM measured in the current study in Zostera natural community. This suggests that even though Z. marina possesses an effective CCM the current availability of DIC in the coastal Baltic Sea does not always meet the demand for the species when growing in dense stands. Our results from the DIC experiment showed that the photosynthetic rates of Z. marina were significantly higher than other studied species, allowing greater use of DIC. Also, studies in the Atlantic Ocean have shown that the photosynthesis of Z. marina might be limited by the current DIC availability (Beer and Rehnberg 1997; Zimmerman et al. 1997), despite the fact that the concentration of DIC is higher in the Atlantic Ocean (~ 2.2 mM) (Ohlson 1991) than in the Baltic Sea. Our findings indicate a positive impact of the increase of CO2 concentration on Z. marina, especially in the case of possible downregulation of CCM as referred by Young et al. (2018).
Potential implications for future development of macrophyte communities
The northeastern part of the Baltic Sea has extensive shallows with dense populations of various macrophyte species of marine, brackish and freshwater origin (Kautsky et al. 2017). The current study suggested that the primary productivity of macrophytes is often limited by carbon availability during their active growth period, and additional CO2 delivery into this coastal ecosystem could enhance primary productivity. However, the experiments in this study indicated that macrophytes have different types of carbon-concentrating mechanisms, and as a result various responses to elevated CO2.
Elevated carbon will have a positive impact on sheltered parts of the shallow coastal northern Baltic Sea where charophytes (Chara spp.) grow. By contrast, the habitat-forming brown algae F. vesiculosus will not experience significant photosynthetic benefits under future CO2 conditions. Lower competitive fitness of this species under future carbon conditions could lead to shifts in the distribution of F. vesiculosus habitat, with implications for species richness. Fucus vesiculosus habitat is the most species-rich macroalgal habitat in the Baltic Sea, and a reduction in the area of such habitat will inevitably lead to a reduction in species richness (Schagerström et al. 2014). In the shallowest part of the vegetated zone dominated by green filamentous algae, an expansion of the algal range is expected.
Red macroalgal species that typically grow in deeper waters likely will benefit the most under future elevated CO2 concentrations. However, we predict the likely loss of the drifting form of F. lumbricalis community due to competitive interaction with C. truncatus under elevated CO2 conditions. Thus, the elevated carbon has the potential to influence the efficiency of the wild harvest of this loose-lying red algal community and the quality of the product provided. Other angiosperms that are widespread in sheltered soft-bottom and often form mixed communities with Z. marina are expected to experience a stable range under future conditions. The photosynthesis of Zostera is expected to enhance under elevated CO2 conditions in the northern Baltic Sea where the species grows in its lowest observed salinity levels. Overall, macrophytes which inhabit the shallowest and deepest parts of the vegetated zone are expected to experience physiological benefits under future CO2 conditions, while intermediate communities dominated by the perennial brown alga F. vesiculosus may experience loss of fitness. These fitness differences have implications for competitive interaction and species range under future climate (Fig. 4).
Hotspot areas of the key macrophyte species (obtained from https://gis.sea.ee/adrienne) and their expected trends under elevated CO2 concentrations in the study area (based on the results of the experiments in this article): blue indicates an increase in depth range, yellow a stable range and red a decrease in depth range
Data availability
Data and material will be made available upon reasonable request.
References
Albert G, Hepburn CD, Pajusalu L, Paalme T, Pritchard DW, Martin G (2020) Could ocean acidification influence epiphytism? A comparison of carbon-use strategies between Fucus vesiculosus and its epiphytes in the Baltic Sea. J Appl Phycol 32:2479–2487
Axelsson L, Mercado JM, Figueroa FL (2000) Utilization of HCO3- at high pH by the brown macroalga Laminaria saccharina. Eur J Phycol 35:53–59
Barboza FR, Kotta J, Weinberger F, Jormalainen V, Kraufvelin P, Molis M, Schubert H, Pavia H, Nylund GM, Kautsky L, Schagerström E, Rickert E, Saha M, Fredriksen S, Martin G, Torn K, Ruuskanen A, Wahl M (2019) Geographic trait variation of the bladderwrack Fucus vesiculosus along the Baltic Sea-North Sea salinity gradient. Ecol Evol 9:9225–9238
Bates D, Maechler M, Bolker B, Walker S (2015) lme4: Linear mixed- effects models using Eigen and S4. version 1.1–31. https://cran.rproject.org/web/packages/lme4/lme4.pdf accessed 10 December 2022
Beardall J, Roberts S (1999) Inorganic carbon acquisition by two Antarctic macroalgae, Porphyra endiviifolium (Rhodophyta: Bangiales) and Palmaria decipiens (Rhodophyta: Palmariales). Polar Biol 21:310–315
Bednaršek N, Feely RA, Howes EL, Hunt BP, Kessouri F, León P, Lischka S, Maas AE, McLaughlin K, Nezlin NP, Sutula M, Weisberg SB (2019) Systematic review and meta-analysis toward synthesis of thresholds of ocean acidification impacts on calcifying pteropods and interactions with warming. Front Mar Sci 6:227
Beer S, Björk M, Beardall J (2014) Photosynthesis in the marine environment. Wiley Blackwell, Ames, 224 pp
Beer S, Rehnberg J (1997) The acquisition of inorganic carbon by the seagrass Zostera marina. Aquat Bot 56:277–283
Boström C, Baden S, Bockelmann AC, Dromph K, Fredriksen S, Gustafsson C, Krause-Jensen D, Möllet T, Nielsen SL, Olesen B, Olsen J, Pihl L, Rinde E (2014) Distribution, structure and function of Nordic eelgrass (Zostera marina) ecosystems: implications for coastal management and conservation. Aquat Conserv 24:410–434
Bučas M, Daunys D, Olenin S (2009) Recent distribution and stock assessment of the red alga Furcellaria lumbricalis on the exposed Baltic Sea coast: combined use of field survey and modelling methods. Oceanologia 51:341–359
Carlberg S, Axelsson L, Larsson C, Ryberg H, Uusitalo J (1990) Inducible CO2 concentrating mechanisms in green seaweeds I. Taxonomical and physiological aspects. In: Baltscheffsky M (ed) Current research in photosynthesis. Springer, Dordrecht, pp 3323–3326
Cole JJ, Hararuk O, Solomon CT (2021) The carbon cycle: with a brief introduction to global biogeochemistry. In: Weathers KC, Strayer DL, Likens GE (eds) Fundamentals of ecosystem science, 2nd edn. Academic Press, London, pp 131–160
Cornwall CE, Hepburn CD, Pritchard D, Currie KI, McGraw CM, Hunter KA, Hurd CL (2012) Carbon use strategies in macroalgae: Differential responses to lowered pH and implications for ocean acidification. J Phycol 48:137–144
Cornwall CE, Boyd PW, McGraw CM, Hepburn CD, Pilditch CA, Morris JN, Smith AM, Hurd CL (2014) Diffusion boundary layers ameliorate the negative effects of ocean acidification on the temperate coralline macroalga Arthrocardia corymbosa. PLoS One 9:e97235
Cornwall CE, Revill AT, Hall-Spencer JM, Milazzo M, Raven JA, Hurd CL (2017) Inorganic carbon physiology underpins macroalgal responses to elevated CO2. Sci Rep 7:46297
Desmond MJ, Pritchard DW, Hepburn CD (2015) Light limitation within southern New Zealand kelp forest communities. PLoS One 10:e0123676
Elser JJ, Bracken ME, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Eriksson BK, Bergström L (2005) Local distribution patterns of macroalgae in relation to environmental variables in the northern Baltic Proper. Estuar Coast Shelf S 62:109–117
France RL, Holmquist JG (1997) Delta13C variability of macroalgae: effects of water motion via baffling by seagrasses and mangroves. Mar Ecol Prog Ser 149:305–308
Gattuso J-P, Epitalon J-M, Lavigne H, Orr J (2022) seacarb: Seawater Carbonate Chemistry. R package version 3.3.1 https://CRAN.R-project.org/package=seacarb accessed 10 December 2022
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Graiff A, Bartsch I, Ruth W, Wahl M, Karsten U (2015) Season exerts differential effects of ocean acidification and warming on growth and carbon metabolism of the seaweed Fucus vesiculosus in the western Baltic Sea. Front Mar Sci 2:112
Gustafsson C (2013) Biodiversity and ecosystem functioning in angiosperm communities in the Baltic Sea. PhD Thesis, Åbo Akademi University, Finland 52 pp
Hepburn C, Pritchard D, Cornwall C, McLeod R, Beardall J, Raven J, Hurd C (2011) Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Glob Change Biol 17:2488–2497
Holbrook GP, Beer S, Spencer WE, Reiskind JB, Davis JS, Bowes G (1988) Photosynthesis in marine macroalgae: evidence for carbon limitation. Can J Bot 66:577–582
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J 50:346–363
Hurd CL (2000) Water motion, marine macroalgal physiology, and production. J Phycol 36:453–472
Hussner A, Jahns P (2015) European native Myriophyllum spicatum showed a higher HCO3− use capacity than alien invasive Myriophyllum heterophyllum. Hydrobiologia 746:171–182
Hussner A, Mettler-Altmann T, Weber AP, Sand-Jensen K (2016) Acclimation of photosynthesis to supersaturated CO2 in aquatic plant bicarbonate users. Freshw Biol 61:1720–1732
Invers O, Pérez M, Romero J (1999) Bicarbonate utilization in seagrass photosynthesis: Role of carbonic anhydrase in Posidonia oceanica (L.) Delile and Cymodocea nodosa (Ucria) Ascherson. J Exp Mar Biol Ecol 235:125–133
IPCC (2022) Climate change 2022: impacts, adaptation and vulnerability. Contribution of working group II to the sixth assessment report of the Intergovernmental Panel on Climate change. Pörtner H-O, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, Okem A, Rama B (eds) Cambridge University Press. Cambridge, pp 1–3056
Johnson K, Goody RS (2011) The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry 50:8264–8269
Jonsson PR, Kotta J, Andersson HC, Herkül K, Virtanen E, Sandman AN, Johannesson K (2018) High climate velocity and population fragmentation may constrain climate-driven range shift of the key habitat former Fucus vesiculosus. Divers Distrib 24:892–905
Kautsky H, Martin G, Snoeijs-Leijonmalm P (2017) The phytobenthic zone. In: Snoeijs-Leijonmalm P, Schubert H, Radziejewska T (eds) Biological oceanography of the Baltic Sea. Springer, Dordrecht, pp 387–455
Kennedy H, Beggins J, Duarte CM, Fourqurean JW, Holmer M, Marbà N, Middelburg JJ (2010) Seagrass sediments as a global carbon sink: isotopic constraints. Glob Biogeochem Cycl 24:GB4026
Kersen P (2013) Red Seaweeds Furcellaria lumbricalis and Coccotylus truncatus: community structure, dynamics and growth in the Northern Baltic Sea. PhD Thesis, Tallinn University, Estonia 127 pp
Koch M, Bowes G, Ross C, Zhang XH (2013) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Change Biol 19:103–132
Kram SL, Price NN, Donham EM, Johnson MD, Kelly ELA, Hamilton SL, Smith JE (2016) Variable responses of temperate calcified and fleshy macroalgae to elevated pCO2 and warming. ICES J Mar Sci 73:693–703
Krause-Jensen D, Duarte CM (2016) Substantial role of macroalgae in marine carbon sequestration. Nat Geosci 9:737–742
Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso J-P (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19:1884–1896
Kuffner IB, Andersson AJ, Jokiel PL, Ku‘ulei SR, Mackenzie FT (2008) Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci 1:114–117
Kulinski K, Pempkowiak J (2012) Carbon Cycling in the Baltic Sea. Springer, Heidelberg
Kübler JE, Dudgeon SR (2015) Predicting effects of ocean acidification and warming on algae lacking carbon concentrating mechanisms. PLoS One 10:e0132806
Larsson C, Axelsson L, Ryberg H, Beer S (1997) Photosynthetic carbon utilization by Enteromorpha intestinalis (Chlorophyta) from a Swedish rockpool. Eur J Phycol 32:49–54
Maberly SC (1990) Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J Phycol 26:439–449
Maberly SC, Madsen TV (2002) Freshwater angiosperm carbon concentrating mechanisms: processes and patterns. Funct Plant Biol 29:393–405
McLeod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560
Madsen TV, Sand-Jensen K (1991) Photosynthetic carbon assimilation in aquatic macrophytes. Aquat Bot 41:5–40
Martin G, Paalme T, Torn K (2006) Seasonality pattern of biomass accumulation in a drifting Furcellaria lumbricalis community in the waters of the West Estonian Archipelago, Baltic Sea. J Appl Phycol 18:557–563
Murru M, Sandgren CD (2004) Habitat matters for inorganic carbon acquisition in 38 species of red macroalgae (Rhodophyta) from Puget Sound, Washington, USA. J Phycol 40:837–845
Müller JD, Schneider B, Rehder G (2016) Long-term alkalinity trends in the Baltic Sea and their implications for CO2-induced acidification. Limnol Oceanogr 61:1984–2002
Ohlson M (1991) On the carbonate systems in the Baltic and Weddell seas: Inventories and influences by man. PhD Thesis. University of Gothenburg, Sweden
Paalme T, Kotta J, Kersen P, Martin G, Kukk H, Torn K (2011) Inter-annual variations in biomass of loose lying algae Furcellaria-Coccotylus community: The relative importance of local versus regional environmental factors in the West Estonian Archipelago. Aquat Bot 95:146–152
Pajusalu L, Martin G, Põllumäe A, Paalme T (2013) Results of laboratory and field experiments of the direct effect of increasing CO2 on net primary production of macroalgal species in brackish-water ecosystems. Proc Estonian Acad Sci 62:148–154
Pajusalu L, Martin G, Põllumäe A, Torn K, Paalme T (2015) Direct effects of increased CO2 concentrations in seawater on the net primary production of charophytes in a shallow, coastal, brackish-water ecosystem. Boreal Environ Res 20:413–422
Pajusalu L, Albert G, Fachon E, Hepburn DC, Kotta J, Liversage K, Paalme T, Peterson A, Pritchard WD, Põllumäe A, Torn K, Martin G (2020) Ocean acidification may threaten a unique seaweed community and associated industry in the Baltic Sea. J Appl Phycol 32:2469–2478
Raven JA, Johnston AM, Kübler JE, Korb R, McInroy SG, Handley LL, Scrimgeour CM, Walker DI, Beardall J, Vanderklift M, Fredriksen S, Dunton KH (2002) Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Funct Plant Biol 29:355–378
Raven JA, Ball LA, Beardall J, Giordano M, Maberly SC (2005) Algae lacking carbon-concentrating mechanisms. Can J Bot 83:879–890
Schagerström E, Forslund H, Kautsky L, Pärnoja M, Kotta J (2014) Does thalli complexity and biomass affect the associated flora and fauna of two co-occurring Fucus species in the Baltic Sea? Estuar Coast Shelf Sci 149:187–193
Schubert H, Blindow I (eds) (2003) Charophytes of the Baltic Sea. Koeltz Botanical Books, Köningstein/Taunus, p 326
Stephens TA, Hepburn CD (2014) Mass-transfer gradients across kelp beds influence Macrocystis pyrifera growth over small spatial scales. Mar Ecol Prog Ser 515:97–109
Surif MB, Raven JA (1989) Exogenous inorganic carbon sources for photosynthesis in seawater by members of the Fucales and the Laminariales (Phaeophyta): ecological and taxonomic implications. Oecologia 78:97–105
Torn K, Krause-Jensen D, Martin G (2006) Present and past depth distribution of bladderwrack (Fucus vesiculosus) in the Baltic Sea. Aquat Bot 84:53–62
van der Loos LM, Schmid M, Leal PP, McGraw CM, Britton D, Revill AT, Virtue P, Nichols PD, Hurd CL (2019) Responses of macroalgae to CO2 enrichment cannot be inferred solely from their inorganic carbon uptake strategy. Ecol Evol 9:125–140
Wheeler WN (1980) Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera. Mar Biol 56:103–110
Wittmann AC, Pörtner HO (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Change 3:995–1001
Young CS, Peterson BJ, Gobler CJ (2018) The bloom-forming macroalgae, Ulva, outcompetes the seagrass, Zostera marina, under high CO2 conditions. Estuar Coast 41:2340–2355
Zimmerman RC, Kohrs DG, Steller DL, Alberte RS (1997) Impacts of CO2 enrichment on productivity and light requirements of eelgrass. Plant Physiol 115:599–607
Acknowledgements
This study was carried out during the training workshop ‘Diversity of carbon use strategies in different macrophyte communities in the NE Baltic Sea’, held in the Kõiguste Field Station of the Estonian Marine Institute, University of Tartu on Saaremaa Island in July 2018. We would also like to thank Holar Sepp for help with determine the natural carbon isotope values from the Department of Geology, University of Tartu.
Funding
This study was supported by Estonian National Marine Monitoring programme and development fund of the Department of Marine Biology, Estonian Marine Institute. MARBEFES (MARine Biodiversity and Ecosystem Functioning leading to Ecosystem Services) project, funded by the European Union under the Horizon Europe Programme, “HORIZON-CL6-2021-BIODIV-01” Theme, Grant Agreement no. 101060937, [marbefes.eu].
Author information
Authors and Affiliations
Contributions
LP, CH, JK, TP, DP, GM conceptualized the study; all authors participated in the field study; LP, GA, EF, AK, DP, AP, KT performed the laboratory experiments; LP, GA, JK, TP, DP, AP performed the data analyses; LP wrote the first draft of the manuscript; all authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pajusalu, L., Albert, G., Fachon, E. et al. Species-specific responses of macrophyte production to the increasing CO2 environment with potential ecosystem implications involved in the Baltic Sea. J Appl Phycol 36, 983–994 (2024). https://doi.org/10.1007/s10811-023-03047-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03047-3