Abstract
The aim of the present investigation was to study the impact of foliar spraying with Chlorella vulgaris, Arthrospira platensis, and Tetradesmus dimorphus suspensions as biostimulants on growth and yield characteristics of common beans such as Phaseolus vulgaris. Seven treatments were tested during the study: T1 (soil amended with N-urea but no microalgae foliar application), T2 (foliar spraying with C. vulgaris but no N-urea added to the soil), T3 (foliar spraying with T. dimorphus but no N-urea added to the soil), T4 (foliar spraying with A. platensis but no N-urea added to the soil), T5 (foliar spraying with C. vulgaris and soil amended with N-urea), T6 (foliar spraying with T. dimorphus and soil amended with N-urea), T7 (foliar spraying with A. platensis and soil amended with N-urea) and control (untreated). Foliar spraying was applied after 7, 25, and 77 days from sowing using the test microalgae suspensions in concentration of 10 g 100 mL−1. Plant growth and biochemical parameters were measured at the end of both vegetative and fruiting growth stages. Compared with control, the treatments from T1 to T7 showed noticeable increase in all growth parameters and yield attribute. The foliar application with C. vulgaris and chemical fertilizer treated plants (T5) exhibited the maximum increase in total plant height (26.9%), dry weight (37.28%), protein content (48.06 ± 2.403 mg g−1 fresh wt.), and total carbohydrate (394 ± 19.7 mg g−1 dry wt.) during vegetative stage as well as number of pods per plant (5.2 ± 0.26), number of seed/pod (3.5 ± 0.18), pods dry weight (0.95 ± 0.05 g plant−1) during fruiting stage. Thus, it is advisable to use C. vulgaris as a biostimulant for enhancing P. vulgaris growth and crop production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Common beans are among the world’s largest cultivated crops used for direct human consumption. About 20 leguminous species are consumed as dry grains in substantial amounts, considered poor man’s meat in third world countries where protein energy malnutrition considered major nutrition problem (Lin et al. 2008; Priya and Manickavasagan 2020). Among the most consumed legumes in the world, Phaseolus vulgaris occupies an important position in human nutrition, with a commercial value exceeding that of all other bean crops (Porch et al. 2013). Despite being low in methionine and cysteine, P. vulgaris dried seeds, or “pulses.” are a major source of nutritional protein which is 2–3 times that of cereal grains (Siddiq et al., 2010). It also contains high amounts of starch, dietary fiber, minerals, vitamins, and variety of phytochemicals such as anthocyanins, flavonoids, proanthocyanidins, phenolic acids, and isoflavones that play important roles in the prevention of cardiovascular disease, obesity, diabetes mellitus, and cancer (Díaz-Batalla et al. 2006; Lin et al. 2008).
Despite their economic and nutritional importance, common beans are low yield crops that cannot meet food demands of growing populations. In recent years, numerous studies have been carried out to develop substances of biological origin as alternatives to chemical inputs and able to enhance plant growth, crop yields, and quality with less environmental damage (Mourice and Tryphone 2012; Dias et al. 2016). Biofertilizers and biostimulants are alternatives to chemical fertilizers that, when applied at low concentrations to soil, seeds, or crops, regulate the physiology of plants through different pathways such as enhancing crop growth, increasing nutrient uptake, resistance to abiotic stresses, and longer the shelf life of harvested products (Kawalekar 2013). Moreover, they are eco-friendly products that contain living microorganisms such as bacteria, actinomycetes, fungi, or algae alone or in combination.
In this regard, microalgal biomass is rich in different biomolecules that are essential for an optimal crop growth and development such as free amino acids, organic acids, and phytohormones. It is widely known (Shaaban 2001a, b; Tarakhovskaya et al. 2007; Khan et al. 2009; Battacharyya et al. 2015; Mógor et al. 2018) that different microalgae species are rich in growth-promoting substances such as auxins, cytokinins, gibberellins that are essential for plant growth and sustainably.
Several studies have investigated the effect of microalgae fertilizers on different crops. For example, foliar spraying with Arthrospira platensis and Scenedesmus sp. hydrolysates increased the flowers number, the root dry weight, number of leaves, and shoots of Petunia x hybrida (Plaza et al. 2018). Similarly, Mógor et al. (2018) observed cytokine-like effects of A. platensis hydrolysate on lettuce seedlings and Bumandalai and Tserennadmid (2019) found a stimulatory effect of foliar spraying with Chlorella vulgaris suspension on tomato and cucumber seeds germination. Other studies have investigated the impact of C. vulgaris and A. platensis fertilizers on rice and maize plants and they found an increase in seed quality, seed germination, yield production, and plant growth parameters (Dineshkumar et al. 2018, 2019).
Based on the above information, the present study aimed primarily at investigating the effect of foliar application with C. vulgaris, Tetradesmus dimorphus, and A. platensis suspensions on different vegetative growth and yield aspects of P. vulgaris plants.
Material and methods
Algal culture
Freshwater samples were collected from the River Nile system at Delta region in the front of Mansoura University (31° 2′ 45.7620″ N, 31° 21′ 18.6444″ E) (Raschke and Schultz 1987) and centrifuged at 2688 × g for 10 min then supernatant was discarded and the pellets were picked up by sterile needle and streaked on Bold Basal Medium (BBM) (Bischoff 1963) solidified with 1.5% (w/v) of bacteriological agar for purification. The plates were incubated for 2 weeks at 25 ± 2 °C under continuous light of 45 μmol photons m−2 s−1 and then examined. Unialgal colonies were transferred to liquid BBM and left to grow to obtain enough biomass for identification (Stein et al. 1973).
Three species were isolated and identified according to Komárek et al. (1983) and Anagnostidis and Komárek (1988): Chlorella vulgaris Beijerinck, Tetradesmus dimorphus (Turpin) M.J.Wynne, and Arthrospira platensis Gomont.
Modified Navicula medium (Starr 1978) was used to grow C. vulgaris and T. dimorphus and Zarrouk’s medium (Zarrouk 1966) was used for A. platensis growth. Cultures were incubated under continuous illumination of 45 μmol photons m−2 s−1 at 25 ± 2 °C, pH = 7 for C. vulgaris and T. dimorphus, and at 35 ± 2 °C, pH = 9 for A. platensis.
Preparation of algal suspension
Samples of each of the test microalgae were harvested at the end of growth stationary phase by centrifugation then pellets were collected, spread as a thin layer on surface of glass plates, and left to dry under mild air current. The air-dried biomass was then collected, put in a porcelain crucible, and left to dry at 50 °C until constant weight. A weight of 10 g dry weight biomass dried at 50 °C was suspended in 100 mL distilled water for foliar spray of the tested potted plants.
Phaseolus vulgaris experimental cultivation
The experiment was carried out in the green house of Botany Department Faculty of Science Mansoura University. The experiment was conducted for 85 days. Seeds of Phaseolus vulgaris were selected then surface sterilized by soaking in 0.01% HgCl2 solution for 3 min. The seeds were washed thoroughly with tap water, divided into eight equal groups in 10 replicates; each pot contains 15 seeds. All sets of seeds were sown in similar earthenware pots (30 cm diameter and 25 cm depth), filled with clay-sand soil (2:1 w/w). Before planting some physical and chemical analysis was carried out on the used soil according to Black (1965): moisture; 1.9%, pH; 8.44, maximum water capacity (MWC); 60%, CaCO3; 2%, electric conductivity; 2.15 ds m−1, total salts; 19.5%, total nitrogen; 44.9%, sand; 22.13%, clay; 60.09%, silt; 19.79%, soil texture class; clay.
The pots were kept in the greenhouse under a normal day/night condition. According to the announcement of Ministry of Agriculture of Egypt, the following was done: (a) super phosphate fertilizer (0.5 g per pot) was added to the soil before sowing, (b) no Rhizobia were added to the soil, (c) for urea-treated plant, N-urea as nitrogen fertilizer (1 g per pot) was added to the soil during the first week of cultivation. After 7 days from sowing thinning took place with 5 uniform seedlings left to grow in each pot.
Plant foliar application was carried out three times using one-hand pressure sprayer containing different microalgae suspensions separately, in concentration of 10 g (100 mL)−1; the first spray was done after the first week of sowing with 50 mL, the second after 25 days with 100 mL, and the third after 77 days from sowing with 150 mL of each microalgae suspension (at vegetative, flowering, and fruiting stages, respectively). Spraying was done in the early morning when the stomata were open, allowing for better foliar penetration. Throughout the experiment, all plants were watered every 72 h, except after foliar application, when they were not watered for 24 h.
The experiment design was as follows:
-
Control (untreated): no addition of N-urea and no foliar spraying with microalgae.
-
T1: soil amended with N-urea but no foliar spraying with microalgae.
-
T2: foliar spraying with C. vulgaris but soil without N-urea addition.
-
T3: foliar spraying with T. dimorphus but soil without N-urea addition.
-
T4: foliar spraying with A. platensis but soil without N-urea addition.
-
T5: foliar spraying with C. vulgaris and soil amended with N-urea.
-
T6: foliar spraying with T. dimorphus and soil amended with N-urea.
-
T7: foliar spraying with A. platensis and soil amended with N-urea.
Analyses of algae biomass
Total protein content was determined by the method of Bradford (1976) as modified by Stoscheck (1990), total carbohydrate content was determined according to Hedge et al. (1962), and total lipid was determined by the method of Sadasivam and Manickam (1996). Fresh frozen samples of the tested microalgal biomass, after extraction according to Shindy and Smith (1975), were sent to the Arid Land Agricultural Research and Services Center Faculty of Agriculture Ain Shams University, for phytohormone analysis (auxins; IAA, gibberellins; GA3, cytokinin; CK and abscisic acid; ABA).
Phaseolus vulgaris growth parameters and yield attribute
Shoot length, number of leaves per plant, shoot fresh weight, shoot dry weight per plant, root length, number of nodules, root fresh weight, and root dry weight of P. vulgaris were recorded at the vegetative stage (14 days from sowing). At the time of harvesting (fruiting stage), number of pods per plant, number of seeds, fresh weight of pods per plant, and dry weight of pods per plant were recorded in addition to shoot length, shoot fresh weight, shoot dry weight per plant, root length, root fresh weight, and root dry weight of P. vulgaris plant (84 days from sowing).
Biochemical parameters such as chlorophyll a, b were measured at 14 days from sowing according to the method described by Arnon (1949), and carotenoids were measured according to Horvath et al. (1972) as modified by Kissimon (1999). Protein content was determined according to Bradford (1976), and total soluble sugars, polysaccharides, and total carbohydrates determined according to Yemm and Willis (1954) and van Handel (1968) at 14 days and 84 days from sowing.
Statistical analysis
Means of data obtained were analyzed by least significant difference (L.S.D) test at probability of 0.05. ANOVA analysis was done with Costat (CoHort software, USA).
Results
Biochemical composition of the test microalgae
Weight % of total lipid, total protein, and total carbohydrate of the microalgae are shown in Fig. 1. The highest percent of total lipid was recorded for Chlorella vulgaris (10.8 ± 0.54%) and the lowest was recorded for Tetradesmus dimorphus (3.8 ± 0.19%). Arthrospira platensis had relatively the highest total protein content (57.55 ± 2.87%) compared with C. vulgaris and T. dimorphus. Tetradesmus dimorphus had the highest percentage total carbohydrate content (29.6 ± 1.48%) compared to C. vulgaris and A. platensis.
Phytohormone content was assessed at the end of the stationary phase of growth of each microalgae species. Figure 2 shows that phytohormone content is species dependent. For instance, T. dimorphus followed by C. vulgaris had the highest content of cytokinins (19.99 ± 0.87 µg (100 mL)−1), auxins (IAA) (58.08 ± 2.7 µg (100 mL)−1), and abscisic acid (ABA) (3.79 ± 0.18 µg (100 mL)−1), while A. platensis had the highest content of gibberellins (GA3) (130.05 ± 6.50 µg (100 mL)−1).
Effect of microalgae treatments on the fresh and dry weight, length, and number of leaves of Phaseolus vulgaris plant during vegetative and fruiting stage growth
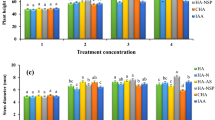
The changes in the estimated growth parameters (shoot length, number of leaves, shoot fresh weight, shoot dry weight, root length, root fresh weight, root dry weight) of P. vulgaris plant in response to different treatments during vegetative and fruiting stages are displayed in Tables 1 and 2. Compared with the control, a noticeable significant increase in all growth parameters were observed in the case of T2 (foliar spraying with C. vulgaris but soil without N-urea addition), T4 (foliar spraying with A. platensis but soil without N-urea addition), T5 (foliar spraying with C. vulgaris and soil amended with N-urea), T6 (foliar spraying with T. dimorphus and soil amended with N-urea), and T7 (foliar spraying with A. platensis and soil amended with N-urea) at the vegetative stage, except for T4 which showed a non-significant increase in shoot dry weight and root length. T1 (soil amended with N-urea but no foliar spraying with microalgae) and T3 (foliar spraying with T. dimorphus but soil without N-urea addition) showed non-significant increase in all parameters except shoot length which increased significantly. During fruiting stage, all parameters increased significantly except T1 in shoot fresh and dry weight, T3 in shoot and root fresh weight and root length, and T4 in shoot fresh weight. The foliar application of C. vulgaris with chemical fertilizer treated plants (T5) caused the maximum increase in shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, shoot length, and root length during both vegetative and fruiting stages (Tables 1 and 2). In addition to the previous growth parameters, number of leaves was highest in the T5 treatment (4.3 ± 1.1) during the vegetative stage (Table 1).
Effect of microalgae treatments on biochemical composition of Phaseolus vulgaris shoot during vegetative growth
Figure 3 illustrates the levels of total protein, total soluble sugar, polysaccharides, and total carbohydrates of P. vulgaris shoot during vegetative growth. There was significant increase in all parameters of P. vulgaris leaves in response to treatments (T1 to T7) compared to the control during early-stages P. vulgaris growth. T5 had the highest protein, total soluble sugar, polysaccharide, and total carbohydrate content during the vegetative stage.
Figure 4 shows a significant increase in chlorophyll a, chlorophyll b, and carotenoids content in the case of treatments T2 to T7 compared to the control. T1 treatment showed a non-significant increase in chlorophyll a and chlorophyll b but a significant increase in carotenoids. The highest value in chlorophyll a, chlorophyll b, and carotenoids content was recorded in the treatment with C. vulgaris and chemical fertilizer (T5).
Seed yield characteristics and biochemical composition of Phaseolus vulgaris after harvest
The yield attributes of P. vulgaris are given in Table 3. The seed yield characters including number of pods, number of seeds, and pods fresh and dry weight increased significantly with treatments T2, T5, T6, T7 compared to control. T3 showed non-significant increase in number of seeds and pods fresh weight, and T4 showed non-significant increase in pods fresh weight. T1 showed significant increase in number of pods and non-significant increase in number of seeds and pods fresh and dry weight. The maximum of these parameters observed in the T5 treated plants.
Figure 5 shows the levels of protein, total soluble sugar, polysaccharides, and total carbohydrates of P. vulgaris seeds during fruiting growth. There was significant increase in the biochemical parameters of P. vulgaris seeds in the case of treatments T1 to T7 compared to the control during late stage of Phaseolus vulgaris growth except T1 in total soluble sugar which increase non-significantly. T5 exhibited the highest protein content (159.25 ± 7.962 mg g−1 fresh wt.), total soluble sugar (128.65 ± 6.43 mg g−1 dry wt.), polysaccharide (379.58 ± 18.979 mg g−1 dry wt.), and total carbohydrate (508.23 ± 25.41 mg g−1 dry wt.) during fruiting stage.
Discussion
The use of microalgae biostimulants has become a global approach for obtaining environmentally friendly, high-yield crops with good quality that are safe for human. It is evident from the results obtained (Figs. 1 and 2) that C. vulgaris, T. dimorphus, and A. platensis biomass are rich in protein, carbohydrate, lipid, and phytohormones. In this context, identifying and selecting microalgae which are high in primary metabolites (carbohydrates, proteins, and lipids) and natural phytohormones particularly auxins and cytokinins is considered a key factor for plant biostimulants to enhance plant growth and productivity (Du Jardin 2015; Chiaiese et al. 2018; Ronga et al. 2019). Thus, the potential of C. vulgaris, T. dimorphus, and A. platensis as plant biostimulants on the vegetative growth and yield production of P. vulgaris was investigated in the present study.
Several studies have revealed foliar spray application as an effective technique alternative to soil application in fertilization for satisfying plant with nutrients sprays required for achieving high yield crops through leaf surface where they can penetrates easier and faster via cuticular cracks, stomata, trichomes, or lenticles reaching target cells where nutrients are required (Fernández et al. 2013; Battacharyya et al. 2015; Dias et al. 2016; Plaza et al. 2018).
In the present study (Tables 1 and 2), foliar application treatments with C. vulgaris, T. dimorphus, and A. platensis suspensions significantly enhanced the plant height, fresh weight, dry weight, and number of leaves at both vegetative growth and fruiting stage of P. vulgaris compared with the control. These obvious positive changes in both vegetative growth and yield of P. vulgaris can be outcome to the effect of protein, carbohydrate, lipid, and phytohormones of the test microalgae biomass singly or in combination. It has become evident that the effects of mixed ingredients are the sum synergetic, antagonistic, and/or addition interactions including obvious stimulation in growth and yield of P. vulgaris.
Additionally, the phytohormones present in C. vulgaris, T. dimorphus, and A. platensis biomass particularly auxins, cytokinins, and gibberellins are contributing to several plant growth and development aspects. It has been reported (Plaza et al. 2018; Bayona-Morcillo et al. 2020; Chookalaii et al. 2020) that both auxins and cytokinins play a prominent role in cell division, cell elongation, and root and shoot development. Gibberellins regulate seed germination, stem elongation, leaf expansion, early flowering, seed development, and inhibition of seed dormancy.
Our results are similar to those obtained by A. platensis foliar spraying of red beat (Ronga et al. 2019), tomato and pepper (Elarroussia et al. 2016), aubergine (Solanum melongena) (Dias et al. 2016), lettuce (Mogor et al. 2018), and Petunia x hybrida (Plaza et al. 2018) as well as C. vulgaris foliar application of grapes (Nagy and Pintér 2015), tomato and cucumber (Bumandalai and Tserennadmid 2019), and rice and maize (Dineshkumar et al. 2018, 2019).
The considerable significant increase in root and shoot fresh weight and dry weight of P. vulgaris in response to the application of C. vulgaris and chemical fertilizer (T5) among other treatments during both vegetative and fruiting stages could be due to the combined effect of both biostimulants along with chemical fertilizer in increasing nutrients use efficiency of P. vulgaris for macro and micronutrients added by the test microalgae that are essential for growth and development (Osman et al. 2010; Garcia-Gonzalez and Sommerfeld 2016; Barone et al. 2019).
The significant increase in total pigment content of P. vulgaris leaves during vegetative growth (Fig. 4) with the C. vulgaris and chemical fertilizer (T5) treatment compared with other treatments may contribute to the growth regulators added by C. vulgaris, particularly cytokinins, which play a crucial role in increasing the leaf chlorophyll content and consequently decreasing senescence or it may be due to the increase the number of leaves and/or the changes in the pigment biosynthesis in response to the used treatment. These results are in accordance with other findings which shown increase in chlorophyll content of grapevine and strawberry plants treated with algae (Dineshkumar et al. 2019; Puglisi et al. 2020).
Additionally, the treatment with C. vulgaris suspension and chemical fertilizer (T5) induced the highest and most significant increase in total protein and total carbohydrate contents of P. vulgaris leaves during vegetative growth (Fig. 3) and seeds during fruiting stage (Fig. 5). These results are similar to those obtained by Puglisi et al. (2020). The high protein content in P. vulgaris plant may be due to the increase in nitrogen uptake by P. vulgaris roots which is then translocated to shoot and other plant parts. It has been reported (Gorelova 2006) that microalgae can play an important role in symbiosis with other organisms, including higher plants as well as microalgal extracts, when applied as foliar spray, showed an increased N-content in root and shoot tissues (Kim et al. 2018). Moreover, the enhancement of pigments production is reflected on carbohydrate synthesis, whereas the increase in carbohydrate contents may contribute to the increase in photosynthesis rate of P. vulgaris.
The overall increase in P. vulgaris yields attribute in response to combined application of biostimulant (C. vulgaris) and chemical fertilizer (T5) may be largely attributed to an adequate and balanced provision of nutrients to the plant throughout the growth period from both the microalgal biomass and the chemical fertilizer resulting in the maximum number of pods per plant, seeds per pods, and pods weight. Our findings confirmed the observations of Osman et al. (2010) who noted an increased yield of pea plants using a combined dose of nitrogen fertilizer and microalgae, as well as Dineshkumar et al. (2018) who found higher yield in rice growth and seed yield and Dineshkumar et al. (2019) who found higher yield in maize using microalgae and cow dungs.
In conclusion, results show that C. vulgaris, T. dimorphus, and A. platensis biomass are potential candidate plant biostimulants. The combination of C. vulgaris and chemical fertilizer is the most effective in enhancing the plant height, fresh weight, dry weight, and number of leaves at both vegetative growth and fruiting stage of P. vulgaris as well as yield compared with other treatments. Thus, foliar spraying with C. vulgaris can be proposed as a plant biostimulant enhancing plant growth and crop production. More in-depth future studies are required to study the effect of C. vulgaris inoculation on soil quality hoping to reduce further the use of chemical fertilizers by biofertilizers.
Availability of data
The authors confirm that all the data are available within the article.
References
Anagnostidis K, Komárek J (1988) Modern approach to the classification system of cyanophytes. 3-Oscillatoriales. Algol Stud / Arch Hydrobiol Suppl 50–53:327–472
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Barone V, Puglisi I, Fragalà F, Stevanato P, Baglieri A (2019) Effect of living cells of microalgae or their extracts on soil enzyme activities. Arch Agron Soil Sci 65:712–726
Bayona-Morcillo P, Plaza B, Gómez-Serrano C, Rojas E, Jiménez-Becker S (2020) Effect of the foliar application of cyanobacterial hydrolysate (Arthrospira platensis) on the growth of Petunia x hybrida under salinity conditions. J Appl Phycol 32:4003–4011
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hort 196:39–48
Bischoff H (1963) Some soil algae from enchanted rock and related algal species. Phycological Studies IV. University of Texas Publ No 6318:1–95
Black C (1965) Method of soil analysis part 2. Chem Microbiol Properties 9:1387–1388
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bumandalai O, Tserennadmid R (2019) Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int J Aquat Biol 7:95–99
Chiaiese P, Corrado G, Colla G, Kyriacou MC, Rouphael Y (2018) Renewable sources of plant biostimulation: microalgae as a sustainable means to improve crop performance. Front Plant Sci 9:1782
Chookalaii H, Riahi H, Shariatmadari Z, Mazarei Z, SeyedHashtroudi M (2020) Enhancement of total flavonoid and phenolic contents in Plantago major L. with plant growth promoting cyanobacteria. J Agric Sci Technol 22:505–518
Dias GA, Rocha RHC, Araújo JL, Lima JF, Guedes WA (2016) Growth, yield, and postharvest quality in eggplant produced under different foliar fertilizer (Arthrospira platensis) treatments. Semina Ciênc Agrár 37:3893–3902
Díaz-Batalla L, Widholm JM, Fahey GC, Castaño-Tostado E, Paredes-López O (2006) Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). J Agric Food Chem 54:2045–2052
Dineshkumar R, Kumaravel R, Gopalsamy J, Sikder MNA, Sampathkumar P (2018) Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Valor 9:793–800
Dineshkumar R, Subramanian J, Gopalsamy J, Jayasingam P, Arumugam A, Kannadasan S, Sampathkumar P (2019) The impact of using microalgae as biofertilizer in maize (Zea mays L.). Waste Biomass Valor 10:1101–1110
Du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hort 196:3–14
Elarroussia H, Elmernissia N, Benhimaa R, El Kadmiria IM, Bendaou N, Smouni A, Wahbya I (2016) Microalgae polysaccharides a promising plant growth biostimulant. J Algal Biomass Utln 7:55–63
Fernández V, Sotiropoulos T, Brown PH (2013) Foliar fertilization: scientific principles and field practices. IFA, Paris pp 12–70
Garcia-Gonzalez J, Sommerfeld M (2016) Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J Appl Phycol 28:1051–1061
Gorelova O (2006) Communication of cyanobacteria with plant partners during association formation. Microbiology 75:465–469
Hedge J, Hofreiter B, Whistler R (1962) Carbohydrate chemistry. Academic Press, New York
Horvath G, Kissimon J, Faludi-Dániel Á (1972) Effect of light intensity on the formation of carotenoids in normal and mutant maize leaves. Phytochemistry 11:183–187
Kawalekar JS (2013) Role of biofertilizers and biopesticides for sustainable agriculture. J Biol Innov 2:73–78
Khan SA, Hussain MZ, Prasad S, Banerjee U (2009) Prospects of biodiesel production from microalgae in India. Renew Sustain Energy Rev 13:2361–2372
Kim MJ, Shim CK, Kim YK, Ko BG, Park JH, Hwang SG, Kim BH (2018) Effect of biostimulator Chlorella fusca on improving growth and qualities of chinese chives and spinach in organic farm. Plant Pathol J 34:567
Kissimon J (1999) Analysis of the photosynthetic pigment composition. International workshop and training course on microalgal biology and biotechnology. Mosonmagyarouar, Hungary, pp 13–26
Komárek J, Fott B, Huber-Pestalozzi G (1983) Das Phytoplankton des Süßwassers Systematik und Biologie-Teil 7, 1. Schweizerbart Science Publishers, Stuttgart
Lin L-Z, Harnly JM, Pastor-Corrales MS, Luthria DL (2008) The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem 107:399–410
Mógor ÁF, Ördög V, Lima GPP, Molnár Z, Mógor G (2018) Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J Appl Phycol 30:453–460
Mourice SK, Tryphone GM (2012) Evaluation of common bean (Phaseolus vulgaris L.) genotypes for adaptation to low phosphorus. Int Schl Res Not 2012:309614
Nagy PT, Pintér T (2015) Effects of foliar biofertilizer sprays on nutrient uptake, yield, and quality parameters of Blaufrankish (Vitis vinifera L.) Grapes. Comm Soil Sci Plant Anal 46:219–227
Osman MEH, El-Sheekh MM, El-Naggar AH, Gheda SF (2010) Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol Fertil Soils 46:861–875
Plaza BM, Gómez-Serrano C, Acién-Fernández FG, Jimenez-Becker S (2018) Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Tetradesmus sp.) on Petunia x hybrida growth. J Appl Phycol 30:2359–2365
Porch TG, Beaver JS, Debouck DG, Jackson SA, Kelly JD, Dempewolf H (2013) Use of wild relatives and closely related species to adapt common bean to climate change. Agronomy 3:433–461
Priya TR, Manickavasagan A (2020) Common bean. In: Manickavasagan A, Thirunathan P (eds) Pulses. Springer, Cham, pp 77–97
Puglisi I, La Bella E, Rovetto EI, Lo Piero AR, Baglieri A (2020) Biostimulant effect and biochemical response in lettuce seedlings treated with a Tetradesmus quadricauda extract. Plants 9:123
Raschke RL, Schultz DA (1987) The use of the algal growth potential test for data assessment. J Water Pollut Control Fed 222–227
Ronga D, Biazzi E, Parati K, Carminati D, Carminati E, Tava A (2019) Microalgal biostimulants and biofertilisers in crop productions. Agronomy 9:192
Sadasivam S, Manickam A (1996) Biochemical methods. New Age International (P) Limited, New Delhi 2:124–126
Shaaban MM (2001a) Green microalgae water extract as foliar feeding to wheat plants. Pak J Biol Sci 4:628–632
Shaaban MM (2001b) Nutritional status and growth of maize plants as affected by green microalgae as soil additives. J Biol Sci 1:475–479
Shindy WW, Smith OE (1975) Identification of plant hormones from cotton ovules. Plant Physiol 55:550–554
Siddiq M, Ravi R, Harte J, Dolan K (2010) Physical and functional characteristics of selected dry bean (Phaseolus vulgaris L.) flours. LWT - Food Sci Technol 43:232–237
Starr RC (1978) The culture collection of algae at the University of Texas at Austin. J Phycol 14:47–100
Stein JR, Hellebust JA, Craigie J (1973) Handbook of phycological methods: culture methods and growth measurements. Cambridge University Press, Cambridge
Stoscheck CM (1990) Quantitation of protein. Meth Enzymol 182:50–68
Tarakhovskaya E, Maslov YI, Shishova M (2007) Phytohormones in algae. Russ J Plant Physiol 54:163–170
Van Handel E (1968) Direct micro determination of sucrose. Anal Biochem 22:280–283
Yemm E, Willis A (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514
Zarrouk C (1966) Contribution a l'etude d'une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur la croissance et la photosynthese de Spirulina maxima. Thesis. University of Paris, France
Acknowledgements
The authors would like to express their gratitude to the Botany Department at Mansoura University in Egypt for allowing them to complete these experiments and for providing financial support.
Author information
Authors and Affiliations
Contributions
D.A.R. prepared the manuscript. D.A.R., E.M.E., M.I.A, and S.A.H conducted the experiments. S.A.H and D.A.R. designed the study. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Refaay, D.A., El-Marzoki, E.M., Abdel-Hamid, M.I. et al. Effect of foliar application with Chlorella vulgaris, Tetradesmus dimorphus, and Arthrospira platensis as biostimulants for common bean. J Appl Phycol 33, 3807–3815 (2021). https://doi.org/10.1007/s10811-021-02584-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02584-z