Abstract
The use of natural bioactive sources to promote plant growth and crop yield gains, such as those obtained from algae, are in evidence as a sustainable agriculture practice. In this sense, recently the calcareous alga Lithothamnium sp. had its bioactive plant growth promoting effect related to the presence of high degree of humic acid with pronounced auxin-like effects, while the cyanobacterium Arthrospira platensis (Spirulina plantensis) has the plant growth promoting effect attributed to a pool of bioactive molecules, such as the free L-amino acids and polyamines. To explore the effect of algae sources combinations as plant biostimulants, the aim of this work was to evaluate the effects of foliar application of micronized Lithothamnium (Lm) associated with A. platensis (Ap) dried biomass in onion (Allium cepa) metabolism, growth and yield. For that, adopting an step by step approach three experiments were conducted: (i) foliar application to onion plants grown in pots in greenhouse comparing algae sources alone and combined, (ii) foliar application to onion plants in the field under organic system testing algae sources combinations (first season), and (iii) foliar application in the field comparing doses and frequencies of selected combinations (second season). The association of algae sources promoted growth and biochemical changes, thus pigment contents, total sugars, amino acids and nitrate reductase enzyme activity were improved, increasing the onion yield by two seasons in a nature friendly way.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rise in the demand for food from nature friendly production systems imposes the necessity to develop new biofertilizers and biostimulants. The biostimulants may contribute to sustainable agriculture (Bayona-Morcillo et al. 2020) due to its composition, such as protein hydrolysates (Colla et al. 2015), humic substances (Gemin et al. 2019), seaweed extracts (Arioli et al. 2015), calcareous algae (Mógor et al. 2021) and microalgae (Colla and Rouphael 2020). In this sense, the combination of algae sources could show synergistic biostimulatory action, helping to design the next generation of plant biostimulants for sustainable agriculture (Rouphael and Colla 2018).
Among the most used algae in agriculture is Lithothamnium sp. – a calcareous rhodophyte—found in marine deposits, which has calcium carbonate, magnesium, micronutrients (Esper Neto et al. 2020) and, if subjected to micronization, may contain humic acid (HA) in its composition (Amatussi et al. 2020). Depending on the calcification of its biomass, Lithothamnium sp. is commonly applied as limestone (Rodrigues Neto et al., 2021). However, the foliar application of micronized Lithothamnium sp. promoted plant growth and yield gains attributed to the presence of a bioactive HA with a high degree of polymerization, showing a significant auxin-like effect (Amatussi et al. 2020; Mógor et al. 2021).
The physiological effect of HA in plants is related to effects similar to those of the hormone auxin (Zandonadi et al. 2014; Canellas et al. 2015, 2020).
Microalgae also have been reported as efficient sources of biofertilizers and biostimulants due to the presence of bioactive molecules in their composition, such us glycosides and the cytokinin zeatin identified in the green microalgae (Chlorophyta) Desmodesmus subspicatus (Mazepa et al. 2021), or polyamines and amino acids in Asterarcys quadricelullare (Cordeiro et al. 2022a; Mógor et al. 2022) and also in the blue-green alga (Cyanophyta) Arthrospira platensis (Spirulina platensis) (Mógor et al. 2017, 2018).
Among the numerous species of microalgae, the cyanobacterium A. platensis has a high content of proteins (Ishaq et al. 2016; Çelekli et al. 2021) and polysaccharides (Trabelsi et al. 2009; Rachidi et al. 2020). The A. platensis enzymatic hydrolysates contain polyamines, such as spermine, which may stimulate cell multiplication and plant growth when applied to plants (Mógor et al. 2017). Foliar application of A. platensis can increase nutrient uptake (Plaza et al. 2018), resulting in more nutritious plants, which is related to food biofortification (Billard et al. 2014; Godlewska et al. 2019).
In addition, the application of microalgae associated with other natural bioactive sources can also be exploited to promote plant growth, as the increase in onion yield of plants subjected to the application of the microalga Scenedesmus subspicatus associated with HA obtained from the mineral leonardite (Gemin et al. 2019), combination that also improved bulbs storage and biofortification (Gemin et al. 2022).
Therefore, considering the potential synergistic biostimulatory action of the combination with calcareous algae and cyanobacterium, the aim of this work was to evaluate the effects of foliar application on onion (Allium cepa) plants grown under organic production system by two seasons, of solutions containing Lithothamnium sp. micronized, an acknowledged source of humic acid, and A. platensis dried biomass, an acknowledged source of proteins, amino acids and polyamines.
Materials and methods
The Lithothamnium sp. sample was obtained off the coast of Espírito Santo State, Brazil (20°19′10″ S – 40°20′16″ W). After micronization (mechanical breakage, caused by the friction between the particles to sizes between 1 to 10 μm) the sample was provided by Valeagro Comércio Importação & Exportação / NaturVita Bioagroindustria (Petrolina, State of Pernambuco, Brazil).
The cyanobacterium Arthrospira platensis was provided by the “Elizabeth Aidar” Microalgae Collection of the Fluminense Federal University (Niterói, State of Rio de Janeiro – Brazil). The axenic autotrophic cultivation of A. platensis was carried out in a semi-continuous system in a photobioreactor (Reichert et al. 2006) in Zarrouk (1966) medium at the Department of Crop Sciences of the Federal University of Paraná (Curitiba, Paraná State—Brazil). After 45-day cultivation, the biomass was separated from the culture medium by centrifugation, attaining 0.95 g L−1 DW (Dry Weight) and was freeze-dried kept a temperature of 50 °C for 24 h (oven) and at – 50 °C, under a vacuum of 1 × 10–6 mBar, for 20 h. The freeze-dried powder was added to spraying solutions, so allowing the cells disruption (Stirk et al. 2020).
The experiments were carried out in the Biofertilizers Lab and Organic production Research Area of the Federal University of Paraná, located in the municipality of Pinhais, Paraná State, Brazil, at 25°23′30″ S and 49°07′30″ W, at an average altitude of 920 m. The climate in the region is a Cfb-type temperate climate (Temperate oceanic climate or subtropical highland climate; coldest month averaging above 0 °C or − 3 °C, all months with average temperatures below 22 °C and at least four months averaging above 10 °C) (Köppen).
For determination of the effect of foliar application of solutions containing Lithothamnium sp. micronized (Lm) separately and associated with Arthrospira platensis (Ap), in terms of growth, development, biochemical alterations, and yield, three experiments were carried out.
Experiment I
It was carried out in greenhouse using pots with a capacity of three liters, filled (in a proportion of 1:1) with commercial substrate based on composted pine bark (Tropstrato®) and organic compost (Provaso®), with: C = 30.3 g kg−1; N = 30.3 g kg−1; P = 8.5 g kg−1; K = 6.6 g kg−1; Ca = 8.1 g kg−1; Mg = 4.1 g kg−1.
At 60 days after sowing (DAS) in nursery beds, the seedlings were transplanted to the pots (experiment I) and simultaneously to the field (experiment II). The seedlings had four leaves, an average pseudostem diameter of approximately 4 mm, showing adequate development. The pots were arranged on benches, with four pots and two plants in each pot, for repetition.
A completely randomized design was used, with two onion (Alliun cepa) hybrid cultivars (Alvará® and Perfecta F1®), each treatment with four replications (n = 4) under a double factorial arrangement (two cultivars x five treatments). A localized irrigation system with a drip tape was used, aiming to maintain the humidity at 80%, controlled with the aid of a tensiometer. The pots contained 2 cm of expanded vermiculite on the surface so that there was no contact of foliar sprayed solutions with the substrate.
Four concentrations of Lithothamnium sp. micronized (Lm) associated or not with A. platensis (Ap) were weekly applied, as well the control sprayed with distilled water. The applications (n = 8) of the treatments were carried out using a 10-L Kawashima electronic sprayer at constant pressure (40 psi). The volume of application varied as a function of plant growth, from 20 mL (first to fourth application) to 36 mL (fifth to eighth application) per pot.
The Lithothamnium sp. (micronized powder) sample was suspended in distilled water at a concentration of 1.5 g L−1 (Amatussi et al. 2020) before being sprayed on leaves. The sample of A. platensis (freeze-dried powder) was suspended in distilled water at a concentration of 0.75 g L−1 and 1.5 g L−1 (Mógor et al. 2018) before being sprayed to the leaves of onion plants. Five treatments were established: Control, Lm (1.5 g L−1 of Lm), LAp1 (1.5 g L−1 of Lm + 0.75 g L−1 of Ap), LAp2 (1.5 g L−1 of Lm + 1.5 g L−1 of Ap) and Ap (1.5 g L−1 of Ap).
After eight applications with a weekly interval, at 90 days after transplanting (DAT) at the beginning of bulbification, leaf material from two plants was collected for biometric and biochemical analysis. Data on plant height determined with a ruler from the base of the pseudostem to the tip of the most expanded leaf (cm), number of leaves, pseudostem diameter determined with digital caliper (mm), fresh and dry mass (g) of the aerial part, and roots (g) using precision scales were obtained. Mass was quantified on a precision scale. The dry mass was quantified after drying in a forced-air circulation oven at 65 °C until reaching a constant value. The biochemical analyzes are described below.
Experiment II
To observe the influence of Lithothamnium sp. (Lm) associated or not with A. platensis (Ap) on the yield of onion bulbs, experiment II was implemented in the field under organic system in August 2018. A completely randomized design was used with two onion cultivars (Alvará® and Perfecta®) where each treatment had four replications (n = 4). The treatments, application frequency, and seedling preparation were the same as described in experiment I.
The chemical analysis of the soil in 0–20 cm layer showed the following mean values: pH (CaCl2) = 5.84; pH H2O = 6.71; Al+3 = 0; H + Al+3 = 2.93 cmolc dm−3; Ca2+ = 5.28 cmolc dm−3; Mg2+ = 3.05 cmolc dm−3; K+ = 1.32 cmolc dm−3; P (Mehlich) = 49.0 mg dm; S = 33.49 mg dm−3; C = 26 g dm−3; %; V% = 76.7 and CEC = 12.58 cmolc dm−3.
The treatments were distributed in beds previously fertilized and prepared seven days before seedling transplanting. Soil preparation was carried out with the incorporation of eight tons per hectare of organic compost with the following mean values: C = 30.3 g kg−1; N = 30.3 g kg−1; P = 8.5 g kg−1; K = 6.6 g kg−1; Ca = 8.1 g kg−1; Mg = 4.1 g kg−1.
The spacing adopted in the experiment was 10 cm between plants and 30 cm between rows, each plot with 40 plants. There were 10 foliar applications of the treatments, with an application volume corresponding to 400 L ha−1.
At harvest, at 135 days after transplanting (DAT), the bulbs were classified according to their diameter (I < 35 mm; II = 35—50 mm; III = 50—70 mm, and IV = 70—90 mm) and their masses were quantified. Yield was extrapolated as a function of the spacing adopted in the experiment (230,000 plants per hectare).
Experiment III
To evaluate the application frequency (weekly and fortnightly) experiment III was implemented in the field, in August 2019. This experiment used the same design, the same population of plants, the same number of replications (n = 4), and the same cultivars used in experiment II.
Applications were performed at two frequencies (weekly and fortnightly), resulting in a triple factorial arrangement (two cultivars x two frequencies x three treatments). The treatments of Lm and Ap associated (LAp1 = 1.5 g L−1 of Lm + 0.75 g L−1 of Ap and LAp2 = 1.5 g L−1 of Lm + 1.5 g L−1 of Ap), in addition to the control, with water application were applied.
Plant material was collected at the beginning of bulbing, at 120 DAT. Plant height (cm), number of leaves, leaf area (cm2), pseudostem volume (cm3), fresh and dry mass (g) of leaves, pseudostem, and bulbs were evaluated. Leaf area and pseudostem volume were obtained using WinRhizo Pro software (Regent Instr Canada). At 135 DAT, the final collection was performed to evaluate yield, as described in experiment II.
Biochemical analyses
Subsequent to the collection of biometric (Experiment I) and yield (Experiment II and III) data, leaves and commercial bulbs (type III and type IV) were collected, macerated in liquid nitrogen and stored (-20° C) for biochemical analyses. Leaf collections were performed between 9 and 10 am.
Analysis of chlorophyll-a (Ch-a), chlorophyll-b (Ch-b) and carotenoids (C), extraction of pigments in the leaves was performed according to Lichtenthaler (1987). The absorbance readings were taken at 663, 647 and 470 nm. The formulas described by Lichtenthaler and Buschmann (2001), were applied, the values were expressed as µg of chlorophyll per g of fresh plant material.
Total sugars were quantified according to Maldonade et al. (2013), preceded by acid hydrolysis of the sample using 3,5-dinitro salicylic acid (DNS). The standard curve for reducing and total sugars was made with glucose (5.5 mM). The values were expressed as µg of sugars per g fresh plant material.
For the analysis of total free amino acids, the amino acids were extracted according to Winters et al. (2002) and the colorimetric reaction with absorbance reading done at 570 nm, according to Magné and Larher (1992). The standard curve was made with glutamine (2 mM). The values were expressed in µg g−1 fresh plant material.
The analysis of phenolic compounds was by the Prussian Blue method (Price and Butler 1977). The absorbance reading was performed at 700 nm, the standard curve performed with gallic acid (0.01 M) in methanol. The values were expressed in µg g−1 fresh plant material.
Nitrate Reductase enzyme activity of leaves was done according to (Jaworski 1971). Reading was performed in a spectrophotometer at 540 nm and values expressed as μ mol of NO2 h−1 g−1 plant material.
All data were tested for homogeneity and then submitted to analysis of variance, when significant (p < 0.05) the data were submitted to Tukey's test. Assistat 7.7 Beta software (Silva and Azevedo 2016) was used.
Results
Experiment I
The number of leaves (NL) did not differ between cultivars and there was no interaction between treatments and cultivars. When comparing the treatments, only LAp2 (1.5 g L−1 of Lm + 1.5 g L−1 of Ap) differed from the control, with an increase of 16% (Table 1). There were no changes in plant height depending on the treatments, only among the cultivars, where ‘Alvará’ was superior to ‘Perfecta F1’.
The Lm application increased root fresh mass (FMR) by 33% (Table 1). The application of the association of calcareous algae in the highest Ap concentration (LAp2), increased by 52% the FMR, in the cultivar ‘Alvará’. The Lm application increased the dry mass of the roots by 57%, while the application of LAp2 promoted an increase of 124% in relation to the control, showing synergic effect of the algal sources combination.
At 90 days the cultivar ‘Alvará’ presented lowest levels of sugars in the leaves (Fig. 1a). The cultivar ‘Perfecta F1’ presented higher values of sugars in the leaves than the control at concentrations LAp1 and LAp2, with an increase of 34 and 30%, respectively. Application of LAp1, in the cultivar ‘Perfecta F1’, increased the content of total free amino acids (Fig. 1a) by 21%. The activity of the enzyme nitrate reductase (NR) showed an interaction between cultivars and treatments (Fig. 1c). The Lm-treated plants showed higher NR activity, with an increase of 24%, in cultivar ‘Alvará’. The NR activity in the ‘Perfecta F1’ cultivar increased by 46%, 45%, 52%, and 45% when Lm, LAp1, LAp2, and Ap were applied, respectively.
Y axis: Total sugar content (a), total free amino acid content (b) and Nitrate reductase enzyme activity in leaves (c). Cultivar factor (FC): ‘Alvará’ and ‘Perfecta’. Factor treatments (FT): X axis: control, (Lm) 1.5 g L−1 of Lm, (LAp1) 1.5 g L−1 of Lm + 0.75 g L−1 of Ap, (LAp2) 1.5 g L−1 of Lm + 1.5 g L−1 of Ap and (Ap) 1.5 g L−1 of Ap. Means followed by the same letter do not differ statistically (p < 0.05), by Tukey's test. Error bars indicate standard deviation. Light gray = ‘Alvará’; dark gray = ‘Perfecta F1’. Lower case letters: between cultivars; upper case letters: between treatments
Experiment II
At the point of harvest, plants subjected to application with LAp2 accumulated 20% more total sugars in the bulbs (Fig. 2a). Furthermore, plants subjected to LAp1 application had the content of total free amino acids (Aa) increased by 40% and application of LAp2 increased by 45% (Fig. 2b). NR activity (Fig. 2c), in cultivar ‘Perfecta F1’, was increased by 33%, 45% and 53% with the application of LAp1, LAp2, and Ap, respectively.
Y axis: Average values of total sugar content (a), total free amino acid content in bulbs (b) and Nitrate reductase enzyme activity in leaves (c). Cultivar factor (FC): Alvará and Perfecta. Factor treatments (FT) X axis: control, (Lm) 1.5 g L−1 of Lm, (LAp1) 1.5 g L−1 of Lm + 0.75 g L−1 of Ap, (LAp2) 1.5 g L−1 of Lm + 1.5 g L−1 of Ap and (Ap) 1,5 g L−1 of Ap. Means followed by the same letter do not differ statistically (p < 0.05), by Tukey's test. Error bars indicate standard deviation. Light gray = ‘Alvará’; dark gray = ‘Perfecta F1’. Lower case letters: between cultivars. Upper case letters: between treatments
The LAp2 applications promoted an increase in commercial onion bulbs (classes III and IV—50 mm to 90 mm), increasing yield by up to 20% in relation to the control (Fig. 3), corresponding to an increase of 4 t ha−1.
Y axis: Average commercial yield. Cultivar factor (CF): ‘Alvará’ and ‘Perfecta F1’. Treatment factor (TF): X axis: control, Lm (1.5 g L−1 of Lm), LAp1 (1.5 g L−1 of Lm + 0.75 g L−1 of Ap), LAp2 (1.5 g L−1 of Lm + 1.5 g L−1 of Ap) and Ap (1.5 g L−1 of Ap). Means followed by the same letter do not differ statistically (p < 0.05), by Tukey's test. Error bars indicate standard deviation
Experiment III
The length of leaves (Fig. 4a) of plants treated with LAp1 and LAp2 increased by 11% and 12%, regardless of the cultivar and the applied frequency. The number of leaves of plants that received LAp1 was increased by 13%. In the weekly application, the leaf area (Fig. 4b) of plants treated with Lap1 and Lap2 was higher in the ‘Alvará’ cultivar by 49% and 43%, respectively. While the leaf area of cultivar ‘Perfecta F1’ was increased by 57% and 47% in the LAp1 and LAp2 biweekly application.
Y axis: Length and number of leaves (a), leaf area (cm2) (b). Cultivar factor (CF): light gray = ‘Alvará’; dark gray = ‘Perfecta F1’. Application frequency factor (FF): weekly (dotted gray); biweekly (gray). Treatment factor (TF) (black bars): X axis: C (control), LAp1 (1.5 g L−1 of Lm + 0.75 g L−1 of Ap) and LAp2 (1.5 g L−1 of Lm + 1.5 g L−1 of Ap). Means followed by the same letter do not differ statistically (p < 0.05), by Tukey test. Error bars indicate standard deviation
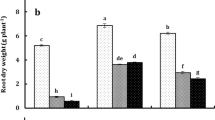
The dry mass of the leaves (Fig. 5a) was superior to the control at 41% and 28% when LAp1 and LAp2 were applied weekly. The application of LAp1 increased the dry mass of onion pseudostems (Fig. 5b) by 24%, regardless of the cultivar and the applied frequency. The volume of the pseudostems (Fig. 5c) of the plants treated with LAp1 was superior to the control in the weekly (41%) and fortnightly (34%) frequencies, while the plants treated with LAp2 had the volume of the pseudostems increased by 44%, in the fortnightly application.
Y axis: Leaf mass (a), pseudostem dry mass (b), pseudostem volume (c) and bulb fresh and dry mass (d). Application frequency factor (FF): weekly (dotted gray); biweekly (gray). Treatment factor (TF) (black bars): X axis: C (control), LAp1 (1.5 g L−1 of Lm + 0.75 g L−1 of Ap) and LAp2 (1.5 g L−1 of Lm + 1.5 g L−1 of Ap). Means followed by the same letter are not statistically different (p < 0.05), by Tukey’s test. Error bars indicate standard deviation. FF x FT interaction (a, c and d): lower case letters = between frequencies, upper case letters = between treatments
At the beginning of the bulb development, the gain in fresh mass of onion bulbs (Fig. 5d) was increased in plants that received LAp1 application, on a weekly (90%) and fortnightly (50%) frequency. The application of LAp2 resulted in an increment 63% higher than the control when the application occurred weekly. In the dry mass, the LAp1 application promoted an increase of 105% and 43% in the weekly and the biweekly frequencies, respectively. Plants submitted to the LAp2 application had the dry mass of the bulbs increased by 56% when the application was performed on a weekly basis.
In the quantification of pigments (Table 2), the levels of chlorophylls a and b were higher than the control in both frequencies when LAp2 concentration was applied. While the LAp1 concentration was superior to the control only in the fortnightly frequency. The content of total chlorophyll and carotenoids was higher than the control when LAp1 and LAp2 were applied fortnightly.
The content of total sugars in the leaves (Fig. 6a), in the weekly application, was higher than the control in the cultivar ‘Alvará’. On the other hand, in the biweekly application, the two cultivars (‘Alvará’ and ‘Perfecta F1’) had an increase in the total sugar content. The non-reducing sugars (Fig. 6b) were higher than the control in both cultivars, regardless of the application frequency. The content of reducing sugars (Fig. 6c) was higher than the control at weekly frequency for cultivar ‘Alvará’ and biweekly for cultivar ‘Perfecta F1’. Applications of LAp concentrations decreased the content of total free amino acids (Aa) by 8% in the leaves and promoted an increase of 22% of Aa in the bulbs (Fig. 6d).
Y axis: Content of total sugars in bulbs (a), non-reducing sugars in bulbs (b), reducing sugars in bulbs (c) and content of total free amino acids in leaves and bulbs (d). Cultivar factor (CF): light gray = ‘Alvará’; dark gray = ‘Perfecta F1’. Application frequency factor (FF): weekly (dotted gray); biweekly (gray). Treatment factor (TF) (black bars): X axis: C (control), LAp1 (1.5 g L−1 of Lm + 0.75 g L−1 of Ap) and LAp2 (1.5 g L−1 of Lm + 1.5 g L−1 of Ap). Means followed by the same letter do not differ statistically (p < 0.05), by Tukey’s test. Error bars indicate standard deviation. FC x FF x FT interaction (a and c): lower case letters = among cultivars, upper case letters = among treatments. (b) FC x FT: lower case letters = among cultivars, upper case letters = among treatments
An interaction among cultivar, frequency, and treatments was found in the activity of the enzyme Nitrate Reductase (Fig. 7). The concentrations of LAp applied to cultivar ‘Perfecta F1’ were higher than the control in both application frequencies, while in ‘Alvará’ cultivar, the increase in enzyme activity was higher than the control in the weekly LAp1 application.
Y axis: Nitrate reductase enzyme activity in leaves. Cultivar factor (CF): light gray = ‘Alvará’; dark gray = ‘Perfecta F1’. Application frequency factor (FF): weekly (dotted gray); biweekly (gray). Treatment factor (TF): X axis: C (control), LAp1 (1.5 g L−1 of Lm + 0.75 g L−1 of Ap) and LAp2 (1.5 g L−1 of Lm + 1.5 g L−1 of Ap). Means followed by the same letter are not statistically different (p < 0.05), by Tukey’s test. Error bars indicate standard deviation. FC x FF x FT interaction: lower case letters = among cultivars, upper case letters = among treatments
At harvest, the application of LAp1 promoted an increase of 17% in the level of total sugars in the bulbs (Fig. 8) when the application occurred every two weeks, while the application of LAp2 promoted an increase of 18% in the concentration of sugars in the bulbs on a weekly frequency. The content of non-reducing sugars (Fig. 8b) increased by 81% in plants treated with LAp2, at a weekly frequency. The fortnightly application of LAp1 promoted an increase of 60% in the non-reducing sugar content. The fortnightly applications of LAp1 and LAp2 promoted an increase in the content of reducing sugars, of 28% and 33% in the cultivar ‘Alvará’, respectively, and a decrease of 59% in the cultivar ‘Perfecta F1’, in the LAp2 treatment. The amino acid content in the bulbs showed an increase of 34% in plants treated with LAp2 at a weekly frequency, and in the fortnightly application of the LAp1 concentration, an increase of 30% was observed in relation to the control.
Y axis: Content of total sugars in bulbs (a), non-reducing sugars in bulbs (b), reducing sugars in bulbs (c) and content of total free amino acids in bulbs (d). Cultivar factor (CF): light gray = ‘Alvará’; dark gray = ‘Perfecta F1’. Application frequency factor (FF): weekly (dotted gray); biweekly (gray). Treatment factor (TF): X axis: C (control), LAp1 (1.5 g L−1 of Lm + 0.75 g L−1 of Ap) and LAp2 (1.5 g L−1 of Lm + 1.5 g L−1 of Ap). Means followed by the same letter are not statistically different from each other (p < 0.05) by Tukey's test. Error bars indicate standard deviation. FF x FT (a, b and d): lower case letters = between frequencies, upper case letters = between treatments. (c) FC x FF x FT: lower case letters = between cultivars, upper case letters = between treatments
The LAp1 and LAp2 foliar applications promoted an increase in onion yield by 14% and 11%, respectively, regardless of cultivar and frequency. These values corresponded to an increase of 4.88 and 4 t ha−1, respectively.
Discussion
The association of Lithothamnium sp. and A. platensis (LAp) presented metabolic effects related to the algal sources combination. The changes in biometric and biochemical variables, and yield gains, may have occurred due to signaling pathways that microalgae can activate in plants (Deviram et al. 2020), resulting in a higher rate of cell division or elongation (Billard et al. 2014; Shahrajabian et al. 2021) probably triggered by free amino acids found in A. platensis biomass (Mógor et al. 2018; Ronga et al. 2019) allied to the high auxin-like bioactivity of HA found in Lithothamnium (Lm) (Amatussi et al. 2020).
The Lm application increased the dry mass of onion roots by 57%, while the application of LAp2 promoted an increase of 124% in relation to the control, showing synergic effect of the algal sources combination, indicating the stimuli to the source-to-sink flow, effect previously reported by Gemin et al. (2019), combining a chlorophyte microalgae with HA obtained from Leonardite mineral.
The results at the bulbs development indicate that there was an accumulation of biomass in the plants that received sprays with LAp (Tab. 1). At harvest, results showed that LAp2 treatment resulted in 20% more accumulation in total sugar content (Fig. 2a) that significantly increased non-reducing sugars (sugars translocated in the phloem), in the weekly application. On the other hand, the increase in reducing sugars (e.g. glucose, fructose) occurred in the LAp—fortnightly application (Fig. 8).
According to Bettoni et al. (2016) foliar sprays using HA results in increases of bulb fresh and dry mass by improving total soluble sugars in plans, with yield gains, as also found in this work, taking into account that the calcareous algae micronization process increases the HA availability (Mógor et al. 2021), allied to the composition of A. platensis, an cyanobacterium rich in bioactive compounds (Ishaq et al. 2016; Mógor et al. 2017; Rachidi et al. 2020; Çelekli et al. 2021) explaining, at less in part, these stimuli in carbon metabolism improving sugar content and resulting in greater onion biomass accumulation and yield.
The results of the LAp1 and LAp2 applications improving the content of total free amino acids (Aa) by 40 and 45% respectively (Fig. 2b) indicate the synergy in the algal sources association related to Nitrogen acquisition. The increase in Aa content in onion plants subjected to Lm foliar applications could be associated with a greater Nitrogen uptake, which may occur due to the effect of HA on the increase of adventitious roots (Canellas et al. 2015; Mógor et al. 2021) and the activity of the enzyme nitrate reductase (NR) (Zandonadi et al. 2007; Mora et al. 2010) that contribute to the N assimilation process, as presented in Figs. 1c, 2c, and 7, with increments in NR activity in onion plants.
The activity of NR, an enzyme that reduces nitrate to nitrite, was superior in plants treated with Lm in cultivar ‘Alvará’. In ‘Perfecta F1’, the treatments with the association (LAp1 and LAp2) and algae sources separately (Lm and Ap) were higher than to the control (Fig. 1c). The application of biostimulants containing HA can regulate important enzymes, such as NR (Mora et al. 2010; Zandonadi et al. 2014), as well as Ap applications have already been associated with increased activity of NR, carotenoids, chlorophylls and proteins (Rachidi et al. 2020), as also found in present work.
Foliar application with microalgae has already been reported to influence the translocation of photoassimilates and increase the caliber and mass of onion bulbs (Cordeiro et al. 2022b), red beet hypocotyl (Mógor et al. 2018), potato tubers (Cordeiro et al. 2022a), and tomato (Lara et al. 2022). The data (Fig. 6) indicate that the applications stimulated the source-to-sink flow with sugars and amino acid accumulation in bulbs, justifying the reduction of the contents in leaves at harvest, confirming the effectiveness of the LAp association. These stimuli to the translocation of photoassimilates from the source (leaf) to the sink (bulbs), providing an increase in the diameter and mass of the bulbs, was well characterized by the yield gains over two seasons (Figs. 2 and 9).
An increase in onion bulb yield after the application of HA associated with microalgae biomass has already been reported (Gemin et al. 2019), relating the gain in yield to the increase in the efficiency of carbon metabolism, as also verified in the present work, by improves on total chlorophyll and carotenoids contents in leaves under biweekly LAp sprays (Tab2) and sugars improvements at different time collections by LAp (Figs. 2, 6, and 8).
These findings show the complexity of the mechanisms of action of both algal sources and the need to understand which phenological stage is the most suitable for application and the concentration of maximum efficiency. Thus, through three experiments, were identified that the foliar sprays with the association of micronized Lithothamnium sp and A. platensis was synergic, promoting plant growth, improving the pigments content, total free amino acids, and NR activity in the leaves, also total free amino acids and total sugar content in the bulbs and increase the yield of onion by two seasons over the algal sources applied separately.
Conclusions
The foliar sprays of the association of micronized Lithothamnium sp calcareous alga and Arthrospira platensis (LAp) allowed uniting different mechanisms of action, increasing plant growth, stimulating plant metabolism increasing yield. Although some variables were dependent on application frequency or cultivar, the both combinations of LAp (LAp1 and LAp2) increased yield for both cultivars and at both application frequencies. Therefore, biweekly application is a more economical option to promote onion yield gains in a nature friendly way.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Amatussi JO, Mógor ÁF, Mógor G, de Lara GB (2020) Novel use of calcareous algae as a plant biostimulant. J Appl Phycol 32:2023–2030
Arioli T, Mattner SW, Winberg PC (2015) Applications of seaweed extracts in Australian agriculture: past, present and future. J Appl Phycol 27:2007–2015
Bayona-Morcillo PJ, Plaza BM, Gómez-Serrano C, Rojas E, Jiménez-Becker S (2020) Effect of the foliar application of cyanobacterial hydrolysate (Arthrospira platensis) on the growth of Petunia x hybrida under salinity conditions. J Appl Phycol 32:4003–4011
Bettoni MM, Mógor AF, Kogerastki JF, Pauletti V (2016) Onion (Allium cepa L.) seedling growth using humic substances. Idesia (Árica) 34:57–62
Billard V, Etienne P, Jannin L, Garnica M, Cruz F, Garcia-Mina JM, Yvin JC, Ourry A (2014) Two biostimulants derived from algae or humic acid induce similar responses in the mineral content and gene expression of winter oil seed rape (Brassica napus L.). J Plant Growth Regul 33:305–316
Canellas LP, Olivares FL, Aguiar NO, Jones DL, Nebbioso A, Mazzei P, Piccolo A (2015) Humic and fulvic acids as biostimulants in horticulture. Sci Hortic (Amsterdam) 196:15–27
Canellas LP, Canellas NOA, Silva ILES, Olivares FL, Piccolo A (2020) Plant chemical priming by humic acids. Chem Biol Technol Agric 7:12
Çelekli A, Gün D, Bozkurt H (2021) Bleaching of olive pomace oil with Spirulina platensis as an eco-friendly process. Algal Res 54:102210
Colla G, Rouphael Y (2020) Microalgae: New source of plant biostimulants. Agronomy 10:1240
Colla G, Nardi S, Cardarelli M, Ertani A, Lucini L, Canaguier R, Rouphael Y (2015) Protein hydrolysates as biostimulants in horticulture. Sci Horte 196:28–38
Cordeiro ECN, Mógor ÁF, Amatussi JO, Mogor G, Marques HMC, de Lara GB (2022a) Microalga biofertilizer improves potato growth and yield, stimulating amino acid metabolism. J Appl Phycol 34:385–394
Cordeiro ECN, Mógor ÁF, Amatussi JO, Mógor G, de Lara GB, Marques HMC (2022b) Microalga biofertilizer triggers metabolic changes improving onion growth and yield. Horticulturae 8:223
de Lara GB, Mógor Á, Amatussi JO, Cordeiro ECN, Marques HMC, Mógor G (2022) Microalga improve the growth, yield, and contents of sugar, amino acid, and protein of tomato. Ciência e Agrotecnologia 46.
Deviram G, Mathimani T, Anto S, Ahamed TS, Ananth DA, Pugazhendhi A (2020) Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J Clean Prod 253:119770
Esper Neto M, Zampar ÉJDO, Cordioli VR, Cassim BMAR, Dias GAR, Inoue TT, Batista MA (2020) Biogenic and common lime characterization in granular and powder forms and their application in sowing furrows for soybean crops. Commun Soil Sci Plant Anal 51:1382–1390
Gemin LG, Mógor ÁF, Amatussi JO, Mógor G (2019) Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci Hortic (Amsterdam) 256:108560
Gemin LG, Mógor ÁF, Amatussi JO, De Lara GB, Mógor G (2022) Organic onion growth, yield and storage improved by foliar sprays of microalgae and fulvic acid as a natural biofertilizer. Biosci J 38:e38045
Godlewska K, Michalak I, Pacyga P, Baśladyńska S, Chojnacka K (2019) Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J Microbiol Biotechnol 35:80
Ishaq AG, Peralta M, Basri HM (2016) Bioactive compounds from green microalga – Scenedesmus and its potential applications: a brief review. Agric Sci 39:1–16
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43:1274–1279
Lichtenthaler HK (1987) Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1:F4.3.1–F4.3.8.
Magné C, Larher F (1992) High sugar content of extracts interferes with colorimetric determination of amino acids and free proline. Anal Biochem 200:115–118
Maldonade IR, Patrícia GBC, Ferreira AN (2013) Protocolo para determinação de acúcares totais em hortaliças pelo método de DNS. Embrapa, Comunicado Tecnico 85, p 4
Mazepa E, Malburg BV, Mógor G, de Oliveira AC, Amatussi JO, Corrêa DO, Lemos JS, Ducatti DRB, Duarte MER, Mógor ÁF, Noseda MD (2021) Plant growth biostimulant activity of the green microalga Desmodesmus subspicatus. Algal Res 59:102434
Mógor ÁF, Ördög V, Lima GPP, Molnár Z, Mógor G (2017) Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J Appl Phycol 30:453–460
Mógor ÁF, Amatussi JO, Mógor G, Lara GB (2018) Bioactivity of cyanobacterial biomass related to amino acids induces growth and metabolic changes on seedlings and yield gains of organic red beet. Am J Plant Sci 09:966–978
Mógor AF, Amatussi JO, Mógor G, Gemin LG (2021) Biostimulant action of Lithothamnium sp. promoting growth, yield, and biochemical and chemical changes on onion. J Appl Phycol 33:1905–1913
Mógor G, Mógor ÁF, Lima GPP, Oliveira RA, Bespalhok JCF (2022) Metabolic changes in sugarcane bud sprouting stimulated by microalga Asterarcys quadricellulare. Sugar Tech 24:930–940
Mora V, Bacaicoa E, Zamarreño AM, Aguirre E, Garnica M, Fuentes M, García-Mina JM (2010) Action of humic acid on promotion of cucumber shoot growth involves nitrate-related changes associated with the root-to-shoot distribution of cytokinins, polyamines and mineral nutrients. J Plant Physiol 167:633–642
Plaza BM, Gómez-Serrano C, Acién-Fernández FG, Jimenez-Becker S (2018) Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. J Appl Phycol 30:2359–2365
Price ML, Butler LG (1977) Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J Agric Food Chem 25:1268–1273
Rachidi F, Benhima R, Sbabou L, El Arroussi H (2020) Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol Rep 25:e00426
Reichert CC, Reinehr CO, Costa JAV (2006) Semicontinuous cultivation of the cyanobacterium Spirulina platensis in a closed photobioreactor. Braz J Chem Eng 23:23–28
Rodrigues Neto J, Pereira DP, Torres JLR, Carvalho FJ, Charlo HCO (2021) Potassium sources and calcium and magnesium doses in carrot crop fertilization. Hortic Bras 39:127–132
Ronga D, Biazzi E, Parati K, Carminati D, Carminati E, Tava A (2019) Microalgal Biostimulants and biofertilisers in crop productions. Agronomy 4:192
Rouphael Y, Colla G (2018) Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front Plant Sci 871:1655
Shahrajabian MH, Chaski C, Polyzos N, Petropoulos SA (2021) Biostimulants application: a low input cropping management tool for sustainable farming of vegetables. Biomolecules 11:698
Silva F de AS e, Azevedo CAV de (2016) The Assistat Software Version 7.7 and its use in the analysis of experimental data. Afr J Agric Res 11:3733–3740
Stirk WA, Balint P, Vambe M, Lovasz C, Molnar Z, van Staden J, Ordog V (2020) Effect of cell disruption methods on the extraction of bioactive metabolites from microalgal biomass. J Biotech 307:35–43
Trabelsi L, M’sakni NH, Ben Ouada H et al (2009) Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol Bioprocess Eng 14:27–31
Winters AL, Lloyd JD, Jones R, Merry RJ (2002) Evaluation of a rapid method for estimating free amino acids in silages. Anim Feed Sci Technol 99:177–187
Zandonadi DB, Canellas LP, Façanha AR (2007) Indolacetic and humic acids induce lateral root development through a concerted plasmalemma and tonoplast H+ pumps activation. Planta 225:1583–1595
Zandonadi DB, Santos MP, Medici LO, Silva J (2014) Ação da matéria orgânica e suas frações sobre a fisiologia de hortaliças. Hortic Bras 32:14–20
Zarrouk C (1966) Contribution a l’etude d’une cyanobacterie: influence de divers facteurs physiques et chimiques sur la croissanceet la photosynthese de Spirulina maxima (Setchell et Gardner) Geitler. PhD thesis, University of Paris, France
Acknowledgements
The Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarship granted (Code 001) to the first author.
Author information
Authors and Affiliations
Contributions
Conceptualization, J.d.O.A., Á.F.M. and G.M.; methodology, J.d.O.A., Á.F.M. and G.M; software, E.C.N.C.J and G.B.d.L.; validation, H.M.C.M., G.M. and Á.F.M.; formal analysis, E.C.N.C. and J.d.O.A.; investigation, J.d.O.A., Á.F.M. and G.M.; resources, Á.F.M. and G.M.; data curation, G.B.d.L. and H.M.C.M.; writing—original draft preparation, E.C.N.C., J.d.O.A., H.M.C.M. and G.B.d.L.; writing—review and editing, Á.F.M. and G.M.; visualization, J.d.O.A.; E.C.N.C. and Á.F.M.; supervision, Á.F.M.; project administration, G.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Amatussi, J., Mógor, Á.F., Cordeiro, E.C.N. et al. Synergic combination of calcareous algae and cyanobacteria stimulate metabolic alterations improving plant growth and yield. J Appl Phycol 35, 483–493 (2023). https://doi.org/10.1007/s10811-022-02873-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02873-1