Abstract
The chrysophyte Poterioochromonas malhamensis has potential for controlling algal blooms through rapid grazing of toxic Microcystis cells and efficient degradation of microcystin. However, this method has not been used in practice because a high-cell-density method for cultivating P. malhamensis has not yet been established and the actual effect of the chrysophyte in controlling Microcystis blooms in the field is still unknown. To achieve the application of this method, high-cell-density heterotrophic cultivation of P. malhamensis was established through optimizing the carbon/glucose concentration, C:N ratio, temperature, pH, and dissolved oxygen concentration. Under optimized conditions, the cell concentration of P. malhamensis reached more than 3 × 108 cells mL−1, which exceeds that reported in other studies by more than an order of magnitude. The ability of the chemoheterotrophic P. malhamensis to graze unicellular Microcystis cells was comparable to that of autotrophic and phagotrophic P. malhamensis. A controlled field experiment showed that chemoheterotrophic P. malhamensis could live in the aquatic environment with a Microcystis bloom and decrease the Microcystis biomass on the surface of the water by promoting the sedimentation of colonial Microcystis cells. This study offers an opportunity to drive the development of methods to control Microcystis blooms using predatory P. malhamensis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chrysophyte Poterioochromonas is a mixotrophic flagellate microalga that can either live by autotrophy (i.e., by photosynthesis through chloroplasts) or heterotrophy (including directly assimilating dissolved organics and utilizing ingested food particles). It can graze on a diverse range of prey (e.g., other microalgae, bacteria, and organic particles) with the help of its two unequal flagella (Zhang & Watanabe, 1996). This grazing ability poses a great threat to commercial, mass culture of other microalgal species (Touloupakis et al., 2016; Ma et al., 2018), although fortunately the early detection of Poterioochromonas by real-time PCR (Wang et al., 2021b) and diverse methods of controlling Poterioochromonas in microalgal culture have been successively established (Ma et al., 2017; Wang et al., 2018; He et al., 2021; Toda et al., 2021). In contrast to these negative impacts, Poterioochromonas also has potential for many beneficial applications. For example, the potential effects of chemical compounds on the cell reproduction and viability of Poterioochromonas malhamensis can be easily examined, making it a good test system in ecotoxicology, toxicology, and pharmacology (Roderer, 1986). Furthermore, P. malhamensis cells can produce many active substances, such as chrysolaminarin (i.e., a β-glucan) serving as an immunopotentiator (Kauss & Kriebitzsch, 1969; Zeković et al., 2005) and malhamensilipin A exhibiting antiviral and antimicrobial activity (Chen et al., 1994).
To realize the potential application of Poterioochromonas, the acquisition of enough Poterioochromonas cells is a critical first step. The growth rate of Poterioochromonas under autotrophic conditions is extremely low, which may be due to the lack of CO2 concentrating mechanisms (Sanders et al., 1990; Giordano et al., 2005). Therefore, Poterioochromonas has been described as a predominantly heterotrophic mixotroph (Holen & Boraas, 1995). The addition of prey microorganisms, such as microalgae and bacteria, can markedly improve the growth rate of Poterioochromonas (Zhang & Watanabe, 2001). However, this method is not suitable for large-scale production of Poterioochromonas because of the difficulty in providing a persistent supply of stable-quality prey. An alternative is chemoheterotrophic cultivation. Up to now, many species of microalgae, such as species of Chlorella, Euglena, and Scenedesmus, have been cultivated on an industrial scale to achieve high cell densities using fermenters (Jin et al., 2020). Generally, the biomass of these microalgae achieved by chemoheterotrophic cultivation is 10 times more than that achieved by autotrophic cultivation (Liu et al., 2014). For instance, a maximum biomass of 32.5 g L−1 for Ochromonas danica has been achieved by heterotrophic fermentation (Lin et al., 2014). Consequently, chemoheterotrophy should also be the most efficient way to cultivate Poterioochromonas on a large scale. However, although Poterioochromonas has been successfully cultivated using chemoheterotrophic medium containing glucose (Lewitus & Caron, 1991; Rottberger et al., 2013), the fermentation technology for cultivation of Poterioochromonas has not been systematically studied.
In recent decades, the promising prospect of using Poterioochromonas to control algal blooms has also been widely studied. Considerable laboratory data have suggested that Poterioochromonas can rapidly graze on toxic Microcystis cells (Kim & Han, 2007; Zhang et al., 2009). More importantly, Poterioochromonas cells can also efficiently degrade microcystins (Ou et al., 2005; Zhang et al., 2008). Therefore, Poterioochromonas is one of the few microorganisms that can simultaneously reduce Microcystis cells and microcystins. It is notable that although the feeding characteristics and scavenging activity of Poterioochromonas on toxic Microcystis cells in the laboratory have been well studied, its viability and feeding behavior in Microcystis blooms in the field remain unknown. Furthermore, autotrophic Poterioochromonas has usually been used in these studies of Poterioochromonas feeding on Microcystis, while the grazing ability of chemoheterotrophic Poterioochromonas on Microcystis remains to be investigated.
This study therefore had two main aims: to establish a method for the high-cell-density chemoheterotrophic cultivation of P. malhamensis by optimizing heterotrophic culture conditions; and to evaluate the effects of chemoheterotrophic P. malhamensis in controlling unicellular Microcystis under laboratory conditions and on outdoor Microcystis blooms. The feasibility of controlling Microcystis blooms using P. malhamensis is also discussed.
Materials and methods
Organisms and culture conditions
The chrysophyte Poterioochromonas malhamensis was isolated from contaminated Chlorella sorokiniana GT-1 culture, purified using a combination of antibiotics according to the methods of Corno and Jurgens (2006) and Destain et al. (2014), and then maintained in axenic culture. The axenic P. malhamensis was cultivated with a “specific flask medium” at a temperature of 25 °C under dark conditions. The “specific flask medium”, adapted from Blom and Pernthaler (2010), contained 10 g L−1 glucose, 3 g L−1 yeast extract, 1.0 g L−1 beef liver infusion, 0.5 g L−1 KH2PO4, and 0.5 g L−1 MgSO4·7H2O. Toxic Microcystis aeruginosa FACHB 942 was obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology, Chinese Academy of Sciences, China. Microcystis aeruginosa was cultivated using BG-11 medium (Rippka et al., 1979) at a temperature of 30 ± 1 °C under continuous illumination at 25 ± 10 μmol photons m−2 s−1.
Optimizing the chemoheterotrophic culture conditions for P. malhamensis
The inoculant for large-scale microalgal fermentation is taken from cultures grown at the bench scale in shake flasks. A stable chemoheterotrophic cultivation method at the flask scale is essential for the large-scale fermentation of P. malhamensis. In this study, optimal levels for most of the P. malhamensis culture conditions were determined using 250-mL shake flasks with a working volume of 100 mL (Table 1). A total of seven culture parameters, including the concentration of glucose, ratio of carbon to nitrogen, temperature, pH of the medium, light intensity, initial cell concentration, and shaker speed, were studied. All experiments were performed in triplicate using shaking incubators (HYL-C3, Qiang Le, China). The basal culture medium for chemoheterotrophic P. malhamensis was the “specific flask medium” as described in the “Organisms and culture conditions” section. The P. malhamensis cells were pre-stained with 1% Lugol’s iodine (Leakey et al., 1994) before counting. The cell concentration of P. malhamensis was counted every day using a hemocytometer (Improved Neubauer, USA) under a light microscope (BX53, Olympus, Japan). The semi-quantitative analysis of glucose concentration was conducted using a Safe-AccuUG Blood Glucose Monitoring System (Model BGMS-1; Sinocare Inc., Changsha, China), with a lower limit of detection of 0.4 g L−1. Generally, the glucose concentration would decrease to zero within 5 days, and therefore, the growth data of P. malhamensis were only collected for 4–5 days.
In addition, the effect of dissolved oxygen (DO) concentration (0, 20, and 40%) on the growth of P. malhamensis was also studied in 1.0-L fermenters (My-control, Applikon Biotechnology, Netherlands). For the 20% and 40% treatments, the DO was controlled automatically by additional delivery of pure oxygen. For the 0% treatment, which received no delivery of pure oxygen, the DO was only monitored by a probe and decreased from 100% to zero within 24 h. The other culture conditions for P. malhamensis in the fermenters were based on the optimal conditions identified in the aforementioned flask experiments. The pH of the medium was set at 6.0 (± 0.2) as determined by the pH probe (Z001018510, Applisens) and was automatically maintained by the addition of 1 M NaOH or 1 M HCl solution. The temperature was set at 28 °C with a fluctuation range of ± 0.5 °C. The stirring speed was 400 rpm. Aeration was maintained at an airflow rate of 1 L min−1. The basal medium used during the fermentation process was adapted from a combination of the “specific flask medium” and Endo growth medium (Jin et al., 2020). It contained 10 g L−1 glucose, 6 g L−1 yeast extract, 2.0 g L−1 liver extract powder, 0.3 g L−1 KH2PO4, 0.5 g L−1 MgSO4·7H2O, 73.5 mg L−1 CaCl2·2H2O, 4.4 mg L−1 Na2·EDTA, 3.15 mg L−1 FeCl3·6H2O, 0.97 mg L−1 H3BO3, 0.02 mg L−1 ZnSO4·7H20, 0.18 mg L−1 MnCl2·4H2O, 0.006 mg L−1 NaMoO4·2H2O, and 0.012 mg L−1 Co(NO3)2·6H2O, with a ratio of carbon to nitrogen source of 5:1. The feeding medium was the 20-fold concentrated basal medium, except for the nitrogen sources (yeast extract (76 g L−1) and liver extract powder (24 g L−1)). The glucose concentration of the medium was monitored using the Safe-AccuUG Blood Glucose Monitoring System every 6–8 h and then adjusted to 5–10 g L−1 by adjusting the flow velocity of the feeding medium. The initial cell concentration of P. malhamensis was 2.8 × 106 cells mL−1, and the cell concentration of P. malhamensis was determined daily. Each treatment was performed in duplicate. The experiment was repeated three times and the same results were obtained.
Grazing ability of chemoheterotrophic Poterioochromonas malhamensis on unicellular Microcystis aeruginosa
To estimate the grazing ability of chemoheterotrophic P. malhamensis cells on unicellular M. aeruginosa, P. malhamensis cells cultivated with three different modes of nutrition (i.e., autotrophy, chemoheterotrophy, and phagotrophy) were used as the predator of M. aeruginosa. Chemoheterotrophic P. malhamensis was grown in the “specific flask medium” as described in the “Organisms and culture conditions” section, while autotrophic P. malhamensis was cultivated in BG-11 medium. For phagotrophy, the P. malhamensis was cultivated in BG-11 medium and fed with M. aeruginosa in advance, and then used in this experiment when the M. aeruginosa was almost cleared out. The feeding experiment was carried out in 250-mL flasks with a working volume of 100 mL BG-11 medium. Each treatment was performed in triplicate. The initial cell concentrations of P. malhamensis and M. aeruginosa were 1.0 × 105 cells mL−1 and 1.0 × 107 cells mL−1, respectively, in all treatments. For the control, the medium was inoculated with only M. aeruginosa, to give a concentration of 1.0 × 107 cells mL−1. Cell concentrations of P. malhamensis and M. aeruginosa were counted every 12 h using a hemocytometer (Improved Neubauer, USA) under a light microscope. The P. malhamensis cells were pre-stained with 1% Lugol’s iodine before counting. The micro-morphology and feeding behavior of P. malhamensis were recorded using a light microscope (BX53, Olympus, Japan). The culture flasks were incubated in a culture room at 25 ± 1 °C under continuous illumination at 25 ± 10 μmol photons m−2 s−1.
Control of Microcystis outdoors using chemoheterotrophic Poterioochromonas malhamensis
The chemoheterotrophic P. malhamensis was further trialed for the control of outdoor colonial Microcystis in the field. To obtain enough P. malhamensis, it was cultivated using 7.5-L bioreactors (BioFlo & CelliGen 310, New Brunswick) with an initial working volume of 3 L. The basal medium and feeding medium were the same as those media used in the 1.0-L bioreactors as described in the “Optimizing the chemoheterotrophic culture conditions for P. malhamensis” section. The DO concentration was automatically maintained at 20% by gradually increasing the stirring speed from 50 to 400 rpm and the aeration rate from 4 to 7 L min−1 as the cultivation progressed. The temperature and pH value were set at 28 °C and 6.0, respectively. After a cultivation phase of 3 days, the cell concentration of P. malhamensis reached 3 × 108 cells mL−1. To prepare the inoculum, the P. malhamensis cells were harvested by centrifugation at 1500 × g for 5 min and washed with double-distilled water (ddH2O) three times to remove the residual organics.
Otdoor Microcystis control experiments were conducted around a fish pond (30° 31′ N, 114° 23′ E; Wuhan, China) with a Microcystis bloom (Fig. 1A–C) from October 16 to October 22 in 2018. The Microcystis sp. suspension (more than 1200 L) was first pumped from the pond into a 1400-L white plastic bucket (Fig. 1D, bucket 0). After homogeneous mixing, a volume of 200 L Microcystis suspension was then successively transferred into six further buckets, comprising three control repetitions and three experiment repetitions. This was repeated four times, giving a final working volume of Microcystis suspension of 800 L in each of the six buckets. For the experiment group, P. malhamensis was added to give a final cell concentration of 3.0 × 105 cells mL−1. To avoid P. malhamensis sinking, a circular aerated conduit with a diameter of 65 cm was placed in the bottom of each of the six buckets (Fig. 1E). Samples were collected daily from a depth of 10–20 cm below the surface of the liquid. On the first 3 days, the Microcystis suspensions were not agitated before sampling; from days 4 to 6, the suspensions were evenly stirred by club before sampling. Collected samples were used to determine cell concentrations of P. malhamensis and Microcystis sp., as well as the chlorophyll-a concentration of the suspension. To determine the cell concentration of Microcystis, the collected sample was pre-heated at 60 °C for 10 min to disperse the colonial Microcystis (Humphries & Widjaja, 1979). The concentration of chlorophyll-a was determined according to the method of Ma et al. (2017). A YSI Professional Plus meter (USA) was used to determine the DO and pH value of the Microcystis suspension. The micro-morphology of Microcystis and P. malhamensis was observed and recorded daily by light microscopy (BX53, Olympus, Japan). The color change of the Microcystis suspension was also recorded by camera (Canon EOS 60D, Japan). Light intensity and temperature (14–24 °C) changed with local weather conditions.
The equipment and Microcystis sources for the outdoor experiment using P. malhamensis. A, Distant view of the fish pond with outbreak of Microcystis; B, close view of the Microcystis bloom; C, microscopic observation of Microcystis (scale bar = 10 μm); D, plastic buckets used in the study (the bucket labeled No. 0 was used for pre-mixing and the other six buckets were used in the control group (Nos. 1–3) and the experiment group (Nos. 4–6)); E, specification of the plastic bucket
Statistical analyses
All data are presented as mean values ± standard deviations. The statistical significance of differences in different sample groups was analyzed using one-way ANOVA (analysis of variance). Tukey’s multiple comparison tests were used when more than two sample groups were present. The results were considered to be significantly different at P < 0.05. All statistical analyses were performed using the software SPSS 16.0.
Results
Optimization of culturing conditions for chemoheterotrophic Poterioochromonas malhamensis in the shake flask system
In the shake flask system, the optimal levels of the four main culture parameters—glucose concentration, C:N ratio, temperature, and pH of the medium—were determined. The absence of glucose (0 g L−1) led to stagnation in the growth of P. malhamensis (Fig. 2A). The highest biomass (3.2 × 107 cells mL−1) was obtained when P. malhamensis was cultivated with 10 g L−1 glucose after 4 days of cultivation. However, in the first 2 days during which glucose was abundant, P. malhamensis at the glucose concentration of 5 g L−1 grew faster than that at 10 g L−1 (P > 0.05). Once the glucose had been consumed in the 5 g L−1 treatment, the cell concentration of P. malhamensis stopped increasing. The two highest glucose concentrations, 20 g L−1 and 30 g L−1, were found to inhibit the growth of P. malhamensis. The growth of P. malhamensis was also greatly affected by the C:N ratio (i.e., the nitrogen concentration). The growth rate of P. malhamensis increased with a decrease in the C:N ratio within the range from 30:1 to 5:1 (Fig. 2B). A lower C:N ratio of 2:1 contributed little to improving the growth of P. malhamensis. The cell concentrations of P. malhamensis cultivated with a C:N ratio of 5:1 and 2:1 also stopped increasing after the glucose had been consumed. Poterioochromonas malhamensis at 28 °C and 32 °C grew faster than at 24 °C and 36 °C (P < 0.05), while the growth rates of P. malhamensis at 28 and 32 °C were similar (P > 0.05) (Fig. 2C). The optimal initial pH value for the growth of P. malhamensis was 6.0, followed by pH 5.0 and 7.0 in the first 3 days. However, the biomass of P. malhamensis after 4 days of cultivation was similar for pH 5.0, 6.0, and 7.0 (P > 0.05). Growth of P. malhamensis was considerably inhibited when the initial pH value increased to 8.0 (Fig. 2D). In the shake flask system, the maximum cell concentration of P. malhamensis could achieve 4.8 × 107 cells mL−1 (Fig. 2B).

source at this point is running out and the glucose concentration is too low to be detected. Error bars represent the standard deviation (n = 3)
Growth of chemoheterotrophic P. malhamensis in 250-mL flasks under different culture conditions. A, Glucose concentration; B, ratio of carbon to nitrogen source; C, temperature; D, initial pH value of medium. Arrows mean that the glucose
To ensure the stable quality of inoculants for subsequent fermentation, the effects of shaker speed, illumination, and initial inoculation concentration on the growth of P. malhamensis in the flasks were also studied. Poterioochromonas malhamensis grew at an extremely low rate under stationary culture conditions, whereas a shake significantly promoted its growth (P < 0.05) (Fig. S1-A). Poterioochromonas malhamensis grew much better when shaken at 180 rpm than at 90 rpm (P < 0.05), and exhibited a similar growth rate in dark or dim light (P > 0.05) (Fig. S1-B). The time for P. malhamensis to reach the maximum cell concentration decreased with an increase in inoculation concentration (Fig. S1-C). For P. malhamensis inoculated to give an initial concentration of 106 cells mL−1, the maximum cell concentration was obtained in 2 days, whereas the time for reaching maximum biomass was longer when the inoculation concentration decreased to 105 cells mL−1 or lower.
Optimization of culturing conditions for chemoheterotrophic Poterioochromonas malhamensis in the fermenter system
Based on the optimized culture conditions of P. malhamensis in shake flasks (i.e., 10 g L−1 glucose, C:N ratio of 5:1, temperature 28 °C, and pH 6.0), the effect of DO concentration on the growth of P. malhamensis was investigated in the fermenter system using a feeding medium to periodically replenish the nutrient (mainly glucose) supply. The glucose concentrations in different treatments were maintained in the range 0 to 10 g L−1 during the whole cultivation period, with the exception of one instance of 12 g L−1 at 40% DO (Fig. 3A). The cell concentration of P. malhamensis cultivated with 0% DO increased from 2.8 × 106 cells mL−1 to 6.6 × 107 cells mL−1 after a cultivation time of 72 h. However, the cell concentrations of P. malhamensis cultivated with 20 and 40% DO increased to 3.0 × 108 cells mL−1 and 3.6 × 108 cells mL−1, respectively (Fig. 3B). It is notable that the cell concentration of P. malhamensis after 72 h of cultivation stopped increasing in all three treatments, with a dramatic reduction in the 40% DO treatment.
Grazing ability of chemoheterotrophic Poterioochromonas malhamensis cells on unicellular Microcystis aeruginosa
The grazing ability of chemoheterotrophic P. malhamensis cells on unicellular M. aeruginosa was compared with those of autotrophic and phagotrophic P. malhamensis. Poterioochromonas malhamensis using different modes of nutrition varied greatly in cell morphology (Fig. 4). Chemoheterotrophic P. malhamensis had the largest cell size and had an obvious chrysolaminarin vacuole, but the chloroplast was inconspicuous. The cell size of autotrophic P. malhamensis was smallest, but its chloroplast was obvious and regular. The cell size of phagotrophic P. malhamensis fell between that of chemoheterotrophic and autotrophic P. malhamensis. The chloroplast in phagotrophic P. malhamensis cell was also obvious. After being grazed for 84 h, cell concentrations of M. aeruginosa co-cultured with P. malhamensis had decreased to extremely low levels (< 104 cells mL−1) for all three modes of nutrition, while the cell concentrations of M. aeruginosa in the control had increased to 1.5 × 107 cells mL−1 (Fig. 5A). However, chemoheterotrophic and phagotrophic P. malhamensis showed greater grazing ability than that of autotrophic P. malhamensis in the first 24 h (P < 0.05). Growth of P. malhamensis showed similar trends for all three modes of nutrition in the first 24 h, but the final cell concentration of chemoheterotrophic P. malhamensis (7 × 105 cells mL−1) was higher than that of phagotrophic and autotrophic P. malhamensis (both around 5 × 105 cells mL−1) (P < 0.05) (Fig. 5B).
Population dynamics of prey (A) and predator (B) when M. aeruginosa was grazed by P. malhamensis cultivated with different modes of nutrition: chemoheterotrophy, phagotrophy, and autotrophy. The curves represented by the cross ( ×) and circle (○) symbols in “A” are coincident. Error bars represent the standard deviation (n = 3)
Outdoor control of Microcystis using chemoheterotrophic Poterioochromonas malhamensis
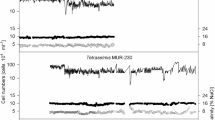
From a macro-observational perspective, the color of the Microcystis suspension in the experiment group (inoculated with P. malhamensis) turned from blue-green to slightly yellow–brown by the third day, while the water color of the Microcystis suspension in the control group maintained a blue-green color (Fig. 6). The chlorophyll-a concentration of the experiment group correspondingly decreased and reached 0.6 mg L−1 on the third day, while the chlorophyll-a concentration of the control group stayed at around the initial level of 1.1 mg L−1. According to our observations, a large proportion of the Microcystis cells sank to the bottom of the buckets in the experiment groups. Therefore, the chlorophyll-a concentration of the experiment group increased substantially after an additional agitation before sampling on the fourth day (Fig. 7A). The cell concentration of Microcystis in the experiment group was significantly lower than that in the control group after 3 days (P < 0.05) (Fig. 7B). The cell concentration of P. malhamensis was substantially unchanged in the first 4 days, but decreased to an extremely low value by the sixth day (Fig. 7B). Microscopic observation showed that P. malhamensis in the experiment group was incapable of grazing on colonial Microcystis and could only ingest a few unicellular Microcystis at any one time (Fig. S2-A & D). Furthermore, the cell size of P. malhamensis had decreased by the end of the experiment (Fig. S2-B & C). The DO concentration in the control group showed an increase in the first 2 days followed by a gradual decrease, while the DO concentration in the experiment group dramatically decreased within the first day and then remained stable after a short time of increase (Fig. 7C). The pH value of the experiment group fluctuated in the range 8.0–9.0, while the pH of the control group increased gradually to 10.8 (Fig. 7D).
Changes in chlorophyll-a concentration (A), cell concentrations of Microcystis and P. malhamensis (B), dissolved oxygen (C), and pH value (D) in the control group and the experiment group. In A and B, the samples on the first 3 days were collected from the suspensions without agitation, while the samples collected on days 4 and 6 were from evenly stirred suspensions
Discussion
Effects of nutrients and environmental factors on the chemoheterotrophic growth of Poterioochromonas malhamensis
The present study provides a promising fermentation technology for acquiring abundant P. malhamensis cells, and the maximum biomass achieved (more than 3 × 108 cells mL−1) in this study exceeds that reported in other studies (Lewitus & Caron, 1991; Rottberger et al., 2013) by more than an order of magnitude. In recent years, fermentation of microalgae has attracted increasing interest due to the ultrahigh cell densities produced, fast growth, and low occurrence of contaminants (Liu et al., 2014). Our results provide useful insights into the optimal culture conditions, in terms of nutrients and environmental factors, for high-cell-density fermentation of P. malhamensis.
Although other carbon sources (e.g., glycerol and ethanol) have been used to cultivate P. malhamensis (Lewitus & Caron, 1991), glucose is the most common carbon source in microalgal heterotrophic cultivation. The concentration of glucose and the strategy adopted for adding it to the culture have a great impact on the growth of microalgae. For example, the growth rate of the diatom Nitschia laevis has been found to decrease with an increase of glucose concentration from 1 to 40 g L−1 (Wen & Chen, 2000), which is consistent with the trends for P. malhamensis in this study. For Chlorella saccharophila, an optimum glucose concentration of 2.5 g L−1 has been reported, with growth inhibition occurring once the glucose concentration exceeds 25 g L−1 (Tan & Johns, 1991). To ensure a high growth rate of P. malhamensis in this study, the glucose concentration in the fermenter was maintained at a relatively low level (< 10 g L−1) by periodic addition of a feeding medium (Fig. 5). The same strategy has also been used in the fermentation of unicellular Scenedesmus acuminatus, yielding the highest levels of biomass (286 g L−1) achieved in microbial heterotrophic cultivation to date (Jin et al., 2020).
The type of nitrogen source and ratio of C:N are also critical nutritional factors affecting microalgal fermentation (Huang et al., 2010; Zheng et al., 2013). Yeast extract and liver powder are commonly used as nitrogen sources in the chemoheterotrophic cultivation of Poterioochromonas and its “sister genus” Ochromonas (Blom & Pernthaler, 2010). In fact, an inorganic nitrogen source (e.g., NH4Cl) could also be used to cultivate Poterioochromonas, but additional vitamin B1 and B12 would be required (data not shown). Based on our shake flask experiment, P. malhamensis grows faster at low ratios of C:N (i.e., 2:1 and 5:1) than at high ratios (i.e., 10:1, 20:1, and 30:1) (Fig. 2B). However, the ratio of C:N has no effect on the maximum biomass of P. malhamensis (P > 0.05). To reduce the cost of the nitrogen sources, a C:N ratio of 10:1 is therefore recommended for further large-scale fermentation of P. malhamensis.
Our results also provide useful comparative data on the growth of P. malhamensis utilizing different modes of nutrition. Heterotrophy in Poterioochromonas comprises phagotrophy (grazing on other particle organics/organisms) and chemoheterotrophy (utilizing dissolved organics). The effects of environmental factors (e.g., temperature, pH value, and light intensity) on the feeding behavior of Poterioochromonas have been well studied (Ma et al., 2018). On the whole, phagotrophic and chemoheterotrophic Poterioochromonas have similar requirements regarding optimal environmental conditions, including temperature, pH, and illumination. The optimal temperature and pH for Poterioochromonas grazing on microalgae have been found to be 25–30 °C and 5.0–6.0, respectively (Ma et al., 2018), which is consistent with the results of this study. Furthermore, illumination has been found to have little effect on the growth rate of Poterioochromonas feeding on other microalgae (Zhang & Watanabe, 2001), which is similar to our results regarding the chemoheterotrophic growth of P. malhamensis (Fig S1-B). These results could be explained by previous findings that photosynthesis only contributes ca. 7% of the total carbon budget of P. malhamensis in mixotrophic conditions (Sanders et al., 1990). However, it is noteworthy that previous studies have revealed that Poterioochromonas could not live for more than 4 days in continuous dark conditions and is presumed to be a light-dependent protist (i.e., some factor(s) supplied by photosynthesis in Poterioochromonas are required for heterotrophy) (Zhang & Watanabe, 2001; Zhang et al., 2009). In our study, we found that the cell concentration of P. malhamensis in dark conditions decreased dramatically with the depletion of added glucose (Fig. 2A–B and S1-C). However, during the long-term subculturing of P. malhamensis in continuous dark conditions, P. malhamensis grew well provided that abundant glucose was continuously supplied. This finding overturns the previous hypothesis.
In addition to needing sufficient glucose, the rapid growth and metabolism of P. malhamensis at high cell concentrations will also require a large amount of oxygen. Although mixotrophic protists (e.g., Poterioochromonas) can tolerate very low DO concentrations as their internal supply of pure oxygen through photosynthesis in chloroplasts (Fenchel, 2014), the photosynthetic capacity of Poterioochromonas decreases dramatically with increasing environmental organic concentration (Lewitus & Caron, 1991). Any increase in DO (either in flasks by shaking or in fermenters by addition of purified oxygen) would therefore be expected to enhance the biomass of P. malhamensis, and this is borne out by our results (Fig. 3 and S1-A).
Feasibility of controlling Microcystis blooms using Poterioochromonas malhamensis
Frequent outbreaks of cyanobacterial blooms have caused great damage to global biodiversity and the equilibrium of aquatic ecosystems (Zhang et al., 2009; Rigosi et al., 2014). In classical biological manipulation to control a cyanobacterial population, large zooplanktons (e.g., Daphnia) are presumed to be one of the main predators (Sarnelle, 1992; Boon et al., 1994). However, the practical application of large-sized zooplankton for biological control is difficult because of their selective grazing on high-food-quality eukaryotic algae (Ger et al., 2019), lower tolerance to toxic cyanobacteria (Lyu et al., 2019), and inability to degrade cyanotoxins (Shams et al., 2014). On the other hand, small-sized protists are generally considered the major predators of phytoplankton in natural plankton ecology (Sherr & Sherr, 2002), typically accounting for 60–70% of daily phytoplankton consumption (Calbet & Landry, 2004). Therefore, it is theoretically feasible to control Microcystis blooms using the mixotrophic flagellate P. malhamensis. Moreover, compared to other zooplankton, P. malhamensis has many other advantages for controlling Microcystis blooms, as well as the high-cell-density cultivation method developed in this study and its ability to degrade microcystins (Ou et al., 2005).
First, the grazing ability of P. malhamensis on unicellular Microcystis is comparatively strong. We found that the number of Microcystis cells that could be consumed by P. malhamensis in 24 h (107 cells mL−1) was 100 times the initial number of P. malhamensis cells (105 cells mL−1), and the cell concentration of P. malhamensis increased up to sevenfold during the same 24-h grazing phase (Fig. 5). Second, recent studies have shown that the chrysophytes Poterioochromonas and Ochromonas favor toxic Microcystis over chlorophytes, which should help the efficient recovery of chlorophytes upon a reduction in the Microcystis population either in the laboratory or in in situ microcosms (Zhang et al., 2009, 2018, 2020). Furthermore, Poterioochromonas is very adaptable to environmental conditions. In fact, Poterioochromonas can survive under conditions of temperature from 10 to 36.7 °C, light intensity from 0 to 1100 μmol photons m−2 s−1, and a pH from 2.5 to 10.0 (Ma et al., 2018). Under extreme conditions, such as low temperature and lack of nutrients, Poterioochromonas can form siliceous cysts (stomatocysts) and germinate again when the environmental conditions are suitable (Andersen et al., 2017). In this study, P. malhamensis was found to be capable of living in the aquatic environment with Microcystis blooms, but a rapid decrease of P. malhamensis cell concentration was observed on the sixth day, which might have been due to the lack of available prey (Fig. 7). So, in this respect, it seems unlikely that P. malhamensis would form another algal bloom after grazing on a large number of prey Microcystis cells. Last but not least, the addition of P. malhamensis appears to promote the sedimentation of Microcystis cells, which could reduce the dominance of Microcystis in surface waters, thereby facilitating the growth of other microalgal species. Considering that pH fluctuation is generally considered one of the vital reasons causing the flocculation of microalgal cells (Nguyen et al., 2019; Tan et al., 2020), this beneficial effect may be due to the addition of P. malhamensis being able to inhibit the increase in the pH value of Microcystis suspensions (Fig. 7).
However, we have to admit that there are still critical issues that remain to be resolved before successful control of Microcystis blooms using P. malhamensis can be achieved. First and foremost, P. malhamensis can only graze on microalgal prey with a cell size less than 10 μm (Ma et al., 2018), while Microcystis in the field is mostly colonial with a colony size larger than 100 μm (Xiao et al., 2018). Therefore, P. malhamensis has been presumed to be incapable of grazing on colonial Microcystis (Van Wichelen et al., 2016), which was also verified by our observations (Fig. S2). To solve this problem, the key point is to effectively disaggregate the colonial Microcystis into unicellular or small-sized colonies using physical methods such as high-turbulent mixing and ultrasonic radiation (Ahn et al., 2003; Li et al., 2018; Wang et al., 2021a), chemical methods such as chlorination (He & Wert, 2016), or biological methods such as bacterial degradation (Wang et al., 2015). These colony disaggregation technologies should allow P. malhamensis to achieve its greatest potential in controlling Microcystis blooms. Furthermore, the timing of P. malhamensis addition might also influence how well it can control Microcystis growth. Poterioochromonas malhamensis should best be able to inhibit Microcystis growth during the early stage of a Microcystis bloom when Microcystis is dominated by unicellular or small-sized colonies (Liu et al., 2017). Finally, the ecological security of releasing cultured P. malhamensis into aquatic ecosystems should be carefully evaluated. Previous studies (Boxhorn et al., 1998; Boenigk & Stadler, 2004; Zhang et al., 2011) have revealed that many large-sized zooplankton (e.g., Daphnia magna and Brachionus angularis) would die after feeding on P. malhamensis, which shows that P. malhamensis might be harmful for certain higher trophic zooplankton. Consequently, more field experiments should be carried out to explore the effect of P. malhamensis on the community structures of zooplankton and phytoplankton. The high-cell-density cultivation method of P. malhamensis established in this study will, at the very least, offer the opportunity to research these issues and to investigate further the potential for controlling Microcystis blooms using predatory P. malhamensis.
Conclusions
A high-cell-density chemoheterotrophic method of cultivating P. malhamensis was established. Under the optimized conditions of 10 g L−1 glucose, a C:N ratio of 5:1, temperature of 28 °C, pH of 6.0, and 20% DO, the cell concentration of P. malhamensis reached more than 3 × 108 cells mL−1, which exceeds that reported in other studies by more than an order of magnitude. Furthermore, the chemoheterotrophic P. malhamensis proved to be effective in grazing unicellular Microcystis cells and decreasing the Microcystis biomass on the surface of the water by promoting the sedimentation of colonial Microcystis cells. This study will play an important role in driving the development of methods to control Microcystis blooms using predatory P. malhamensis.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahn CY, Park MH, Joung SH, Kim HS, Jang KY, Oh HM (2003) Growth inhibition of cyanobacteria by ultrasonic radiation: laboratory and enclosure studies. Environ Sci Technol 37:3031–3037
Andersen RA, Graf L, Malakhov Y, Yoon HS (2017) Rediscovery of the Ochromonas type species Ochromonas triangulata (Chrysophyceae) from its type locality (Lake Veysove, Donetsk region, Ukraine). Phycologia 56:591–604
Blom JF, Pernthaler J (2010) Antibiotic effects of three strains of chrysophytes (Ochromonas, Poterioochromonas) on freshwater bacterial isolates. FEMS Microbiol Ecol 71:281–290
Boenigk J, Stadler P (2004) Potential toxicity of chrysophytes affiliated with Poterioochromonas and related ‘Spumella-like’flagellates. J Plankton Res 26:1507–1514
Boon PI, Bunn SE, Green JD, Shiel RJ (1994) Consumption of cyanobacteria by freshwater zooplankton: implications for the success of ‘top-down’ control of cyanobacterial blooms in Australia. Mar Freshwater Res 45:875–887
Boxhorn JE, Holen DA, Boraas ME (1998) Toxicity of the chrysophyte flagellate Poterioochromonas malhamensis to the rotifer Brachionus angularis. Hydrobiologia 387:283–287
Calbet A, Landry MR (2004) Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol Oceanogr 49:51–57
Chen JL, Proteau PJ, Roberts MA, Gerwick WH, Slate DL, Lee RH (1994) Structure of malhamensilipin A, an inhibitor of protein tyrosine kinase, from the cultured chrysophyte Poterioochromonas malhamensis. J Nat Prod 57:524–527
Corno G, Jurgens K (2006) Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity. Appl Environ Microbiol 72:78–86
Destain J, Haubruge É, Thonart P, Portetelle D, Francis F, Bauwens J, Tarayre C, Brasseur C, Mattéotti C, Vandenbol M (2014) Isolation of an amylolytic chrysophyte, Poterioochromonas sp., from the digestive tract of the termite Reticulitermes santonensis. Biotechnol Agron Soc Environ 18:19–31
Fenchel T (2014) Protozoa and oxygen. Acta Protozool 53:3–12
Ger KA, Naus-Wiezer S, De Meester L, Lürling M (2019) Zooplankton grazing selectivity regulates herbivory and dominance of toxic phytoplankton over multiple prey generations. Limnol Oceanogr 64:1214–1227
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
He X, Wert EC (2016) Colonial cell disaggregation and intracellular microcystin release following chlorination of naturally occurring Microcystis. Water Res 101:10–16
He Y, Ma M, Hu Q, Gong Y (2021) Assessment of NH4HCO3 for the control of the predator flagellate Poterioochromonas malhamensis in pilot-scale culture of Chlorella sorokiniana. Algal Res 60:102481
Holen DA, Boraas ME (1995) Mixotrophy in chrysophytes. In: Sandgren CD, Smol JP, Kristiansen J (eds) Chrysophyte algae: ecology, phylogeny and development. Cambridge University Press, Cambridge, pp 119–140
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Humphries S, Widjaja F (1979) A simple method for separating cells of Microcystis aeruginosa for counting. Br Phycol J 14:313–316
Jin H, Zhang H, Zhou Z, Li K, Hou G, Xu Q, Chuai W, Zhang C, Han D, Hu Q (2020) Ultrahigh-cell-density heterotrophic cultivation of the unicellular green microalga Scenedesmus acuminatus and application of the cells to photoautotrophic culture enhance biomass and lipid production. Biotechnol Bioeng 117:96–108
Kauss H, Kriebitzsch C (1969) Demonstration and partial purification of A β-(1→3)-glucan phosphorylase. Biochem Biophys Res Comm 35:926–930
Kim BR, Han MS (2007) Growth and grazing of the mixotrophic flagellate Poterioochromonas malhamensis on the cyanobacterium Microcystis aeruginosa. Kor J Nat Conserv 5:183–194
Leakey R, Burkill P, Sleigh M (1994) A comparison of fixatives for the estimation of abundance and biovolume of marine planktonic ciliate populations. J Plankton Res 16:375–389
Lewitus AJ, Caron DA (1991) Physiological responses of phytoflagellates to dissolved organic substrate additions. 1. Dominant role of heterotrophic nutrition in Poterioochromonas malhamensis (Chrysophyceae). Plant Cell Physiol 32:671–680
Li M, Xiao M, Zhang P, Hamilton DP (2018) Morphospecies-dependent disaggregation of colonies of the cyanobacterium Microcystis under high turbulent mixing. Water Res 141:340–348
Lin Z, Raya A, Ju LK (2014) Microalga Ochromonas danica fermentation and lipid production from waste organics such as ketchup. Process Biochem 49:1383–1392
Liu J, Sun Z, Chen F (2014) Heterotrophic production of algal oils. In: Pandey A, Lee D-J, Chisti Y, Soccol CR (eds) Biofuels from algae. Elsevier, Amsterdam, pp 111–142
Liu M, Shi X, Chen C, Yu L, Sun C (2017) Responses of Microcystis colonies of different sizes to hydrogen peroxide stress. Toxins 9:306
Lyu K, Gu L, Wang H, Zhu X, Zhang L, Sun Y, Huang Y, Yang Z (2019) Transcriptomic analysis dissects the mechanistic insight into the Daphnia clonal variation in tolerance to toxic Microcystis. Limnol Oceanogr 64:272–283
Ma M, Gong Y, Hu Q (2018) Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res 29:142–153
Ma M, Yuan D, He Y, Park M, Gong Y, Hu Q (2017) Effective control of Poterioochromonas malhamensis in pilot-scale culture of Chlorella sorokiniana GT-1 by maintaining CO2- mediated low culture pH. Algal Res 26:436–444
Nguyen TDP, Tran TNT, Le TVA, Phan TXN, Show PL, Chia SR (2019) Auto-flocculation through cultivation of Chlorella vulgaris in seafood wastewater discharge: influence of culture conditions on microalgae growth and nutrient removal. J Biosci Bioeng 127:492–498
Ou D, Song L, Gan N, Chen W (2005) Effects of microcystins on and toxin degradation by Poterioochromonas sp. Environ Toxicol 20:373–380
Rigosi A, Carey CC, Ibelings BW, Brookes JD (2014) The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol Oceanogr 59:99–114
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61
Roderer G (1986) Poterioochromonas malhamensis-a unicellular alga as test system in ccotoxicology, toxicology, and pharmacology. Environ Toxicol 1:123–138
Rottberger J, Gruber A, Boenigk J, Kroth PG (2013) Influence of nutrients and light on autotrophic, mixotrophic and heterotrophic freshwater chrysophytes. Aquat Microb Ecol 71:179–191
Sanders RW, Porter KG, Caron DA (1990) Relationship between phototrophy and phagotrophy in the mixotrophic chrysophyte Poterioochromonas malhamensis. Microb Ecol 19:97–109
Sarnelle O (1992) Nutrient enrichment and grazer effects on phytoplankton in lakes. Ecology 73:551–560
Shams S, Cerasino L, Salmaso N, Dietrich DR (2014) Experimental models of microcystin accumulation in Daphnia magna grazing on Planktothrix rubescens: implications for water management. Aquat Toxicol 148:9–15
Sherr EB, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 81:293–308
Tan CK, Johns MR (1991) Fatty acid production by heterotrophic Chlorella saccharophila. Hydrobiologia 215:13–19
Tan X, Duan Z, Duan P, Parajuli K, Newman J, Shu X, Zhang D, Gao L, Li M (2020) Flocculation of Microcystis unicells induced by pH regulation: mechanism and potential application. Chemosphere 263:127708
Toda N, Murakami H, Kanbara A, Kuroda A, Hirota R (2021) Phosphite reduces the predation impact of Poterioochromonas malhamensis on cyanobacterial culture. Plants 10:1361
Touloupakis E, Cicchi B, Benavides AM, Torzillo G (2016) Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl Microbiol Biotechnol 100:1333–1341
Van Wichelen J, Vanormelingen P, Codd GA, Vyverman W (2016) The common bloom-forming cyanobacterium Microcystis is prone to a wide array of microbial antagonists. Harmful Algae 55:97–111
Wang H, Tao Y, Li Y, Wu S, Li D, Liu X, Han Y, Manickam S, Show PL (2021a) Application of ultrasonication at different microbial growth stages during apple juice fermentation by Lactobacillus plantarum: investigation on the metabolic response. Ultrason Sonochem 73:105486
Wang W, Zhang Y, Shen H, Xie P, Yu J (2015) Changes in the bacterial community and extracellular compounds associated with the disaggregation of Microcystis colonies. Biochem Syst Ecol 61:62–66
Wang X, Li H, Zhan X, Ma M, Yuan D, Hu Q, Gong Y (2021b) Development and application of quantitative real-time PCR based on the mitochondrial cytochrome oxidase subunit I gene for early detection of the grazer Poterioochromonas malhamensis contaminating Chlorella culture. Algal Res 53:102133
Wang Y, Gong Y, Dai L, Sommerfeld M, Zhang C, Hu Q (2018) Identification of harmful protozoa in outdoor cultivation of Chlorella and the use of ultrasonication to control contamination. Algal Res 31:298–310
Wen Z, Chen F (2000) Heterotrophic production of eicosapentaenoid acid by the diatom Nitzschia laevis: effects of silicate and glucose. J Ind Microbiol Biotechnol 25:218–224
Xiao M, Li M, Reynolds CS (2018) Colony formation in the cyanobacterium Microcystis. Biol Rev 93:1399–1420
Zeković DB, Kwiatkowski S, Vrvić MM, Jakovljević D, Moran CA (2005) Natural and modified (1→ 3)-β-D-glucans in health promotion and disease alleviation. Crit Rev Biotechnol 25:205–230
Zhang L, Gu L, Hou X, Kong Q, Chen K, Zhu X, Huang Y, Chen Y, Yang Z (2018) Chlorophytes prolong mixotrophic Ochromonas eliminating Microcystis: temperature-dependent effect. Sci Total Environ 639:705–713
Zhang L, Wang Z, Wang N, Gu L, Sun Y, Huang Y, Chen Y, Yang Z (2020) Mixotrophic Ochromonas addition improves the harmful Microcystis-dominated phytoplankton community in in situ microcosms. Environ Sci Technol 54:4609–4620
Zhang X, Hu HY, Men YJ, Yang J, Christoffersen K (2009) Feeding characteristics of a golden alga (Poterioochromonas sp.) grazing on toxic cyanobacterium Microcystis aeruginosa. Water Res 43:2953–2960
Zhang X, Hu HY, Hong Y, Yang J (2008) Isolation of a Poterioochromonas capable of feeding on Microcystis aeruginosa and degrading microcystin-LR. FEMS Microbiol Lett 288:241–246
Zhang X, Hu HY, Warming TP, Christoffersen KS (2011) Life history response of Daphnia magna to a mixotrophic golden alga, Poterioochromonas sp., at different food levels. Bull Environ Contam Toxicol 87:117–123
Zhang X, Watanabe MM (2001) Grazing and growth of the mixotrophic chrysomonad Poterioochromonas malhamensis feeding on algae. J Phycol 37:738–743
Zhang X, Watanabe MM (1996) Light and electron microscopy of grazing by Poterioochromonas malhamensis (Chrysophyceae) on a range of phytoplankton taxa. J Phycol 32:37–46
Zheng Y, Li T, Yu X, Bates PD, Dong T, Chen S (2013) High-density fed-batch culture of a thermotolerant microalga Chlorella sorokiniana for biofuel production. Appl Energy 108:281–287
Acknowledgements
Thanks are due to Dr. Binliang Wang and Qingyang Song for helping with the outdoor control experiment. The authors also thank the National Aquatic Biological Resource Center (NABRC) at the Institute of Hydrobiology, Chinese Academy of Sciences, for providing support.
Funding
This work was funded by the National Key Research and Development Program of China (No. 2019YFD0900302), the National Natural Science Foundation of China (No. 31772419, No. 31872201, and No. 32002413), the National Key Research and Development Project (No. 2017YFE0125700), the China Postdoctoral Science Foundation (No. 2019M662749), and the Agricultural Science and Technology Innovation Action Project of Hubei Province of China (2018).
Author information
Authors and Affiliations
Contributions
Mingyang Ma and Fuchen Wang performed the experiments, analyzed the data, and wrote the paper. Chaojun Wei and Hongxia Wang participated in the outdoor experiment. Jianping Chen and Hu Jin participated in the fermentation of P. malhamensis in the 7.5-L bioreactors. Lirong Song provided algal cultures and offered crucial suggestions on the analysis of the results. Qiang Hu and Yingchun Gong contributed to the design of the experiments, the drafting of the paper, and revising it critically. All authors gave approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, M., Wang, F., Wei, C. et al. Establishment of high-cell-density heterotrophic cultivation of Poterioochromonas malhamensis contributes to achieving biological control of Microcystis. J Appl Phycol 34, 423–434 (2022). https://doi.org/10.1007/s10811-021-02659-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02659-x