Abstract
This work aimed to improve the techniques for cultivating the species Gelidium floridanum, testing different sources of calcium. Tetraspores and explants were grown in the laboratory for 20 days with seawater enriched with 50% von Stosch solution and oyster or mussel shell powder (252 mg L-1 and 336 mg L-1), or calcium chloride (147 mg L-1 and 295 mg L-1). Tetraspores or explants cultivated with no calcium salts or shell powder were used as control. Tetraspore germination rate and morphology, and germling morphology, average length, and growth rate were evaluated. Besides, the morphology, upright axes formation and growth rate of explants were also evaluated. There was no difference in tetraspore germination among treatments and control. Treatments with shell powder impaired tetraspore viability, indicated by a greenish color, cellular disorganization, and delay in germ tube development. Germlings cultivated with CaCl2 show better initial development but with no improvement compared to control. Concerning explants, those cultivated with mussel shell powder showed the highest growth rate (3.92 and 4.28% day-1, according to concentration) in relation to other treatments. In particular, those cultivated with 252 mg L-1 mussel shell powder had a significant formation of upright axes (10.15 ± 0.85 upright axes) compared to the control (5.18 ± 0.42 upright axes). Based on these results, we recommend using mussel shell powder at a concentration of 252 mg L-1 to optimize explant cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gelidium species have commercial importance due to their higher grade and purified agar used in pharmacological, biomedical, biotechnological industries, and other specific applications (Armisén 1991; Santos and Melo 2018). Approximately 14,500 t of agar are produced each year, yielding around US$ 246 million (Porse and Rudolph 2017). Although agar extracted from Gelidium currently represents only about 1.6% of the world's production of phycocolloids, its high resistance to natural gelation and low gelation temperatures makes it difficult to replace it with agar extracted from other species (Santos and Melo 2018).
One of the limiting factors in agar from Gelidium production is that there are no commercial crops. The industry depends on natural stocks worldwide, which are being depleted at an alarming rate. Massive cultivation of Gelidium is still in the experimental stage and few attempts have been made in the laboratory and field (Porse and Rudolph 2017). Strategies for small-scale cultivation of Gelidium in tanks have been carried out for a long time in Chile (Santelices 1976), India (Mairh and Rao 1978), USA (Hansen 1980), and Norway (Fredriksen and Rueness 1989). Long-line cultivation using the vegetative propagation method has been carried out in China on a small and large scale (Fei and Huang 1991), in South Africa on experimental farms of G. robustum and G. nudifrons (Melo et al. 1991), and in India with G. pristoides and G. pusillum (Aken et al. 1993, Veeragurunathan et al. 2018). However, none of those experiments have expanded to a large scale, even with methods showing results justifying these attempts (Yarish and Pereira 2008).
Most of these Gelidium cultivation studies are based on vegetative propagation or spore germination. In both cases, the presence of calcium ion (Ca2+) has been demonstrated to be an important factor for the development of these earlier stages (Santelices 1991; Otaiza et al. 2019; Bouvie 2018). Ca2+ enters through calcium channels, increasing its concentration gradient in the region where the germ tube is formed; it regulates actin filaments and induces seaweed growth (Derksen et al. 1995; Filipin et al. 2019).
In plants and animals it is well established that cell polarization occurs due to the involvement of the cytoskeleton, mainly in the growth and early development of eukaryotic organisms (Green et al. 2013). Cytoskeletal proteins, such as microtubules and filamentous actin (F-actin), are responsible for organelle movement, vesicle targeting, and cell organization (Cárdenas et al. 2008, Cai and Cresti 2010). In experiments using cytoskeletal blockers in tetraspores of G. floridanum, Filipin et al. (2019) concluded that F-actin directed the cell wall components and contributed to maintaining chloroplast shape and elongation during the germ tube formation. Therefore, it seems that extracellular calcium is essential for the organization of microtubules and consequent organization and cell growth. In fact, Bouvie (2018) observed an acceleration in germ tube formation of G. floridanum spores cultivated with different concentrations of CaCl2, compared to the control, with no CaCl2 addition in the culture medium.
However, when considering large-scale production, the use of CaCl2 can be costly. Considering that the mollusks shell is a cheap and available source of calcium, this work aimed to evaluate the effect of the calcium salts from these shells in tetraspores and explants development of the G. floridanum species.
Material and methods
Gelidium floridanum tetrasporophytes were collected during low tide, on the rocky shore of Sambaqui beach (27°29′18.8″ S and 48°32'12.9″ W), located in Florianopolis, State of Santa Catarina, and transported in dark containers to the Seaweed Laboratory (LCM Macroalgas) of the Federal University of Santa Catarina (UFSC). Once in the laboratory, samples were brushed and cleaned with sterilized seawater. The seawater was passed through 25, 10, and 5 mm filters and sterilized by an ultraviolet light (UV).
Culture conditions
Tetrasporophytic thalli were acclimated in 1 L conical flasks with sterile seawater enriched with 50% von Stosch solution (McLachlan 1973), 35‰ salinity, temperature 24 ± 1 °C, 12 h photoperiod, 50 μmol photons m-2 s-1 irradiance and constant aeration one week before the experiment for acclimation.
Shell processing
Shells of Crassostrea gigas oysters and Perna perna mussels were collected in the Marine Farms Atlântico Sul and Marpesca, located at Florianopolis. They were cleaned, sanitized with 2% sodium hypochlorite, rinsed abundantly in freshwater, and placed in the oven for 48 h at 100 °C. After drying, they were ground in the Laboratory of Vitroceramic Materials (VitroCer) at the Mechanical Engineering Department of UFSC, using a Hammer Mill (Servitech CT-058) to obtain smaller parts and a Planetary Mill (Servitech CT-242) to transform these particles into powder.
Culture medium
Two standard solutions (1 and 2 mM) of calcium chloride (Ca1 and Ca2, respectively) were prepared with sterilized seawater, based on Bouvie (2018). Two mussel powder (Mx1 and Mx2) and two oyster powder (Os1 and Os2) solutions were also prepared with sterilized seawater, based on the best concentrations (252 mg L-1 and 336 mg L-1, respectively) found by Zhou et al. (2016). These solutions were placed in beakers with aeration for five days. After this period, they were filtered in low pressure with a 47 mm glass fiber microfilter to remove residues. The calcium ion concentration (Ca2+) was quantified by the Analysis Center of the Chemistry Laboratory (EQA) of UFSC, as follows:
\(\begin{array}{c}-\;\mathrm{Ca}1:\;39.2\; \mathrm{mg}\;\mathrm {L}^{-1}\; \mathrm{and} \; \mathrm{Ca}2:\;78.4\; \mathrm{mg}\;{\mathrm L}^{-1};\\-\; \mathrm{Mx}1:\;68\; \mathrm{mg}\;{\mathrm L}^{-1}\; \mathrm{and}\; \mathrm{Mx}2:\;106\; \mathrm{mg}\;{\mathrm L}^{-1}\\-\; \mathrm{Os}1:\;84\; \mathrm{mg}\;{\mathrm L}^{-1}\; \mathrm{and}\; \mathrm{Os}2:133\; \mathrm{mg}\;{\mathrm L}^{-1}.\end{array}\)
The culture medium was composed of sterilized seawater enriched with 50% von Stosch solution supplemented with one of these solutions (treatments). Sterilized seawater only enriched with 50% von Stosch solution was used as a control.
Cultivation of tetraspores and explants
Fertile tetrasporophytes were placed on four slides inside 2 L white plastic boxes with 500 mL of culture medium for tetraspore release and germination according to each treatment and control, in triplicates (n = 3). After 24 h, thalli were carefully removed and one slide from each replicate was collected for morphological analysis and germination quantification. The remaining slides were cultivated for 20 days under the following conditions: 24 °C ± 1 °C, 37 ± 3 μmol photons m-2 s-1 irradiance, 33‰ salinity, and 12 h photoperiod. The culture medium was changed every five days. After this period, the slides were removed for morphological analyses and growth rate and seedling average length calculations.
For explants, healthy thalli were selected and acclimated for seven days in the culture media. In the last two days, 2 mL L-1 of antimycotic and antibiotic solution (composed of 10,000 units mL-1 penicillin G, 10 mg mL-1 sulfate of streptomycin, and 25 μg mL-1 amphotericin B - SIGMA) were added. Subsequently, the thalli were washed several times in sterilized seawater. The lateral branches were removed. The main thalli were sectioned in 5 mm (explants), following the methodology described by Yokoya and Handro (1997). According to the treatment or control, ten explants were cultivated in 250 mL conical flasks with culture media in the same conditions described for tetraspores cultivation for 20 days in quadruplicates (n = 4). Once a week the culture medium was changed. At the end of the experimental period explants were removed for morphological analyses and growth rate and seedling average length calculations.
Morphology and data analysis
Released spores were analyzed under a Leica DM500 microscope with an image capture system (OPTHD 3.7 software) at the Plant Cell Biology Laboratory (LABCEV - UFSC). One hundred tetraspores of each treatment and control (n = 3) were selected at random and classified according to the following characteristics: a) germinated tetraspores (with the formation of the germ tube), b) non-germinated but viable tetraspores, and c) non-viable tetraspores, with green pigmentation.
After 20 cultivation days germlings and explants of each treatment and control were evaluated under an Olympus SZX16 stereoscope with an image capture system (CellSens Dimension 1.12) at the Multi-User Laboratory for Studies in Biology (LAMEB - UFSC). The growth rate was calculated according to Altamirano et al. (2003):
where GR = growth rate; Il = initial length; Fl = final length and t = days.
The length of 30 germlings from each repetition (n = 3) was measured. The length and number of upright axes at each end of the explants (n = 4) were also evaluated.
Statistical analysis
All results were submitted to a Shapiro-Wilk test to verify the normality and an F-test for homoscedasticity. One-way analysis of variance (ANOVA) was used, followed by Tukey's test. All statistical analyzes were performed using the Statistica software package (version 10.0), considering p <0.05.
Results
Tetraspores germination rate and morphology
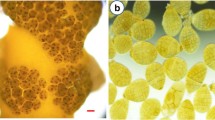
After 24 hours germ tubes were observed both in control and treatments (Fig. 1). Control and Ca2 presented a significantly higher germination percentage (53.3 ± 10% and 66 ± 7%, respectively) (average ± standard deviation) than the other treatments (Fig. 2) (ANOVA: F6 = 9.89, p<0.01). Tetraspores treated with shell powder showed few germinated tetraspores, and among them, most delayed the germ tube's formation (Figs. 1d to g).
Light microscopy of G. floridanum tetraspores cultivated in different sources of calcium after 24 hours. (a) Control (no calcium supplementation); (b) Ca1 (147 mg L-1 CaCl2); (c) Ca2 (295 mg L-1 CaCl2); (d) Mx1 (252 mg L-1 mussel shell powder); Mx2 (336 mg L-1 mussel shell powder); (f) Os1 (252 mg L-1 oyster shell powder); (g) Os2 (336 mg L-1 oyster shell powder). Arrows (developed germ tubes); Arrowhead (viable spores); asterisk (unviable spores). Scale: 15 μm
Germination rate of G. floridanum tetraspores cultivated in different sources of calcium after 24 hours. (C) Control (no supplementation); Ca1 (147 mg L-1 CaCl2); Ca2 (295 mg L-1 CaCl2); Mx1 (252 mg L-1 mussel shell powder); Mx2 (336 mg L-1 mussel shell powder); Os1 (252 mg L-1 oyster shell powder); Os2 (336 mg L-1 oyster shell powder). Values are presented as each treatment's average, and the vertical bars represent the standard deviation (n = 3). Letters indicate significant differences among treatments and control in relation to germination rate viable or inviable spores (considering p<0.05)
Viable tetraspores that did not germinate but showed reddish coloration (Fig. 1) presented significant differences in Ca1 and Ca2 to the Mx1 and control, presenting the lowest viable rate tetraspores (ANOVA F6 = 3.82, p = 0.0049). Unviable tetraspores were observed in all treatments and control, indicated by the greenish color (Fig. 1). Tetraspores of Mx1 showed an unviable tetraspore rate significantly higher them those from Os1 treatment, but with no difference among other treatments and control (Fig. 2) (ANOVA F6 = 2.90, p = 0.021).
Seedling morphology, average length, and growth rate
After 20 cultivation days, well-developed seedlings in clusters formation were observed. Two specific patterns were identified among seedlings treated with shells powder and CaCl2 (Fig. 3). The first pattern was characterized by dense clusters with homogeneous germlings observed in control and treatments Ca1 and Ca2 (Fig. 3a to c). The second pattern showed seedlings of several sizes, observed in shell powder treatments (Fig. 3d to g). Control and treatments presented rhizoid formation.
Light microscopy of G. floridanum germlings after 20 cultivation-days in different sources of calcium. (a) Control (no supplementation); (b) Ca1 (147 mg L-1 CaCl2); (c) Ca2 (295 mg L-1 CaCl2); (d) Mx1 (252 mg L-1 mussel shell powder at); Mx2 (336 mg L-1mussel shell powder); (f) Os1 (252 mg L-1 oyster shell powder); (g) Os2 (336 mg L-1 oyster shell powder). Arrows show the germlings clusters. Scale: 500 μm
Germlings from Ca2 treatment showed length significantly higher (847.7 ± 139.6 μm) than those from shell powder treatments but similar to control (805.9 ± 143 μm) and Ca1 treatment (697.1 ± 26.7 μm) (average ± standard deviation) (ANOVA F6 = 8.42, p < 0.01). On the other hand, germlings from Os1 were significantly shorter (389.7 ± 82.8 μm) than control and those from treatments with CaCl2 but similar to the other treatments with shell powder (Fig. 4A). In general, growth rates were similar among the treatments and control, except again for germlings from Os1 treatment, which show growth rates significantly lower than control and CaCl2 treatments but similar to the shell powder treatments (Fig. 4B) (ANOVA F6 = 9.14, p < 0.01).
Length and growth rates of G. floridanum germlings after 20 days of cultivation in different sources of calcium. (C) Control (no supplementation); Ca1 (147 mg L-1 CaCl2); Ca2 (295 mg L-1 CaCl2); Mx1 (252 mg L-1 mussel shell powder); Mx2 (336 mg L-1 mussel shell powder); Os1 (252 mg L-1 oyster shell powder); Os2 (336 mg L-1 oyster shell powder). A) germling length; B) growth rate. Values are presented as the average of each treatment and control, and the vertical bars represent the standard deviation (n = 3). Letters indicate significant differences among treatments and control (considering p<0.05)
Explants morphology, upright axes formation, and growth rate
Explants of control, Ca1, and Ca2 treatments formed the upright axes mainly at one end (Fig. 5a – c). Explants from Mx1 and Mx2 formed a greater amount and visibly larger upright axes at both ends than the other treatments (Fig. 5d-e). However, explants treated with oyster shell powder showed thalli degradation and subsequent death (Fig. 5f-g). For this reason, there are no Os1 and Os2 results for quantifying formed upright axes and growth rates.
Light micrographs of G. floridanum explants after 20 days of cultivation. (a) Control (no calcium supplementation); (b) Ca1 (147 mg L-1 CaCl2); (c) Ca2 (295 mg L-1 CaCl2); (d) Mx1 (252 mg L-1 mussel shell powder); e: Mx2 (336 mg L-1 mussel shell powder); (f) Os1 (252 mg L-1 oyster shell powder); (g) Os2 (336 mg L-1 oyster shell powder). Arrows show the highest formation of upright axes. Scale: 1 mm
Explants from Mx1 presented a significantly higher formation of upright axes (10.15 ± 0.85 upright axes) in relation to explants from control (5.18 ± 0.42 upright axes), Ca1 (5.87 ± 0.62 upright axes), and Ca2 (5.18 ± 0.40 upright axes) and similar to them from Mx2 (8.37 ± 0.45 upright axes) (Fig. 6A) (ANOVA F6 = 11.31, p < 0.01). Growth rates were also significantly higher in explants from Mx1 and Mx2 in relation to the other treatments and control (Fig. 6B) (ANOVA F6 = 10.49, p < 0.01).
Number of upright axes and growth rates of G. floridanum explants after 20 days of cultivation in different sources of calcium. (C) Control (no supplementation); Ca1 (147 mg L-1 CaCl2); Ca2 (295 mg L-1 CaCl2); Mx1 (252 mg L-1 mussel shell powder); Mx2 (336 mg L-1mussel shell powder). A) number of upright axes in the end; B) growth rate. Letters indicate significant differences. Values are presented as the average of each treatment and control, and vertical bars represent the standard deviation (n = 4), considering p<0.05
Discussion
The germination of Gelidium floridanum tetraspores was not stimulated by adding CaCl2, as there was no significant difference compared to the control. This result differs from Bouvie (2018) where tetraspores treated with 2 mM CaCl2 showed a significantly higher germination rate than the control and germ tube formation. This difference can be explained by the analysis period; Bouvie (2018) evaluated the germination after 6 h of germination, while the present study evaluated it after 24 hours. In normal conditions, once established on the substrate, the tetraspore started a polarized disorganization of the cytoplasmic content, resulting in cell expansion and a rapid formation of the germ tube. This occurs approximately in the first 6 h and the adhesion and initiation of germ tube formation are dependent on Ca2+, as demonstrated by Bouvie (2018). The cytoplasmic content present in the initial tetraspore is then transferred to the germ tube. At the same time, a thin layer of the cell wall is synthesized around the tube, and a septum is formed, separating the initial spore from the tube (Fig. 1a to c). The germ tube begins successive cell divisions, and in the distal region, there is the emission of a rhizoid cell, which will give rise to the rhizoids. From the fixation of the rhizoid to the substrate, the opposite region originates the erect part of the seedling (Bouzon et al. 2005, 2006). Since the present work aimed to evaluate the tetraspores' germination rate and morphology, rather than the cellular development of the germination process, we decided to analyze after 24 hours, so we could have a higher possibility of having more tetraspores germinated.
This germ tube formation pattern was not observed in treatments with shell powder. Tetraspores treated with shell powder showed few germinated tetraspores, and among them, most delayed the germ tube's formation (Fig. 1d to g), showing a change in cell division direction, alteration in the migration of cytoplasmic content, and in the formation of rhizoids. In particular, tetraspores treated with oyster powder started germination but died in the process, indicated by the presence of a greenish mass. This greenish mass probably comes from tetraspores that did not adhere to the substrate, dying and disintegrating (Simioni et al. 2015). Possibly, even after filtration, the shell powder solution presented microparticles deposited on the slides, making it difficult for tetraspores to adhere, causing their degradation.
Moreover, the oyster shell has calcite in its composition, making the powder finer than mussel powder because of the less organized molecular structure and fragile bonds. This finer powder could also influence tetraspore development since there was an apparent difference between them and those cultivated in mussel powder. For instance, after 20 cultivation days, germlings cultivated in Ca2 showed dense clusters, while those from shell powder showed small low-density clusters. In particular, germlings from Os1, Mx1, and Mx2 were shorter than other treatments and control. Probably, these results are a consequence of what happened in the germination process, confirming the adhesion of tetraspores cultivated in shell powder.
The explants from Mx1 and Mx2 developed upright axes in both ends with significantly higher numbers and growth rates. On the other hand, explants treated with CaCl2 showed upright axes in only one extremity. The calcium gradient in the cytoplasm is responsible for F-actin organization, which results in the correct cell polarization (Hable and Hart, 2010). The higher calcium concentration CaCl2 treatment (78.4 mg L-1) is slightly higher than the lowest mussel powder (68 mg L-1) treatment. In this case, it seems that calcium availability is not the only factor influencing the polarization. Oyster powder treatments, in turn, resulted in the degradation of the thallus. This degradation probably is not caused by calcium since the concentration (84 and 133 mg L-1) is slightly higher than mussel powder. Since the main component of the powder is calcium carbonate, the toxicity of carbon dioxide should be considered. Hamester et al. (2012) characterized the calcium carbonate of the same species used in the present work, finding that there are no significant differences in the percentage of calcium oxide (CaO) and sulfur trioxide (SO3); however, these oxides were the only common ones, and mussel powder showed more diversity in other oxides, including K2O, SiO2, SrO, Fe2O3, MgO and Al2O3. Probably, these cations are working as a buffer in the seawater, avoiding the toxicity caused by the carbonate excess. More detailed studies are needed to understand the effects of these oxides in the growth of G, floridanum.
Santa Catarina is the leading Brazilian producer of mollusks (EPAGRI 2019). Thus, there is high consumption of mussels and oysters, generating a high amount of shell waste. These wastes can be obtained free of charge. There is no need to use a chemical process during the transformation into powder under the conditions described, offering a sustainable solution if it could be used in Gelidium farming, replacing CaCl2.
In conclusion, the shell powder did not improve tetraspore germination, but it can be considered for the cultivation of explants since it stimulates new upright axes. Mussel powder at 252 mg L-1 is recommended rather than oyster powder.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aken ME, Griffin NJ, Robertson BL (1993) Cultivation of agarophyte Gelidium pristoides in Algoa Bay. South Africa Hydrobiologia 268:169–178
Altamirano M, Flores-Moya F, Kuhlenkamp R, Figueroa FL (2003) Stage-dependent sensitivity to ultraviolet radiation in zygotes of the brown alga Fucus serratus. Zygote 11:101–106
Armisén R (1991) Agar and agarose biotechnological applications. Hydrobiologia 221:157–166
Bouvie F (2018) Effects of extracellular calcium on the Gelidium floridanum germination. Dissertation. Universidade Federal de Santa Catarina, Brazil. (in Portuguese). P 64.
Bouzon ZL, Ouriques LO, Oliveira EC (2005) Ultrastructure of tetraspore germination in the agar-producing seaweed Gelidium floridanum (Gelidiales, Rhodophyta). Phycologia 44:409–415
Bouzon ZL, Ouriques LC, Oliveira EC (2006) Spore adhesion and cell wall formation in Gelidium floridanum (Rhodophyta, Gelidiales). J Appl Phycol 18:287–294
Cai G, Cresti M (2010) Microtubule motors and pollen tube growth still an open question. Protoplasma 247:131–143
Cárdenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK (2008) Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol 146:1611–1621
Derksen J, Rutten T, Amstel TV, Win A, Doris F, Steer M (1995) Regulation of pollen tube growth. Acta Bot Neerl 44:93–119
EPAGRI (2019) Annual Summary of Agriculture in Santa Catarina 2018-2019 (In Portuguese). Available at: https://docweb.epagri.sc.gov.br/website_cepa/publicacoes/Sintese_2018_19.pdf (last accessed 20 January 2022)
Fei XG, Huang LJ (1991) Artificial sporeling and field cultivation of Gelidium in China. Hydrobiologia 221:119–124
Filipin EP, Pereira DT, Ouriques LC, Bouzon ZL, Simioni C (2019) Participation of actin filaments, myosin and phosphatidylinositol 3-kinase in the formation and polarisation of tetraspore germ tube of Gelidium floridanum (Rhodophyta, Florideophyceae). Plant Biol 21:352–360
Fredriksen S, Rueness J (1989) Culture studies of Gelidium latifolium (Grev.) Born. et Thur. (Rhodophyta) from Norway. Growth and nitrogen storage in response to varying photon flux density, temperature and nitrogen availability. Bot Mar 32:539–546
Green JJ, Cervantes DC, Peters NT, Logan KO, Kropf DL (2013) Dynamic microtubules and endomembrane cycling contribute to polarity establishment and early development of Ectocarpus mitospores. Protoplasma 250:1035–1043
Hable WE, Hart PE (2010) Signaling mechanisms in the establishment of plant and fucoid algal polarity. Mol Reprod Dev 77:751–758
Hamester MRR, Balzer PS, Becker D (2012) Characterization of calcium carbonate obtained from oyster and mussel shells and incorporation in polypropylene. Mater Res 15:204–208
Hansen JE (1980) Physiological considerations in the mariculture of red algae. J World Mariculture Soc 14:1–4
McLachlan J (1973) Growth media-marine. In: Stein JR (ed) Handbook of Phycological methods. Culture Methods and Growth Measurements. Cambridge University Press, Cambridge, pp 25–55
Mairh O, Rao P (1978) Culture studies on Gelidium pusillum (Stackh.) Le Jolis. Bot Mar 21:169–174
Melo RA, Harger BWW, Neushul M (1991) Gelidium cultivation in the sea. Hydrobiologia 221:91–106
Otaíza RD, Cáceres JH, Rodriguez CY, Sanhueza AG (2019) Seeding of fragments of the agarophyte Gelidium lingulatum (Rhodophyta, Gelidiales) for the repopulation of lower levels of wave exposed, intertidal rocky shores. J Appl Phycol 31:2133–2143
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Santelices B (1976) Note on mass cultivation of some species of Gelidiales (Rhodophyta). Rev Biol Mar Oceanogr 16:27–33 (In Spanish)
Santelices B (1991) Production ecology of Gelidium. Hydrobiologia 221:31–44
Santos R, Melo RA (2018) Global shortage of technical agars: back to basics (resource management). J Appl Phycol 30:2463–2473
Simioni C, Schmidt ÉC, Rover T, Santos R, Filipin EP, Pereira DT, Costa GB, Oliveira ER, Chow F, Ramlov F, Ouriques L, Maraschin M, Bouzon ZL (2015) Effects of cadmium metal on young gametophytes of Gelidium floridanum: metabolic and morphological changes. Protoplasma 252:1347–1359
Veeragurunathan V, Vadodariya N, Chaudhary JP, Gogda A, Saminathan KR, Meena R (2018) Experimental cultivation of Gelidium pusillum in open sea along the south east Indian coast. Indian J Geo Mar Sci 47:336–345
Yarish C, Pereira R (2008) Mass production of marine macroalgae. In: Jørgensen SE, Fath BD (eds) Encyclopedia of ecology. Elsevier, Oxford, pp 2236–2247
Yokoya NS, Handro W (1997) Thallus regeneration and growth induced by plant growth regulators and light intensity in Grateloupia dichotoma (Rhodophyta). In: Kitamura T (ed) Proceedings of the I.T.I.T. International Symposium on New Technologies from Marine-Sphere, Kagawa, Japan, pp 83–86
Zhou W, Sui Z, Wang J, Hu Y, Kang KH, Hong HR, Niaz Z, Wei H, Du Q, Peng C, Mi P, Que Z (2016) Effects of sodium bicarbonate concentration on growth, photosynthesis, and carbonic anhydrase activity of macroalgae Gracilariopsis lemaneiformis, Gracilaria vermiculophylla, and Gracilaria chouae (Gracilariales, Rhodophyta). Photosynth Res 128:259–270
Acknowledgments
CPB thanks Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) for the master's scholarship. LH thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the Productivity Fellowship (Process number 311680/2020-8). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. We also thanks Fernando Zwierzikowski da Silva for the technical support and Prof. Zenilda L. Bouzon for the Plant Cell Biology Laboratory infrastructure.
Funding
This work was funding by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001, Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) for the master's scholarship of Camila Pereira Bruzinga and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the Productivity Fellowship (Process number 311680/2020-8) of Leila Hayashi.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission
1) made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work;
2) drafted the work or revised it critically for important intellectual content;
3) approved the version to be published; and
4) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bruzinga, C.P., Simioni, C. & Hayashi, L. Shell powder as source of calcium in the development of Gelidium floridanum (Rhodophyta, Gelidiales) tetraspores and explants. J Appl Phycol 34, 2579–2588 (2022). https://doi.org/10.1007/s10811-022-02796-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02796-x