Abstract
New food sources are urgently needed due to population growth. The production of microalgae biomass with high protein content is particularly of interest. Galdieria sulphuraria is an extremophilic red microalgae that can grow in acidic environments (pH 0 to 4) and above 40 °C. The aim of this work was to study the photoautotrophic growth and the biochemical composition of five G. sulphuraria strains to potentially produce single-cell protein. A kinetic study of cell growth and macromolecule content was performed in batch mode under controlled conditions: 42 °C, pH 2, constant illumination at 100 μmol photons m−2 s−1, 150 rpm, 0.5 vvm, and atmospheric CO2 (0.04%). The G. sulphuraria CCMEE 5587.1 reached 2.33 g L−1 of dry cell weight (DCW) (~ 9 × 107 cells mL−1) in 20 days, i.e., a productivity of ~ 110 mgDCW L−1 day−1 was achieved. The biomass from this strain shows a high protein content (~ 44% w/wDCW), 42.7% of essential amino acids, 4.7% (w/wDCW) of phycocyanin, 5.9% (w/wDCW) total carbohydrates, and 14.1% (w/wDCW) total lipids. A productivity of 21 t ha−1 year−1 could be attained assuming a straightforward scale-up in open ponds and reaching half the protein productivities obtained in the laboratory. This biomass composition is suitable for food purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The food industry has recently focused on new food and feed sources (e.g., carbohydrates, lipids, proteins, PUFAs, vitamins, and nutraceuticals among others) that satisfy dietary and nutritional needs (Nalage et al. 2016). Proteins are one of the most studied food groups due to their importance as a source of essential amino acids. The unicellular protein or single-cell protein (SCP) refers to protein obtained from the cultivation of various microorganisms using organic or inorganic carbon sources such as bacteria, yeasts, fungi, and algae (Najafpour 2007) that contain more than 40% of crude protein on a dry weight basis (García-Garibay et al. 2014).
SCP production with microorganisms (bacteria and yeasts) under heterotrophic culture conditions has been performed using a wide variety of organic carbon sources such as monosaccharides (glucose), fermentation products (ethanol), and industrial residues (whey) (García-Garibay et al. 2014). However, the use of such carbon sources raises the total economic cost of production due to the high cost of commercial carbon sources or pretreatments made of industrial wastes. Therefore, the use of microalgae biomass as a protein source becomes relevant due to the ability of microalgae to grow in autotrophic culture conditions.
Microalgae are a diverse group of photoautotrophic organisms present in many ecosystems (Gaignard et al. 2019). Microalgae have recently gained great biotechnological and industrial importance due to their ability to fix atmospheric carbon dioxide using solar energy through photosynthesis and produce biomass and other compounds such as storage carbohydrates (e.g., starch or glycogen) (Brányiková et al. 2011; Martinez-Garcia et al. 2017), lipids (e.g., PUFAs) (Koller et al. 2014), and proteins with higher productivities than those obtained with terrestrial plants (Bajpai et al. 2014).
Red algae (Rhodophyta) are a phylum of photosynthetic organisms that contains multicellular and unicellular species that can colonize a wide range of habitats including marine and fresh waters, hot sulfur springs, and volcanic environments. These microorganisms share diverse characteristics such as eukaryotic cells, absence of flagella, production of floridean starch as storage carbohydrate, synthesis of phycobiliprotein pigments (phycocyanin and phycoerythrin), and non-stacked thylakoids (Sheath and Vis 2015). Cyanidiophyceae (subphylum Cyanidiophytina) belongs to the Rhodophyta, which are unicellular algae that live in extremophile conditions in acidic environments and at high temperatures (Gaignard et al. 2019). This class includes two families in the order Cyanidiales (Cyanidiaceae and Galdieriaceae) and the genera Cyanidium, Cyanidioschyzon, and Galdieria.
Galdieria sulphuraria is an extremophile red microalga that thrives in acidic environments with pH values from 0 to 4 and temperatures up to 56 °C (Martinez-Garcia and van der Maarel 2016). It can grow under autotrophic, mixotrophic, and heterotrophic culture conditions using many organic carbon sources (e.g., sugars, polyols, disaccharides, amino acids, and organic acids) (Oesterhelt et al. 2007). In addition, G. sulphuraria has great potential for the biotechnological production of pigmented proteins like phycocyanin (Wan et al. 2016) and functional carbohydrates such as glycogen (Martinez-Garcia et al. 2017) and floridoside (Pade et al. 2015).
The biotechnological potential of microalgae for obtaining high-value products has been explored. However, one of the main limitations is the potential culture contamination with other microorganisms; this issue is relevant when mesophilic temperatures and pH values around neutrality are used (e.g., 5 to 9) (Malavasi et al. 2020). Therefore, the use of acidophilic microalgae becomes relevant because the low pH prevents the growth of other autotrophic microorganisms or other microorganisms that could colonize the culture medium (Varshney et al. 2015).
There are proteins synthesized by G. sulphuraria of biotechnological interest including phycobiliproteins (phycoerythrin, allophycocyanin, and phycocyanin). The latter are pigmented protein complexes involved in the collection and transport (antenna complex) of solar energy during the light phase of photosynthesis (Masojídek et al. 2007). The phycobiliprotein content in G. sulphuraria can reach up to 10% of the total dry weight of the biomass in photoautotrophic cultures (Graziani et al. 2013). Moreover, the potential of G. sulphuraria as a source of protein has been reported with a protein content of 33% (G. sulphuraria 064/309) based on dry weight (Graziani et al. 2013). However, to date, there are few reports regarding the cultivation of G. sulphuraria under autotrophic growth conditions. The aim of this work was to study the photoautotrophic growth and the biochemical macromolecular composition of five different G. sulphuraria strains to potentially produce SCP.
Materials and methods
Algae strains and culture conditions
Different strains of Galdieria sulphuraria were obtained from the algae collection of the Department of Experimental Phycology and Culture Collection of Algae from the University of Göttingen, Germany (SAG 107.79, SAG 108.79, and SAG 21.92), from the Algae Culture Collection of the University of Texas, USA (UTEX 2919), and from the microbial collection of extreme environments of the University of Oregon, USA (CCMEE 5587.1). Stock cultures were maintained by sub-cultivation in 500-mL shake flasks containing 300 mL of medium described by Ford (1979) as well as Gross and Schnarrenberger (1995). One liter of culture medium contained 1.5 g (NH4)2SO4, 300 mg MgSO4·7H2O, 300 mg K2HPO4·3H2O, 20 mg CaCl2·2H2O, 20 mg NaCl, 1.5 mL of Fe-EDTA-solution (690 mg FeSO4, 930 mg EDTA per 100 mL), and 2 mL trace-element solution (2.86 g H3BO3, 1.82 g MnCl2·4H2O, 220 mg ZnSO4·7H2O, 30 mg (NH4)6Mo7O24·4H2O, 80 mg CuSO4·5H2O, 40 mg NaVO3·4H2O, and 40 mg CoCl2·6H2O per liter). The pH was adjusted to 2.0 with H2SO4.
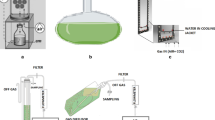
The experimental cultures were evaluated in photobiological systems containing 900 mL of mineral media. A comparative study of cell growth and protein, carbohydrate, and lipid content was performed under controlled growth conditions: 42 °C, pH 2, constant illumination of 100 μmol photons m2 s−1 (white lamps), 150 rpm, and an air flow rate of 0.5 vvm with atmospheric CO2 (0.04%) and filtered through a 0.2 µm sterile membrane. Axenic batch autotrophic cultures were performed in 1-L glass photobioreactors (Schott-Duran 1-L glass bottles with a diameter of 101 mm) (Fig. 1). The bioreactor included a sample port, an air inlet with a sintered stainless steel sparger (2 μm pore size), a gas venting outlet, and an illuminated incubator with temperature control. Agitation was provided with a magnetic stir bar at the bottom of the photobioreactors (50 × 7 mm) and bubbled air through the sparger placed above the stir bar. It was necessary to moisten the air that entered the system using a humidifier due to the temperature used in cultures. All experimental cultures were started with an optical density of 0.1 at 800 nm and a cell density of ~ 5 × 106 cells mL−1. This was performed in triplicate and monitored every 2 days for 20 days.
Analytical methods
Biomass concentration, cell density, and biomass productivity
The biomass concentration was determined by spectrophotometry at 800 nm using distilled water as a blank. The measurements were made in triplicate and interpolated in a dry weight curve.
The cell density (cells mL−1) of the cultures was determined by direct count in a Neubauer chamber and a light microscope equipped with a 40 × objective. The counts were in triplicate, and the results are represented as mean values. The cell density was determined using Eq. 1:
where N is the total cell count, Df is the dilution factor, and n is the total counted quadrants.
The doubling time (Dt) (day) was calculated using Eq. 2 and assuming exponential growth during 20 days of cultivation or when the cells grew exponentially.
where the specific growth rate was determined by
where DCW is the dry cell weight (in gDCW L−1) and f and i correspond to the final and initial values for DCW and time (in days), respectively.
Biomass productivity (QDCW) was calculated using Equation 4:
Carbohydrate content
The glucose-carbohydrate hydrolysis was done by a thermochemical treatment: 10 mg of dry algal biomass was resuspended in 0.5 mL of 2 N hydrochloric acid (HCl), homogenized by vortexing, incubated at 99 °C for 1 h and cooled at room temperature (Garibay-Hernández et al. 2013). For glucose determination, the samples were neutralized with 4 N NaOH and centrifuged at 16,800 × g. The supernatant was retained and the glucose measurements were made with a biochemical analyzer (model YSI 2700, YSI Inc., USA). All determinations were done in triplicate.
Gravimetric lipid determination
The lipid extraction was done by the methodology described by Bligh and Dyer (1959) with some modifications. Thirty mg of dry biomass was resuspended by vortexing in 6 mL of a methanol and chloroform (2:1 v/v) solution and sonicated for 60 min (Branson Model B200 Ultrasonic, USA). Next, 4 mL of a chloroform and 1% NaCl solution (1:1 v/v) were added to obtain a final ratio of methanol, chloroform, and 1% NaCl of 2:2:1 v/v. Finally, the organic phase (chloroform and lipids) was recovered and dried at room temperature, and the remaining portion was considered as lipids. All extractions were performed in triplicate.
Protein content
Protein extraction followed the methodology developed by Slocombe et al. (2013) based on Price (1965) with some modifications. Five mg of dry algae biomass was resuspended by vortexing in 200 μL of 24% (w/v) trichloroacetic acid (TCA). The homogenates were incubated at 95 °C for 15 min and cooled at room temperature. Next, the samples were diluted to 6% TCA (w/v) in ultra-pure water, centrifuged at 15,600 × g for 20 min at 4 °C, and their supernatants discarded. The pellets were resuspended in 500 μL of Lowry D reagent (48:1:1 ratio solution of Lowry reagents A (2% (w/v) Na2CO3 (anhydrous) in 0.1 N NaOH), B (1% (w/v) NaK tartrate tetrahydrate), and C (0.5% (w/v) CuSO4.5H2O in H2O); samples were then incubated for 3 h at 55 °C. The samples were cooled at room temperature and centrifuged at 16,800 × g for 20 min, and the supernatant was retained for quantification via the colorimetric Lowry method (Lowry et al. 1951). Next, 950 μL of Lowry D reagent was added to 50 μL of the above protein extract, mixed by inversion, and incubated for 10 min at RT. Next, 0.1 mL of the diluted Folin–Ciocalteu phenol reagent (1:1 ratio of 2 N Folin–Ciocalteu phenol reagent: ultra-pure) was added to each sample and vortexed immediately. After 30 min at RT, the absorbance of each sample was read at 600 nm. The measurements were made in less than 1 h, and the tests were done in triplicate. The results were interpolated using a bovine serum albumin (BSA) standard curve.
Phycobiliprotein extraction and quantification
For cell disruption and extraction of phycobiliproteins, 10 mg of lyophilized G. sulphuraria biomass was resuspended in 1 mL of extraction buffer (CelLytic M solution with cOmplete Protease Inhibitor Cocktail); 300 mg of 450–500 µm glass beads (Sigma Aldrich) was added, mixed by shaking (Daigger Scientific vortex mixer, Vortex-Genie 2,) for 2 min, incubated for 3 h at 37° C with shaking at 300 rpm (ThermoMixer F1.5, Eppendorf, Germany), and finally centrifuged 16,800 × g (room temperature). The quantification of phycobiliproteins was carried out using the spectrophotometric method of Kursar and Alberte (1983). The absorbance of the supernatants containing phycobiliproteins was measured at 618, 650, and 498 nm to calculate the concentration of phycocyanin, allophycocyanin, and phycoerythrin using Eqs. 5, 6, and 7, respectively. Measurements were made in triplicate (1/10 dilution factor), and the results are represented as mean values:
Chlorophyll extraction and quantification
For cell disruption and chlorophyll extraction, 5 mg of lyophilized G. sulphuraria biomass was resuspended in 1 mL of extraction solution (acetone 90%); 500 mg of 450–500 µm glass beads (Sigma Aldrich) was then added and mixed with shaking (Daigger Scientific vortex mixer, Vortex-Genie 2) for 2 min and incubated for 3 h at 37 °C and 300 rpm (ThermoMixer F1.5, Eppendorf, Germany). Samples were centrifuged at 16,800 × g at room temperature. Chlorophyll quantification used the spectrophotometric method proposed by Ritchie (2006). The absorbance of the chlorophyll-containing supernatants was measured from 400 to 700 nm, and Eq. 8 was used to determine the concentration of the pigment. A dilution factor of 1/10 was used for the adequate measurement of absorbance. All determinations were carried out in triplicate, and the results are represented as mean values:
Amino acid profile determination
The hydrolysis of the microalgal biomass was carried out by a thermochemical treatment. Ten milligram of dry G. sulphuraria biomass was resuspended in 1 mL of 3 N HCl, homogenized by vortexing and incubated for 24 h at 99 °C and 200 rpm. The sample was then neutralized with 10 N NaOH and centrifuged for 30 min at room temperature and 16,800 × g, and the supernatant was retained and filtered. The determination of the amino acid profile was performed by high-performance liquid chromatography (HPLC) according to Henderson et al. (2000) with some modifications. Prior to injection, the amino acids present in the samples were derivatized using ortho-phthalaldehyde (OPA) (for primary amino acids). The following volumes were automatically injected and mixed by the autosampler in the following order: 2.5 μL of borate buffer (0.4 N, pH 10.2), 0.5 μL of sample, 0.5 μL of OPA, and 37 μL of water. The HPLC system (1100 series, Agilent Technologies, Germany) consisted of a binary pump, a thermostat-controlled autosampler, a column compartment, and a diode array detector at 338 nm. Analyses were performed using a Synergi 4 µm Max-RP 80 Å, LC 75 × 2 mm column (Phenomenex, USA), and the column temperature was set at 40 °C. The mobile phase was a 40 mM solution of NaH2PO4 (phase A) and a mixture of water, methanol, and acetonitrile (10:45:45; phase B). The elution of the samples was performed at a flow rate of 2.0 mL min−1 by gradient elution and a total run time of 14 min.
Statistical analysis
The experimental error was determined for all triplicate determinations and expressed as standard deviation (SD). The significant difference of the duplication time comparison was determined by a variance analysis one-way ANOVA test followed by a Tukey test using the statistical packages GraphPad Prism version 6.01 and PAST version 3.14, respectively; p < 0.05 was considered statistically significant.
Results
Growth and biomass production
Table 1 shows a summary of results of the five different G. sulphuraria strains cultivated under autotrophic growth conditions. The microalgae were incubated for 3 weeks. The cells did not reach the stationary phase during such period other than SAG 21.92, which grew poorly under the tested conditions, and CCMEE 5587.1 strain, which surpassed all strains in cell density and biomass accumulation (Fig. 2). However, the G. sulphuraria strain CCMEE 5587.1 grew slower after 14 days of culture and reached a final biomass concentration of 2.33 g L−1 of dry cell weight (DCW) at 20 days. Excluding strain SAG 21.92, this value was 2.2 to 3.2-fold higher than that obtained with the other strains (Fig. 2A, Table 1). Most of the SAG 21.92 cells settled even with the agitation provided by the magnetic stirrer and air sparger. Observations with the microscope revealed the formation of flocs: this strain probably excretes a polymer, but other studies are required to determine the factors that promote this behavior.
In relation to the maximum cell density obtained for each strain (Table 1), G. sulphuraria CCMEE 5587.1 (8.97 × 107 mL−1 cells) reached a value that was 4.6- to 5.5-fold higher than that obtained by the other strains (excluding strain SAG 21.92) (Fig. 2B). Furthermore, the values found for doubling times (Dt) were, on average, 2.12-fold lower for most strains compared to CCMEE 5587.1 (Table 1). These values have a significant difference (Dt SAG. 21.92 ≠ Dt SAG 107.79 = Dt SAG 108.79 ≠ Dt UTEX 2919 ≠ Dt CCMEE 5587.1) as represented by the statistic p < 0.05 (p = 0.0001).
Figure 3A shows the cumulative biomass productivity among the four strains: G. sulphuraria CCME 5587.1 reached a maximum biomass productivity between 14 and 20 days of cultivation (111.7 ± 2.7 mg L−1 day−1). This value was 3.5-, 3.0-, and 2.31-fold higher compared to SAG 107.79, SAG 108.79, and UTEX 2919 at 20 days, respectively.
Biochemical composition: carbohydrates, lipids, and proteins
The main reserve carbohydrate accumulated by G. sulphuraria is floridean starch (Martinez-Garcia et al. 2017). Floridean starch is a highly branched glucose homopolymer with a branched percentage of ~ 18% (α-1,6 linkages) and a molecular size of 2.5 × 105 Da (Martinez-Garcia et al. 2016). Hence, the carbohydrate content was determined from the glucose released after thermochemical hydrolysis (see the Materials and Methods section). The G. sulphuraria strains accumulated lower amounts of carbohydrates that ranged from 4 to 9% (w/w); the highest content was obtained with strain SAG 107.79 (Table 1). Throughout the characterization, there were no changes in the content of this macromolecule at different culture times (Fig. 4A), suggesting that the cultures were not found under nutrient limitation or stress condition, which could lead to over-accumulation of reserve glucans. However, G. sulphuraria CCMEE 5587.1 had a slight increase in carbohydrate content as seen at the end of the culture (day 20), which is a characteristic of the deceleration phase observed for this strain (Fig. 2) in which the carbohydrate content can increase as a reserve material.
Biochemical composition, carbohydrates (glucose) (A), lipids (B), and proteins (C), of different G. sulphuraria strains, was determined at 0, 5, 10, 15, and 20 days of cultivation time. The macromolecule content is expressed as (gX gDCW−1) × 100, where X are proteins, carbohydrates, or lipids. * The macromolecule content in G. sulphuraria SAG.2192 was not determined
The lipid content in the five evaluated strains ranged from 7 to 14% (w/w), and G. sulphuraria CCMEE 5587.1 presented a lipid content 2 times higher than the other strains (Fig. 4B). It has been reported that G. sulphuraria is capable of accumulating lipids with values ranging from 1.1 (Graziani et al. 2013) to 5.4% (Sakurai et al. 2016), which are lower than those contents found in all strains in the present study.
All strains showed a high protein content, above 40% (w/w) for most strains, being G. sulphuraria UTEX 2919 the strain that reached the highest value (~ 47%) (Fig. 4C). These values are higher than those reported previously for G. sulphuraria strain 064/309 (Graziani et al. 2013), which has a protein content of 32.5% in autotrophic cultures. However, the cumulative protein productivity is evident wherein strain G. sulphuraria CCMEE 5587.1 has a productivity 2 times higher than that of strain G sulphuraria UTEX 2919, 51.3, and 25.1 mg L−1 day−1, respectively (Fig. 3B).
Photosynthetic pigments: phycobiliproteins and chlorophyll
There was a slight increase in protein content throughout the exponential phase of the different strains of G. sulphuraria (Fig. 4C). The microalgae actively synthesize various protein components involved in the increase of biomass such as enzymes, structural proteins, transporters, etc. Table 2 shows that the phycocyanin is the phycobiliprotein that accumulates at the highest proportion in the phycobilisome protein complex. These results contrast with that reported by Graziani et al. (2013) who found a higher content of allophycocyanin compared to the other phycobiliproteins. Remarkably, strain CCMEE 5587.1 accumulates the highest amount of phycocyanin: 1.4- to 3.04-fold higher that the values obtained with the other strains (Fig. 5). In the same way as the CCMEE 5587.1 strain reached the highest biomass and proteins productivities, this strain is expected to achieve the highest phycocyanin productivity: 3.9 to 8.6 times higher than the values obtained with the other strains (Fig. 3C). The SAG 21.92 strain did not grow under the tested conditions; the photosynthetic pigments (phycobiliproteins and chlorophyll) and amino acid profiling were not performed for this strain.
As expected, the absorption spectrum obtained shows the presence of two signal peaks at 410 nm and 670 nm, which are characteristic of chlorophyll a (Fig. 6). Despite having a high content of phycobiliproteins as part of the photon harvester complex, it also contains chlorophyll a, which acts as a reaction center in the photosystems involved in the light phase of photosynthesis (Masojídek et al. 2007). Table 2 also shows the chlorophyll pigments extracted from the G. sulphuraria biomass, in which chlorophyll a was accumulated from 1.08 to 2.48 mg g−1 with the highest value found in strain CCMEE 5587.1
Amino acid profile
Due to the extraction methodology (thermochemical hydrolysis) and analysis (HPLC) used in this research, some amino acids such as tryptophan, asparagine, and glutamine were not detectable, which suggests that it could be related to the detection limit of the technique or with the thermochemical treatment carried out for the hydrolysis of the analyzed biomass. Table 3 shows the amino acid profile (essential and non-essential) of four G. sulphuraria strains. It was clear that all strains had a similar amino acid profile with eight of the nine amino acids defined as essential and seven of nine non-essential amino acids.
Tryptophan is a primary, aromatic, and essential amino acid, whose biosynthesis in photosynthetic microorganisms (for example, diatoms) is closely related to the biosynthesis of other aromatic amino acids such as phenylalanine and tyrosine; chorismate is shared as a common precursor molecule (Bromke 2013). Various studies have shown that this amino acid may be found in small concentrations in the biomass of various genera of microalgae and corresponds to one of the amino acids in a lower percentage; thus, some studies have shown that it is not detectable (Chronakis and Madsen 2011; Tibbetts et al. 2015; Koyande et al. 2019). Likewise, some conditions for extracting proteins from microalgal biomass using acids and high temperatures (thermochemical conditions) can destroy tryptophan, and thus, tryptophan cannot be subsequently determined by traditional chromatographic methods (Barbarino and Lourenço 2005).
Glutamine and asparagine (non-essential amino acids) could not be quantified with our methodology. However, Salbitani and Carfagna (2020) measured three free amino acids (glutamine, glutamate, and asparagine) from wet biomass of G. sulphuraria strain 011 using 80% ethanol for its extraction. Therefore, the protein present in the different strains evaluated here could contain these non-determinate amino acids.
Theoretical productivity projected for Galdieria sulphuraria cultures under autotrophic conditions
A theoretical calculation of annual productivity was performed to estimate the potential of G. sulphuraria to produce microalgal biomass with a high protein content. The projection was based on the productivities reached over 20 days using the CO2 contained in air, pH 2, and temperature of 42 °C. The calculations were performed using two hypothetical open ponds with the following dimensions: 100 m × 50 m × 0.25 m (length, width, and liquid depth). The projections were made under two scenarios: (A) the productivity values are not affected by the scaling process and (B) 50% reduction in biomass productivity. The results showed that biomass and protein productivity values are 15.49–48.78 tDCW ha−1 year−1 and 6.18–21.44 tproteins ha−1 year−1, respectively, assuming a half reduction of the projected parameters (Table 4).
Discussion
Galdieria sulphuraria CCMEE 5587.1 had better growth characteristics than SAG 21.92, SAG 107.79, SAG 108.79, and UTEX 2919. It offered the highest biomass concentration, the highest cell density, and the shortest doubling time. Comparing our results with those reported for G. sulphuraria 074 W strain (maximum biomass concentration of 1.1 g L−1 in 12 days under autotrophic conditions) (Sakurai et al. 2016), we observed that strain G. sulphuraria CCMEE 5587.1 had a 25% higher DCW with a value of 1.37 g L−1 in the same elapsed culture time.
The maximum biomass concentration and the macromolecular content (proteins, carbohydrates, and lipids) are essential criteria to evaluate the potential of microorganisms to produce high-value metabolites. However, this parameter alone is an inadequate measure to make a correct choice of a useful strain for large-scale industrial applications where several parameters must be considered, e.g., productivity (Maroneze et al. 2016). The biomass productivity determination throughout the culture also allows us to show the time in which the maximum productivity is reached and therefore decrease the cultivation time. Here, the maximum biomass productivity of G. sulphuraria CCMEE 5587.1 was reached on day 14. Doubling time and protein productivity values obtained for this strain, together with the advantages to growth at acidic pH, can be used to design repeated batch, fed-batch, and semicontinuous or continuous cultures at pilot plant aiming to test the technical feasibility to scale-up the culture process for protein production using G. sulphuraria.
All strains exhibit a high protein content (above 40% for most strains), which shows the potential of this microalgal biomass to produce SCP as a potential food or feed. The protein values achieved with this microorganism compare favorably with the reported content of some vegetables used as protein source in the food and feed industry such as soybean flour (Glycine max) whose percentage yield is ~ 36% and is considered a source of protein (Koyande et al. 2019). Likewise, the protein content in this current study is higher than that of other red microalga such as Porphyridium marinum with a content of 15.4% (Ben Hlima et al. 2019), Porphyridium cruentum at 28.0–39.0% (Becker 2007), and Porphyridium purpureum with 15.1% (Assunção et al. 2017). These were also measured under autotrophic conditions. Since microalgal biomass destined for food or feed is normally supplied as a supplement in diets, it is important to consider the entire biomass. In addition to the high protein content that we observed in the G. sulphuraria biomass, there are other types of macromolecules present such as lipids and carbohydrates that collectively provide good nutritional characteristics to the generated biomass (Koyande et al. 2019).
The presence of phycobiliproteins and chlorophyll in the biomass of G. sulphuraria provides a nutraceutical value added to the microalgal biomass obtained in this work using only the CO2 content in the air. Phycocyanin has nutraceutical activities related to a decrease in blood pressure (antihypertensive activity), free radicals and reactive oxygen species (antioxidant activity), inflammatory processes (anti-inflammatory activity), and proliferation of uncontrolled cellular processes (anticancer activity) (Abd El-Hack et al. 2019; Chandra et al. 2020; Lafarga et al. 2020). In addition, chlorophyll provides anticarcinogenic, antioxidant, and antigenotoxic properties. It is an effective nutraceutical for liver illnesses (Bishop and Zubeck 2012; García et al. 2017; Koyande et al. 2019).
Phycocyanin is currently a commercially valuable molecule obtained from photoautotrophic cultures with cyanobacteria such as Arthrospira (Spirulina) platensis. For this microorganism, contents of 56.6 mg g−1 (Manirafasha et al. 2018), 148.1 mg g−1 (Ajayan et al. 2012), and 160 mg g−1 (Xie et al. 2015) have been reached, which are 1.2 to 3.4 times higher than those reported in our study with the strain CCMEE 5587.1 (47 mg g−1) (Table 2), indicating that further research is needed to maximize phycocyanin production with several G. sulphuraria strains. It is worth mentioning that one of the main advantages of the phycocyanin synthesized by G. sulphuraria is its thermostability at 60 °C, unlike that reported for phycocyanin from Spirulina sp., with stability at temperatures below 47 °C (Moon et al. 2014).
Regarding the quality of the protein obtained, the amino acid profile showed that the generated biomass has most essential amino acids. The amino acid profile or amino acid score is a fundamental parameter for the evaluation of protein quality especially protein destined for human and animal consumption (Vanthoor-Koopmans et al. 2013). This protein should preferably contain all or most of the essential amino acids (Koyande et al. 2019). The presence of some amino acids such as methionine and lysine increases the growth rate in broiler chickens (Lima et al. 2008; Vanthoor-Koopmans et al. 2013).
Assuming a straightforward scale-up using strain CCMEE 5587.1 in open ponds and that half of the protein productivities obtained in the laboratory can be reached, a productivity of 21 tproteins ha−1 year−1 could be attained. This value compares favorably with the productivities reported for soybean in Brazil and the USA (main soybean producing countries in the world): 2019–2020 were 1.36 and 1.27 tproteins ha−1 year−1, respectively (estimated values based on protein content of 40%) (Embrapa 2021). This hypothetical productivity with G. sulphuraria is 15.4 to 16.5 times higher than that obtained with this legume. Additionally, one of the main advantages that the cultivation of microalgae presents compared to the cultivation of terrestrial plants is that the cultivation of microalgae does not require the use of agricultural land and therefore does not compete with food or feed production (Benedetti et al. 2018).
The main problem associated with the use of microalgae, especially in open ponds, is the high probability of contamination with other microorganisms. This is one of the most important factors affecting microalgae growth and decreases the productivity of metabolites of interest. Therefore, one of the most effective strategies to counteract this problem is to use extremophilic microalgae species in extreme cultivation conditions and growing in simple mineral media. Galdieria sulphuraria is classified as extremophilic because it could survive in acidic environments with pH values 0–4 (acidophilic) and at temperatures above 40 °C (thermotolerant) (Martinez-Garcia and van der Maarel 2016). Cultivation conditions used for G. sulphuraria also promotes a synergism between the low pH of the culture medium, irradiance, high temperature, and dissolved oxygen (DO), which leads to the appearance of reactive oxygen species (ROS) and a potential negative effect on the growth of contaminating microorganisms (Munasinghe-Arachchige et al. 2019). Finally, temperature control in the microalgae cultivation in open ponds continues to be one of the main technological challenges to implement. In this sense, G. sulphuraria can grow adequately in reactors exposed to sunlight and natural temperature variations from 24 to 50 °C (Henkanatte-Gedera et al. 2017).
Conclusions
We produce G. sulphuraria microalgal biomass with high protein content under phototrophic conditions and using the CO2 content in air. Due to the thermoacidophile conditions, the biomass is free of microbial contamination and suitable for scale-up. Finally, the resulting biomass has a high potential to produce single-cell protein as the result of the biochemical analysis of this red microalga. This biomass composition is suitable for food or feed purposes, but nutritional tests must still be done. Among the five different tested strains, G. sulphuraria CCMEE 5587.1 is proposed as the most appropriate strain for scale-up because it has the highest biomass productivity and the highest percentage content of phycocyanin and chlorophyll as well as a protein and lipid content higher than 40% and 14%, respectively.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abd El-Hack ME, Abdelnour S, Alagawany M, Abdo M, Sakr MA, Khafaga AF, Mahgoub SA, Elnesr SS, Gebriel MG (2019) Microalgae in modern cancer therapy: current knowledge. Biomed Pharmacother 111:42–50
Ajayan KV, Selvaraju M, Thirugnanamoorthy K (2012) Enrichment of chlorophyll and phycobiliproteins in Spirulina platensis by the use of reflector light and nitrogen sources: an in-vitro study. Biomass Bioenerg 47:436–441
Assunção MFG, Varejão JMTB, Santos LMA (2017) Nutritional characterization of the microalga Ruttnera lamellosa compared to Porphyridium purpureum. Algal Res 26:8–14
Bajpai R, Zappi M, Dufreche S, Subramaniam R, Prokop A (2014) Status of algae as vehicles for commercial production of fuels and chemicals. In: Bajpai R, Prokop A, Zappi M (eds) Algal Biorefineries, vol 1. Cultivation of Cells and Products. Springer, Dordrecht, pp 3–24
Barbarino E, Lourenço SO (2005) An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J Appl Phycol 17:447–460
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210
Ben Hlima H, Dammak M, Karkouch N et al (2019) Optimal cultivation towards enhanced biomass and floridean starch production by Porphyridium marinum. Int J Biol Macromol 129:152–161
Benedetti M, Vecchi V, Barera S, Dall’Osto L (2018) Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microb Cell Fact 17:173
Bishop W, Zubeck H (2012) Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J Nutr Food Sci 02:
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brányiková I, Maršálková B, Doucha J, Brányik T, Bišova K, Zachleder V, Vítova M (2011) Microalgae-novel highly efficient starch producers. Biotechnol Bioeng 108:766–776
Bromke MA (2013) Amino acid biosynthesis pathways in diatoms. Metabolites 3:294–311
Chandra P, Sharma RK, Arora DS (2020) Antioxidant compounds from microbial sources: a review. Food Res Int 129:108849
Chronakis IS, Madsen M (2011) Algal proteins. In: Phillips GO, Williams PA (eds) Handbook of Food Proteins. Woodhead Publishing, London, pp 353–394
Embrapa (2021) Soja em números (safra 2019/20). Empresa Brasileira de Pesquisa Agropecuária. https://www.embrapa.br/soja/cultivos/soja1/dados-economicos. Accessed 2 June 2021
Ford TW (1979) Ribulose 1,5-bisphosphate carboxylase from the thermophilic, acidophilic alga, Cyanidium caldarium (Geitler): purification, characterisation and thermostability of the enzyme. Biochim Biophys Acta - Enzymol 569:239–248
Gaignard C, Gargouch N, Dubessay P, Delattre C, Pierre G, Laroche C, Fendri I, Abdelkaf S, Michaud P (2019) New horizons in culture and valorization of red microalgae. Biotechnol Adv 37:193–222
García JL, de Vicente M, Galán B (2017) Microalgae, old sustainable food and fashion nutraceuticals. Microb Biotechnol 10:1017–1024
García-Garibay M, Gómez-Ruiz L, Cruz-Guerrero AE, Bárzana E (2014) Single cell protein Yeasts and Bacteria. Encycl Food Microbiol 431–438
Garibay-Hernández A, Vazquez-Duhalt R, Serrano-Carreón L, Martinez A (2013) Nitrogen limitation in Neochloris oleoabundans: a reassessment of its effect on cell growth and biochemical composition. Appl Biochem Biotechnol 171:1775–1791
Graziani G, Schiavo S, Nicolai MA, Buono S, Fogliano V, Pinto G, Pollio A (2013) Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct 4:144–152
Gross W, Schnarrenberger C (1995) Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol 36:633–638
Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C (2000) Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Agilent Technologies. Application note No. 5980–1193E
Henkanatte-Gedera SM, Selvaratnam T, Karbakhshravari M, Myint M, Nirmalakhandan N, Van Voorhies W, Lammers PJ (2017) Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: laboratory to field scale demonstration. Algal Res 24:450–456
Koller M, Muhr A, Braunegg G (2014) Microalgae as versatile cellular factories for valued products. Algal Res 6:52–63
Koyande AK, Chew KW, Rambabu K, Tao Y, Chu DT, Show PL (2019) Microalgae: a potential alternative to health supplementation for humans. Food Sci Hum Wellness 8:16–24
Kursar TA, Alberte RS (1983) Photosynthetic unit organization in a red alga : relationships between light-harvesting pigments and reaction centers. Plant Physiol 72:409–414
Lafarga T, Fernández-Sevilla JM, González-López C, Acién-Fernández FG (2020) Spirulina for the food and functional food industries. Food Res Int 137:109356
Lima LMB, Lara LJC, Baiao NC, Cancado SD, Michell BC, Ferreira FC (2008) Effect of energy, lysine and methionine and cystine levels on performance and carcass yield of broiler chickens. Braz J Anim Sci 37:1424–1432
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Malavasi V, Soru S, Cao G (2020) Extremophile microalgae: the potential for biotechnological application. J Phycol 56:559–573
Manirafasha E, Murwanashyaka T, Ndikubwimana T, Ahmed NR, Liu J, Lu Y, Zeng X, Ling X, Jing K (2018) Enhancement of cell growth and phycocyanin production in Arthrospira (Spirulina) platensis by metabolic stress and nitrate fed-batch. Bioresour Technol 255:293–301
Maroneze MM, Siqueira SF, Vendruscolo RG, Wagner R, Ragagninde-de-Menezes C, Queiroz-Zepka L, Jacob-Lopes E (2016) The role of photoperiods on photobioreactors – a potential strategy to reduce costs. Bioresour Technol 219:493–499
Martinez-Garcia M, van der Maarel MJEC (2016) Floridoside production by the red microalga Galdieria sulphuraria under different conditions of growth and osmotic stress. AMB Express 6:71
Martinez-Garcia M, Stuart MCA, van der Maarel MJE (2016) Characterization of the highly branched glycogen from the thermoacidophilic red microalga Galdieria sulphuraria and comparison with other glycogens. Int J Biol Macromol 89:12–18
Martinez-Garcia M, Kormpa A, van der Maarel MJEC (2017) The glycogen of Galdieria sulphuraria as alternative to starch for the production of slowly digestible and resistant glucose polymers. Carbohydr Polym 169:75–82
Masojídek J, Torzillo G, Koblížek M (2007) Photosynthesis in microalgae. In: Richmond A, Hu Q (eds) Handbook of Microalgal Culture. John Wiley Blackwell, Oxford, pp 20–39
Moon M, Mishra SK, Kim CW, Suh WI, Park MS, Yang JW (2014) Isolation and characterization of thermostable phycocyanin from Galdieria sulphuraria. Korean J Chem Eng 31:490–495
Munasinghe-Arachchige SP, Delanka-Pedige HMK, Henkanatte-Gedera SM, Tchinda D, Zhang Y, Nirmalakhandan NM (2019) Factors contributing to bacteria inactivation in the Galdieria sulphuraria-based wastewater treatment system. Algal Res 38:101392
Najafpour GD (2007) Single-cell protein. In: Najafpour GD (ed) Biochem Eng Biotechnol. Elsevier Science, New York, pp 332–341
Nalage DN, Khedkar GD, Kalyankar AD, Sarkate AP (2016) Single cell proteins. Encycl Food Heal 790–794
Oesterhelt C, Schmälzlin E, Schmitt JM, Lokstein H (2007) Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J 51:500–511
Pade N, Linka N, Ruth W, Weber APM, Hagemann M (2015) Floridoside and isofloridoside are synthesized by trehalose 6-phosphate synthase-like enzymes in the red alga Galdieria sulphuraria. New Phytol 205:1227–1238
Price CA (1965) A membrane method for determination of total protein in dilute algal suspensions. Anal Biochem 12:213–218
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Sakurai T, Aoki M, Ju X, Ueda T, Nakamura Y, Fujiwara S, Umemura T, Tsuzuki M, Minoda A (2016) Profiling of lipid and glycogen accumulations under different growth conditions in the sulfothermophilic red alga Galdieria sulphuraria. Bioresour Technol 200:861–866
Salbitani G, Carfagna S (2020) Different behaviour between autotrophic and heterotrophic Galdieria sulphuraria (Rhodophyta) cells to nitrogen starvation and restoration. Impact on pigment and free amino acid contents. Int J Plant Biol 11:7–11
Sheath RG, Vis ML (2015) Red Algae. In: Wehr JD, Sheath RG, Kociolek JP (eds) Freshwater algae of North America, 2nd edn. Academic Press, Boston, pp 237–264
Slocombe SP, Ross M, Thomas N, McNeill S, Stanley MS (2013) A rapid and general method for measurement of protein in micro-algal biomass. Bioresour Technol 129:51–57
Tibbetts SM, Milley JE, Lall SP (2015) Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J Appl Phycol 27:1109–1119
Vanthoor-Koopmans M, Wijffels RH, Barbosa MJ, Eppink MHM (2013) Biorefinery of microalgae for food and fuel. Bioresour Technol 135:142–149
Varshney P, Mikulic P, Vonshak A, Beardall J, Wangikara PP (2015) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol 184:363–372
Wan M, Wang Z, Zhang Z, Wang J, Li S, Yu A, Li Y (2016) A novel paradigm for the high-efficient production of phycocyanin from Galdieria sulphuraria. Bioresour Technol 218:272–278
Xie Y, Jin Y, Zeng X, Chen J, Lu Y, Jing K (2015) Fed-batch strategy for enhancing cell growth and C-phycocyanin production of Arthrospira (Spirulina) platensis under phototrophic cultivation. Bioresour Technol 180:281–287
Acknowledgements
The authors thank Mario A. Caro-Bermudez, Juan Manuel Hurtado Ramírez, Roberto Pablo Rodríguez Bahena, and Leonardo Peña-Carranza for technical support. Algae strains were obtained from the University of Göttingen, Germany (SAG 107.79, SAG 108.79, and SAG 21.92); Algae Culture Collection of the University of Texas, USA (UTEX 2919); and the Microbial Collection of Extreme Environments of the University of Oregon, USA (CCMEE 5587.1). CAMH held a scholarship from CONACyT – Mexico (699699).
Funding
This work was supported by Universidad Nacional Autónoma de México (UNAM) – Dirección General de Asuntos del Personal Académico (DGAPA), Grant PAPIIT-DGAPA-UNAM IT201119.
Author information
Authors and Affiliations
Contributions
CAM-H and AM, conception and design of the study and manuscript drafting and editing. CAM-H, cultivation of microalgae, biochemical composition analyses, and data analysis. AM, project administration and funding acquisition. All the authors (CAM-H, FV-LP, GTH-C, and AM) participated in data analyses, discussion of results, and writing and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Montenegro-Herrera, C.A., Vera-López Portillo, F., Hernández-Chávez, G.T. et al. Single-cell protein production potential with the extremophilic red microalgae Galdieria sulphuraria: growth and biochemical characterization. J Appl Phycol 34, 1341–1352 (2022). https://doi.org/10.1007/s10811-022-02733-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02733-y