Abstract
The effects of light and salt stress (NaCl and MgCl2) on the two-stage cultivation of Chlorella vulgaris, with or without a medium replacement, for the simultaneous production of lipid, carotenoids, and antioxidant compounds were investigated. The highest lipid productivity (15.59 ± 0.10 mg L−1 day−1) was obtained at 5 g L−1 MgCl2 and 140 μmol photons m−2 s−1 light intensity. The saturated fatty acids (SFA) ranged from 52.35–81.64%, monounsaturated fatty acids (MUFA) from 7.38–34.26%, and polyunsaturated fatty acids (PUFA) from 7.25–25.10%, with palmitic (C16:0), stearic (C18:0), and oleic (C18:1) acids as predominant fatty acids. Under high light intensity and nitrogen limitation in the two-stage cultivation, supplementation of 10 g L−1 NaCl with a medium replacement caused a marked increase in the total carotenoids (4.37 ± 0.33 μg mL−1). Cultivation of C. vulgaris in a medium containing 5 g L−1 NaCl or Mg Cl2, with or without a medium replacement step and with exposure to 140 μmol photons m−2 s−1 light intensity, led to enhanced antioxidant activities (65–79%). The different levels of antioxidant activities of the C. vulgaris extracts suggested the variation in the phytochemical compounds, a result of the stressed conditions.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are the major producers of lipids, essential fatty acids, carbohydrates, hormones, recombinant proteins, and pigments such as the chlorophylls and carotenoids in the biotechnology and food industries. Therefore, microalgal cultivation is an attractive option for biochemical production with diverse applications in bioenergy, bioremediation, and nutraceutical and biopharmaceutical industries (Borowitzka and Moheimani 2013; Khan et al. 2018; Grubišić et al. 2019; Abdullah and Hussein 2020). Microalgae (Chlorophyta) are superior to the land plants (Streptophyta) as efficient sources of biofuels and bioproducts as they can produce neutral lipids in large quantities per unit area, exceeding the yield of the best oil-seed crops (Khan et al. 2018; Aratboni et al. 2019). Both sequester CO2 through photosynthesis and reduce greenhouse gas emissions, but unlike land plants, microalgae can be cultured in high salinity or brackish water and can utilize wastewater as nutrient sources (Khan et al. 2018). The growth of microalgae can be enhanced in photobioreactors for higher biomass production (Pierre et al. 2019) by downstream processes optimized to harvest the bioproducts (Abdullah et al. 2016, 2017).
Stress involves the disruption of homeostasis as a result of a stressor application. The stress responses are the changes in the cell metabolism as the cells acclimatize and attempt to restore the homeostasis. The different stages in stress responses are alarming stage, regulation, acclimation, and adaptation (Borowitzka 2018). Stress strategies have been adopted to enhance high-value compounds production using single stress factor such as the nutritional factors (e.g., carbon source, nitrogen, phosphorus), environmental factors (high light intensities, temperature, pH, salinity, reactor configurations, and operating conditions) (Sun et al. 2014; Shah and Abdullah 2018; Zappi et al. 2019; Li et al. 2020). Salt stress causes several biochemical and bioenergetic alterations such as increased rates of lipid biosynthesis and enhanced biopolymer and energy production, changes in membrane permeability with ion homeostasis interruption (Alyabyev et al. 2011), and elevated level of reactive oxygen species (ROS). As a response to the increasesd ROS level, microalgae may accumulate antioxidant compounds such as polyphenols, flavonoids, and carotenoids to quench the free radicals (Edge and Truscott 2010; Wani et al. 2016). Nitrogen-limiting conditions can trigger lipid synthesis in some algal species by reducing cell division and shifting the lipid biosynthetic pathways to synthesize more neutral lipids than membrane lipids (Vitova et al. 2015). Light of suitable wavelengths and intensity can also be the key factors that affect or control the biomass and lipid production in micralgae (Sajjadi et al. 2018). The manipulation of light intensities alters the types and levels of secondary metabolites such as phenolics and flavonoids, which consequently influence antioxidant activities. There are strong correlations between the total phenolic and flavonoid contents with the antioxidant activities (Karimi et al. 2013; Ali et al. 2014).
The biomass or lipid production is significantly affected by the mode of cultivation (Ryu et al. 2019). Fed-batch and continuous mode may promote cell growth but do not necessarily lead to high lipid productivity (Ho et al. 2014) unless stressful conditions are adopted throughout the cultivation to induce product formation. However, this at the end may be at the expense of the cell growth. In plant cell culture, intermediary and production medium strategies adopted have resulted in increased hydrogen peroxide level but concomitantly with significant enhancement of cell dry weight, products of interest, and antioxidant compounds (Chong et al. 2004; Abdullah et al. 2005). The implementation of an appropriate cultivation strategy can therefore increase the biomass production with the high-value products. One of the most effective strategies is the cultivation of microalgae through the two-stage cultivation strategy (TSCS) (Aziz et al. 2020). The TSCS and semi-continuous mode could achieve high biomass with simultaneous enhanced lipid yield (Narala et al. 2016). The TSCS typically consists of the biomass production, pre-harvesting, and product induction stages, making it possible to separate the biomass growth phase (vegetative stage) from the lipid accumulation phase (stress stage) during microalgal cultivation (Aziz et al. 2020). Biomass production is enhanced under optimal culture conditions in the first phase (Johnson et al. 2018) and in the second phase microalgae may be subjected to one or more stressors including physical stimuli (temperature, light intensity) and chemical stimuli (nutrient limitations or additions).
A number of large-scale TSCSs have been successfully implemented to produce high-value metabolites (Su et al. 2017; Chew et al. 2018), such as the TSCS developed by Aquasearch to produce both oil and astaxanthin from the green microalga Haematococcus pluvialis in photobioreactors and open ponds (Schenk et al. 2008). However, the high costs and energy in transferring the vegetative cells from nutrient-rich (phase I) to nutrient-deficient (phase II) media are the main obstacles in attaining the commercial scale of production based on the TSCS. Hence, modifications or simplifications of this step can considerably increase the economic feasibility of the TSCS (Aziz et al. 2020). To overcome this drawback, direct addition of inducers such as NaCl to the growth medium (Xia et al. 2013), or plant hormones (Wu et al. 2018), has been investigated. The incorporation of more than one inducer may lead to an increase in the lipid accumulation and other high-value metabolites (Sun et al. 2014). However, the effects of some of these inducers in most microalgae strains are not known.
The objective of this study was to attain enhanced simultaneous production of lipid, carotenoids, and antioxidant compounds in Chlorella vulgaris under combined stress factors involving nutrient (nitrogen), high light intensity, and salt stress (NaCl and MgCl2) in a two-stage cultivation strategy, with or without a medium replacement step. The kinetics of cell growth and product formation were evaluated, and the fatty acids profile was analyzed by the GC analysis.
Materials and methods
Microalgal culture conditions
The experimental design and culture conditions of the two-stage cultivation strategy of Chlorella vulgaris are shown in Fig. 1 and Table 1. The C. vulgaris culture was maintained in standard conditions at 25 ± 1 °C in BG-11 medium (Stanier et al. 1971), under fluorescent white light (Philips, TLD18W/54-765) at 40 μmol photons m−2 s−1, with constant bubbling of air (filtered through a 0.22 μm microporous filter).
Stress treatments
Chlorella vulgaris cells with optical density (OD680 nm) of 1.6 were inoculated into BG-11 medium (10% of the total culture medium) in 1 L Erlenmeyer flasks, and incubated under standard conditions for 15 days (vegetative stage) (Table 1 and Fig. 1). In the stress stage the vegetative cultures were divided into 3 groups: (i) group 1, where the cultures were in the same medium under 40 μmol photons m−2 s−1 light for 15–20 days and designated control 1 (nutrient stress); the cultures were transferred in the same medium under 140 μmol photons m−2 s−1 for 20 days and designated control 2 (light stress); transferred for exposure to 140 μmol photons m−2 s−1 and starvation medium for 20 days and designated control 3 (nutrient + light stress); (ii) group 2, where the flasks were exposed to 140 μmol photons m−2 s−1 with 5 or 10 g L−1 of NaCl or MgCl2 addition and designated C1/2/5/6 (control 2 + salt); and (iii) group 3, where the cultures were harvested, washed, and diluted by 2-fold (OD680 = 0.76), and then re-suspended in BG-11 medium, with nitrogen limitation (0.1 g L−1 NaNO3) and the addition of 5 or 10 g L−1 of NaCl or MgCl2, and exposed to 140 μmol photons m−2 s−1 light intensity, designated as C3/4/7/8 (control 3 + salt). Cultures were incubated at 25 ± 1 °C for 20 days with constant bubbling of air (filtered through 0.22 μm filter).
Cell growth measurements
Cell growth was determined in both the vegetative and stress stages based on 3 mL sample removal every 5 days, by measuring the OD680 nm (Hsieh and Wu 2009).
The dry weight (DW) was evaluated gravimetrically. After centrifugation of 20 mL of culture, the pellets were collected and washed two times using deionized water, dried overnight at 80 °C, and then cooled and weighed. The biomass productivity (BP, mg L−1 day−1) and biomass yield (BY, mg L−1) were determined according to Hempel et al. (2012) and Vidyashankar et al. (2015), respectively as follows:
where Xf and X0 are the final and initial biomass concentrations (g L−1), respectively; t is the duration of the run (day).
Determination of lipid content
Lipids were extracted based on the modified Bligh and Dyer method (1959). Dried biomass (0.5 g) was added to a mixture of chloroform and methanol at the 1:1 ratio (v/v), and heated in a microwave for 1 min. Water was added to achieve the final ratio of chloroform, methanol, and water at the 1:1:0.9 ratio (v/v). The lipid-containing chloroform layer at the bottom of the separating funnel was removed, washed with 5 mL of 5% NaCl, and left to a constant weight in an oven at 60 °C. The lipid dry weight was determined:
where L is the lipid content (%); WL and WB are the weights of the extracted lipids (mg L−1) and the dry biomass (mg L−1), respectively.
The lipid productivity (LP) was calculated as follows (Hempel et al. 2012):
where LP is the lipid productivity (mg L−1 day−1), BP is the biomass productivity (mg L−1 day−1), and L is the lipid content (% dry weight).
The lipid yield was determined as follows (Yang et al. 2014):
where LY is the lipid yield (mg L−1), biomass yield, BY (g L−1), and lipid content, L (% dry weight).
Transesterification and fatty acid analyses
The fatty acid methyl esters (FAMEs) of the extracted lipids were analyzed according to Mandal et al. (2013). The analyses were performed using gas chromatography (Agilent 6890, Model G1530A, USA), with flame ionization detector and a DB-5 silica capillary column (60 m × 0.32 mm i.d.). The oven temperature was initially set at 45 °C to reach 60 °C at 1 °C min−1, before finally programmed from 60 to 240 °C at 3 °C min−1. Helium was the carrier gas at 1 mL min−1 flow rate. The injector temperature was 240 °C (starting at 150 °C for 1 min, ramping to 240 °C at 30 °C min−1, and held at 240 °C for 30 min).
Pigment extraction and quantification
The determination of chlorophylls and carotenoids was according to Lichtenthaler and Wellburn (1983). Culture samples (10 mL) were centrifuged at 2500 rpm for 5 min and the supernatant was discarded. Ten milliliter of methanol (96%) was added and the cells were re-suspended by homogenizing at 1000 rpm for 1 min. The residue was re-extracted several times until the solvent became colorless. The homogenate was centrifuged at 2500 rpm for 10 min, the supernatants collected and finally topped up to 25 mL with 96% methanol. The absorbance between 400 and 700 nm was read where the maximum absorbance of chlorophyll a, chlorophyll b, and total carotenoids was shown at 666 nm, 653 nm, and 470 nm, respectively. The concentration of each pigment (μg mL−1) was calculated as follows (Lichtenthaler and Wellburn 1983):
where A653, A666, and A470 nm are the absorbance at the indicated wavelengths.
Preparation of algal extracts
One gram of freeze-dried microalgal biomass was extracted with 100 mL mixture of methylene chloride and methanol at the 1:1 ratio (v/v) for 40 min at room temperature. The extraction was repeated three times. The supernatant was collected, filtered, and evaporated using rotary vacuum evaporator (40–45 °C). The amount of extractable substances (crude extracts) was determined in mg g−1 DW.
Antioxidant assay
DPPH radical scavenging activity
The free radical scavenging activities of crude methanol:methylene chloride (1:1) extracts were determined (Yen and Chen 1995). Two milliliters of 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution in methanol (0.16 mM), considered as a control, was mixed with 2 mL of extracts or the standard (butyl hydroxyl toluene (BHT) and vitamin C) at 200 μg mL−1 concentration. The mixture was kept for 30 min in the dark at room temperature. The absorbance was later measured at 517 nm, and the radical scavenging activity was calculated:
where At and Ac represent the absorbance of the samples and the DPPH control, respectively.
ABTS radical cation scavenging activity
The potency of the extracts to scavenge 2,2′-azino-bis ethylbenzthiazoline-6-sulfonic acid (ABTS) radical cation was evaluated. The mixture was prepared by adding 7 mM ABTS solution with 2.45 mM potassium persulfate (1:1, v/v). The mixture was left for 4–16 h until the formation of the free radical and the absorbance became stable around 0.700 ± 0.05 at 734 nm using ethanol for dilution (Re et al. 1999). A total of 100 μL of the standard (BHT or vitamin C) or tested extracts at 200 μg mL−1 was mixed with 900 μL of ABTS for 45 s. The decreasing absorbance was determined after 1 min at 734 nm and the antioxidant activitiy determined:
where At and Ac represent the absorbance of the samples and the ABTS control, respectively.
For IC50 determination, the 2-fold dilution of the sample from 500, 250, 125, 62.5, 31.25, 15.65 to 7.81 μg mL−1 concentrations were used.
Statistical analysis
The experiments were carried out in three replicates. The significant difference of variables was determined using one-way ANOVA with 95% confidence (probability limit of p < 0.05). The Tukey’s test was used to identify the differences between each level of treatment. The statistical analyses were performed using Minitab software (V18, Minitab Inc., USA).
Results
Cell growth and lipid productivity
Figure 2 a and b show that all cultures exhibited no significant differences in the growth rate in the vegetative stage, but showed significant variation in the stress stage, based on the different level of salt stressors and light intensity. The control 1 and 2 cultures maintained the highest biomass growth, followed by the C5 and C6 as well as C1 conditions. The lowest cell growth was observed in C3, C4, C7, and C8, attributable to the lower inoculum density (OD680 = 0.76) from the 2-fold dilution carried out and the combined stress conditions of nitrogen deficiency (0.1 g L−1 NaNO3), high salt stress (5 and 10 g L−1 NaCl or MgCl2), and high light intensity (140 μmol photons m−2 s−1). Table 2 shows that the C. vulgaris productivities (C1–C8) for 35 days of cultivation period were affected (p < 0.05) by the type and concentration of salt stress and light intensity as well as the cultivation strategy. The biomass productivity was high when the vegetative cells of C. vulgaris was transferred to grow under 140 μmol photons m−2 s−1 light intensity, with the addition of 5 or 10 g L−1 of NaCl (C1, C2) or MgCl2 (C5, C6). The highest biomass productivity (67.43 ± 0.143 mg L−1day−1) and biomass yield (2.36 ± 0.05 g L−1) were obtained under single-stage cultivation (control 1). Interestingly, the biomass productivity of cultures supplemented with MgCl2 (C5–C6) was higher than the cultures supplemented with NaCl (C1–C4). The maximum lipid productivity (15.59 ± 0.10 mg L−1day−1) and lipid yield (560.76 ± 3.48 mg L−1) were achieved with 5 g L−1 MgCl2 addition and exposure to 140 μmol photons m−2 s−1 light (C5). The lipid content increased up to 37.80 ± 0.80% when the culture was re-grown in a medium with 10 g L−1 NaCl addition and 140 μmol photons m−2 s−1 light (C4).
Fatty acid profile
The fatty acids consisted of saturated (SFA) (52.35–81.64% of total FAME), monounsaturated (MUFA) (7.38–34.26% of total FAME), and polyunsaturated fatty acids (PUFA) (7.25–25.10% of total FAME) (Fig. 3). The highest SFA content (81.64%) was recorded in C7 when the culture was transferred to a medium containing 5 g L−1 MgCl2 with N-limitation. High SFA was also noted in control 2 (82.05%) under light stress; control 1 (78.22%) under single stage cultivation; and control 3 (77.9%) with similar conditions to C7 but without salt stress. The other conditions exhibited SFA in the range of 52–74%. For MUFA, there was a noticeable increase in C5 (34.26%), followed by C6 (22.2%), C8 (18.57%), C3 (18.50%), C1 (16.88%), C2 (15.92%), and C4 (15.06%). In contrast to SFA, the MUFA contents were lower in C7 (7.38%) and the controls (10.07–12.96%). The PUFA in all salt stress conditions also recorded significant increase, reaching a maximum in C6 (25.10%) and others (10.1–14.15%), as compared to the controls (7.75–9.26%). Table 3 suggests that the relative percentage of fatty acids was strongly affected by the variations in the salt stress conditions. The main fatty acid component is palmitic acid (C16:0) at 11.80–41.65%, achieving the highest level under C7 (41.65%), C2 (36.4%), and C8 (26.76%). Stearic acid (C18:0) (10.70–37.90%) and oleic acid (C18:1) (3.93–17.81%) were other major components in all salt stress conditions. Optimal balance between the SFA (55.43%), MUFA (34.26%) and PUFA (10.32%) composition was attained in C5, when the cultures were supplemented with 5 g L−1 MgCl2, under high light intensity.
Grid graph bar showing boundaries of all fatty acid components of C. vulgaris cultured under different salt stress conditions in a two-stage cultivation strategy. SFA saturated fatty acids (C8:0–C18:0); MUFA monounsaturated fatty acids (C16:1–C20:1); and PUFA polyunsaturated fatty acids (C18:2–C18:3)
Chlorophyll and carotenoid production
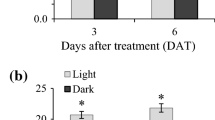
The chlorophyll a and total carotenoid concentrations were simultaneously increased in the vegetative stage (Fig. 4). The highest chlorophyll a content was observed in the control group, having adequate nutrients in the medium but devoid of light or salt stress. During the stress stage (carotenogenesis), the total carotenoid levels increased under all salt stress and light conditions, while chlorophyll a started to decline until day 35, except in control 1 (Fig. 4). The C4 condition, where the culture was supplemented with 10 g L−1 NaCl and with a medium replacement step, recorded the lowest biomass productivity, but the highest carotenoid level (4.37 ± 0.33 μg mL−1) and also the highest lipid content attained (Table 2).
Antioxidant activities
Figure 5 illustrates that the highest antioxidant activities were exhibited, based on the DPPH and ABTS methods, respectively, in C1 (68.15 ± 1.05%, 67.24 ± 1.09%), C3 (75.34 ± 2.18%, 65.59 ± 1.76%), and C5 (71.27 ± 1.8%, 78.76 ± 2.35%). These were only slightly lower than the standards BHT (87.63 ± 1.55%, 91.82 ± 1.68%) and vit. C (88.8 ± 1.8%, 93.2 ± 1.93%), respectively. Based on the DPPH method, the control conditions exhibited the lowest activities (46.25 ± 1.67–51.34 ± 1.016%), and almost comparable to C2, C4, and C6–8. The C1, C3, and C5 culture extracts, respectively, exhibited the IC50 of 25.56, 23.12, and 24.44 μg mL−1 (as compared to 11.2 μg mL−1 for BHT, 12.9 μg mL−1 for vit. C) based on the DPPH; and 25.91, 26.56, and 22.12 μg mL−1 (as compared to 15.1 μg mL−1 for BHT, 14.7 μg mL−1 for vit. C) based on the ABTS method. Phytochemical screening showed the presence of considerable amounts of phenolic compounds, flavonoids, sterols, terpenoids, tannins, and glycosides (data not shown) in the extracts of C1, C3, and C5 cultures which were cultivated in low salt concentration at 5 g L−1 of NaCl and MgCl2, with and without medium replacement step.
Antioxidant activity of C. vulgaris extracts (200 μg mL−1) cultured under different salt stress conditions in a two-stage cultivation strategy as measured by the DPPH and ABTS radical scavenging methods. Different small letters on the bars indicate significant difference (p < 0.05). Error bars represent standard deviation of three replicates
Discussion
Environmental stressors such as high salinity and light intensity are important strategies to enhance lipids and/or carbohydrates, or high-value products such as carotenoids and bioactive compounds in marine and freshwater microalgae (Ishika et al. 2017). However, high lipid production under salinity stress is often associated with cell growth retardation, resulting in lower biomass (Aziz et al. 2020). The TSCS based on salinity stress also may involve pre-harvesting step in between the first and the second stages (Kakarla et al. 2018; Sajjadi et al. 2018). Eliminating or modifying this step could lower the operating cost and increase the TSCS economic feasibility (Narala et al. 2016). In our study, the effect of combined stress factors (salt stresses and light intensity) in the TSCS of C. vulgaris with or without a medium replacement aimed to achieve the economic viability of microalgal cultivation, with simultaneous production of lipid, carotenoids, and antioxidant compounds. The results clearly showed that the TSCS, salt stress (type and concentration), and the light intensity significantly affected the growth and lipid accumulation. The highest lipid productivity was recorded when C. vulgaris was cultured using TSCS without a medium replacement (C5) (Table 2). Similar results have been obtained where the lipid production in Scenedesmus obtusus XJ-15 is improved by direct addition of NaCl into the growth medium, which is more effective than transferring the vegetative cells from the nutrient-replete to nutrient-depleted media (Xia et al. 2013).
The lipid content in microalgal cells can be enhanced by a single inducer such as nitrogen starvation (Rehman and Anal 2019), phosphate limitation (Yu et al. 2016), high salinity (Shah and Abdullah 2018; Gour et al. 2020), high light intensities (He et al. 2018), or by combining the different stress factors (Sun et al. 2014). Hyper-accumulation of lipid content is generally achieved by combining these stimuli in the cultivation process (Guedes et al. 2011), such as combining the physical stress factors and nutrient starvation (Singh et al. 2016), but this may be at the expense of cell growth (Yu et al. 2016). The high light intensity in combination with salt stress in our study had led to an increase in the lipid yield of C. vulgaris (C5), higher than control 2 which was exposed to only light intensity factor in the stress stage. The TSCS of Neochloris oleoabundans HK-129 has been investigated where in the first stage, maximal biomass productivity is achieved under optimal nutrient, and in the second stage, the effects of combined nitrogen starvation, high light intensity, and high concentration of iron (Fe3+) on lipid accumulation are carried out. The biomass/lipid productivities obtained under nitrogen starvation suggest that the effects of light intensities are superior to the effects of Fe3+ (Sun et al. 2014). Similarly, the combined two stress factors have been found to increase the lipid productivity (25–54%), higher than the single stress conditions (Kwak et al. 2016). Nitrogen deficiency as a single stress factor may not improve the lipid productivity in all microalgal species. The combination of two or more types of stressors is not only beneficial but is also needed in some microalgal species (Ho et al. 2017).
Combining high salinity and light intensity as stressors not only affects cell growth and lipid but also the fatty acid composition (Xia et al. 2014), as similarly exhibited in our study (Table 3 and Fig. 3). The exposure of the C. vulgaris vegetative cells in the stress stage to the high salt stress and light intensity significantly alters the fatty acid profile. Various lipid extraction methods have been carried out for transesterification process (Mandal et al. 2013). For biodiesel production, the microalgal species should ideally have high level of lipid and triacylglycerol (TAG), and a balanced fatty acid composition. The lipid produced from green microalgae species is generally similar to that of vegetable oils, which mainly contain C16 and C18 fatty acids, and are therefore appropriate for biodiesel (Francisco et al. 2010; Mondal et al. 2017). The long chain fatty acids (C16–18) is preferable as the increase in carbon chain length leads to an increase in the biodiesel properties such as heat of combustion, cetane number, and viscosity (Francisco et al. 2010). The C. vulgaris culture in our study exhibits predominantly C16 and C18 fatty acids, suggesting its suitability as biodiesel feedstock, whilst at the same time can be harnessed as a source of high-value caroetenoids and antioxidants.
Under optimal conditions (vegetative stage), the level of chlorophyll a in the C. vulgaris cells was significantly high. The accumulation of chlorophyll is favored under conditions that are optimal for cell growth, which is consistent with their role in photosynthesis (Faraloni and Torzillo 2017). On the other hand, the carotenoid accumulation in C. vulgaris cells was induced under stressed conditions, and especially under nitrogen deficiency with high light intensity and/or salt addition (Fig. 4). Dunaliella salina cultivated at extremely high (232 g L−1 NaCl) and high salt stress (58 g L−1 NaCl) has the total carotenoids elevated to 9.67 ± 0.19 μg mL−1 and 1.54 ± 0.08 μg mL−1, respectively (Gallego-Cartagena et al. 2019). Reactive oxygen species (ROS) may be generated under stressed conditions and carotenoids may be induced as a defense mechanism, in a biochemical pathway promoted by the presence of additional salt, mediated by abscisic acid (Yoshida et al. 2004). The resulting increase in abscisic acid concentration has triggered counter responses including with higher level of carotenoids produced (Pancha et al. 2015).
The combined stress conditions (salt stress and light intensity) in the TSCS had resulted in the C. vulgaris cell extracts exhibiting considerably different antioxidant activities as measured by the DPPH and ABTS radical scavenging methods. The C1, C3, and C5 extracts had shown the lowest IC50 values, suggesting the strongest antioxidant activities. These can be correlated to the presence of unsaturated bonds and the hydroxyl groups of the compound extracts which exhibits high ability for scavenging free radicals and preventing the oxidation processes (El-fayoumy et al. 2020). Out of seven phytochemicals estimated quantitatively in the methanolic extracts of C. vulgaris, phenols are found the highest, followed by alkaloids, terpenoids, and glycosides and the tannin are the least (Prabakaran et al. 2018).
The effects of different NaCl salts have been tested on different microalgal species where a dose-dependent upregulation of antioxidant activities have been observed with the elevation of phenolic compounds, flavonoids, and the pigments. Maximum upregulation of the total flavonoid content (71.8 ± 0.21 mg QE g−1 DW) is registered in Pithophora cleveana grown in hypersaline conditions (Mukherjee et al. 2020). The salt stressors have led to changes in the level of antioxidant compounds which could scavenge the highly toxic ROS (Noctor et al. 2016). The imbalance of the cellular ions and the osmotic pressure, under salt stress, could retard the cell growth (Shalaby et al. 2010). The increased phytochemical compounds and the radical scavenging activities of C. vulgaris at low and high salinities therefore indicate its response to the abiotic stressors. There is a need to strike a balance between the level of intracellular oxidant and antioxidant molecules. While stressful conditions could result in the production of oxidizing agents, the hazardous consequences could be avoided by having the cells over-producing the antioxidant molecules (Barsanti et al. 2008). The developed stressful cultivation condition is therefore an effective strategy to promote the microalgal antioxidant machinery (Skjanes et al. 2013), especially if the aim is for industrial production of the high-value antioxidant compounds.
Our study shows that the TSCS of C. vulgaris without a medium replacement, with direct addition of 5 g L−1 MgCl2 to the growth medium and exposure to 140 μmol photons m−2 s−1 light intensity, is a suitable strategy for lipid and fatty acid production for biodiesel. The MgCl2 stress displays a lower effect on the cell growth than the NaCl stress as magnesium is essential for microalgal growth. Magnesium not only constitutes the central atom of chlorophyll, but also as the co-factor of some enzymes in the metabolic pathways (Wang et al. 2014). The highest carotenoid level was obtained when C. vulgaris was cultivated using the TSCS with a medium replacement, addition of 10 g L−1 NaCl, and exposure to 140 μmol photons m−2 s−1 light. The TSCS, with or without a medium replacement, with an addition of 5 g L−1 NaCl or MgCl2, and exposure to 140 μmol photons m−2 s−1 light intensity, are also the appropriate strategies for phytochemical compounds production. These results constitute a further step towards the development of potential and cost-effective technologies for the production of lipid, carotenoid, or antioxidant compound–enriched microalgal biomass.
Conclusion
The oleaginous Chlorella vulgaris cultivated under light and salt stress conditions using two-stage cultivation strategy, with or without a medium replacement step, had shown elevated production of lipid and high-value antioxidant compounds. Low concentrations of MgCl2 enhanced the lipid productivity with high saturated, monounsaturated, and polyunsaturated fatty acids, suggesting the potential for its eventual use as a biodiesel feedstock. High NaCl concentration in combination with nitrogen deficiency and high light intensity induced the carotenoid production in stress phase, with a medium replacement step. The low salt stresses could improve the production of antioxidant phytochemicals. Salt-supplemented cultures in the two-stage cultivation exhibited a promising strategy for simultaneous production of lipids and high-value compounds for bioenergy and biopharmaceutical applications.
References
Abdullah MA, Hussein HA (2020) Integrated algal biorefinery and palm oil milling for bioenergy, biomaterials and biopharmaceuticals. IOP Conf Series: Earth Environ Sci 463:012084
Abdullah MA, Lajis NH, Ali AM, Marziah M, Sinskey AJ, Rha CK (2005) Issues in plant cell culture engineering for enhancement of productivity. J Dev Chem Eng Min Process 13:573–587
Abdullah MA, Ahmad A, Shah SMU, Shanab SMM, Ali HEA, Abo-State MAM, Othman MF (2016) Integrated algal engineering for bioenergy generation, effluent remediation and production of high-value bioactive compounds. Biotechnol Bioprocess Eng 21:236–249
Abdullah MA, Shah SMU, Shanab SMM, Ali HEA (2017) Integrated algal bioprocess engineering for enhanced productivity of lipid, carbohydrate and high-value bioactive compounds. Res Rev J Microbiol Biotechnol 6:61–92
Ali HEA, Shanab SMM, Abo-State MAM, Shalaby EAA, El Demerdash UMN, Abdullah MA (2014) Evaluation of antioxidants, pigments and secondary metabolites contents in Spirulina platensis. Appl Mech Mater 625:160–163
Alyabyev A, Andreyeva I, Rachimova G (2011) Influence of pH shift and salting on the energetics of microalgae Chlorella vulgaris and Dunaliella maritima. J Therm Anal Calorim 104:201–207
Aratboni HA, Rafiei N, Garcia-Granados R, Alemzadeh A, Morones-Ramírez JR (2019) Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb Cell Factories 18:178
Aziz MMA, Kassim KA, Shokravi Z, Jakarni FM, Lieu HY, Zaini N, Tan LS, Islam S, Shokravi H (2020) Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: a review. Renew Sust Energ Rev 119:109621
Barsanti L, Coltelli P, Evangelista V, Frassanito AM, Passarelli V, Vesentini N, Gualtieri P (2008) Oddities and curiosities in the algal world. In: Evangelista V, Barsanti L, Frassanito AM (eds) Algal toxins: nature, occurrence, effect and detection. Springer, New York, pp 353–391
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (2018) The ‘stress’ concept in microalgal biology—homeostasis, acclimation and adaptation. J Appl Phycol 30:2815–2825
Borowitzka MA, Moheimani NR (2013) Sustainable biofuels from algae. Mitig Adapt Strateg Glob Chang 18:13–25
Chew KW, Chia SR, Show PL, Yap YJ, Ling TC, Chang JS (2018) Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: a review. J Taiwan Inst Chem Eng 91:332–344
Chong TM, Abdullah MA, Nor’Aini MF, Lai OM, Lajis NH (2004) Anthraquinone production, hydrogen peroxide level and antioxidant vitamins in Morinda elliptica cell suspension cultures from intermediary and production medium strategies. Plant Cell Rep 22:951–958
Edge R, Truscott TG (2010) Properties of carotenoid radicals and excited states and their potential role in biological systems. In: Landrum JT (ed) Carotenoids: Physical, Chemical, and Biological Functions and Properties. CRC Press, Boca Raton, pp 283–308
El-fayoumy EA, Shanab SM, Shalaby EA (2020) Metabolomics and biological activities of Chlorella vulgaris grown under modified growth medium (BG11) composition. CMU J Nat Sci 19:91–123
Faraloni C, Torzillo G (2017) Synthesis of antioxidant carotenoids in microalgae in response to physiological stress. In: Cvetkovic D, Nikolic G (eds) Carotenoids. IntechOpen, UK, pp 143–157
Francisco ÉC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Gallego-Cartagena E, Castillo-Ramírez M, Martínez-Burgos W (2019) Effect of stressful conditions on the carotenogenic activity of a Colombian strain of Dunaliella salina. Saudi J Biol Sci 26:1325–1330
Gour RS, Garlapati VK, Kant A (2020) Effect of salinity stress on lipid accumulation in Scenedesmus sp. and Chlorella sp.: feasibility of stepwise culturing. Curr Microbiol 77:779–785
Grubišić M, Ivančić Šantek M, Šantek B (2019) Potential of microalgae for the production of different biotechnological products. Chem Biochem Eng Q 33:161–181
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644
He Q, Yang H, Hu C (2018) Effects of temperature and its combination with high light intensity on lipid production of Monoraphidium dybowskii Y2 from semi-arid desert areas. Bioresour Technol 265:407–414
Hempel N, Petrick I, Behrendt F (2012) Biomass productivity and productivity of fatty acids and amino acids of microalgae strains as key characteristics of suitability for biodiesel production. J Appl Phycol 24:1407–1418
Ho SH, Ye X, Hasunuma T, Chang JS, Kondo A (2014) Perspectives on engineering strategies for improving biofuel production from microalgae critical review. Biotechnol Adv 32:1448–1459
Ho SH, Nakanishi A, Kato Y, Yamasaki H, Chang JS, Misawa N, Hirose Y, Minagawa J, Hasunuma T, Kondo A (2017) Dynamic metabolic profiling together with transcription analysis reveals salinity-induced starch-to-lipid biosynthesis in alga Chlamydomonas sp. JSC4. Sci Rep 7:45471
Hsieh CH, Wu WT (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100:3921–3926
Ishika T, Moheimani NR, Bahri PA (2017) Sustainable saline microalgae co-cultivation for biofuel production: a critical review. Renew Sust Energ Rev 78:356–368
Johnson TJ, Katuwal S, Anderson GA, Gu L, Zhou R, Gibbons WR (2018) Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol Prog 34:811–827
Kakarla R, Choi JW, Yun JH, Kim BH, Heo J, Lee S, Cho DH, Ramanan R, Kim HS (2018) Application of high-salinity stress for enhancing the lipid productivity of Chlorella sorokiniana HS1 in a two-phase process. J Microbiol 56:56–64
Karimi E, Jaafar HZ, Ghasemzadeh A, Ibrahim MH (2013) Light intensity effects on production and antioxidant activity of flavonoids and phenolic compounds in leaves, stems and roots of three varieties of Labisia pumila Benth. Aust J Crop Sci 7:1016
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17:36
Kwak HS, Kim JYH, Woo HM, Jin E, Min BK, Sim SJ (2016) Synergistic effect of multiple stress conditions for improving microalgal lipid production. Algal Res 19:215–224
Li T, Wang W, Yuan C, Zhang Y, Xu J, Zheng H, Xiang W, Li A (2020) Linking lipid accumulation and photosynthetic efficiency in Nannochloropsis sp. under nutrient limitation and replenishment. J Appl Phycol 32:1619–1630
Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem Soc Trans 11:591–592
Mandal S, Patnaik R, Singh AK, Mallick N (2013) Comparative assessment of various lipid extraction protocols and optimization of transesterification process for microalgal biodiesel production. Environ Technol 34:2009–2018
Mondal M, Goswami S, Ghosh A, Oinam G, Tiwari ON, Das P, Halder GN (2017) Production of biodiesel from microalgae through biological carbon capture: a review. 3 Biotech 7:99
Mukherjee P, Gorain PC, Paul I, Bose R, Bhadoria PBS, Pal R (2020) Investigation on the effects of nitrate and salinity stress on the antioxidant properties of green algae with special reference to the use of processed biomass as potent fish feed ingredient. Aquac Int 28:211–234
Narala RR, Garg S, Sharma KK, Thomas-Hall SR, Deme M, Li Y, Schenk PM (2016) Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front Energ Res 4:29
Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ 39:1140–1160
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348
Pierre G, Delattre C, Dubessay P, Jubeau S, Vialleix C, Cadoret JP, Michaud P (2019) What is in store for EPS microalgae in the next decade? Molecules 24:4296
Prabakaran P, Moovendhan M, Arumugam A, Matharasi A, Dineshkumar R, Sampathkumar P (2018) Quantitative analysis of phytochemical profile in marine microalgae Chlorella vulgaris. Int J Pharm Biol Sci 8:562–565
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rehman ZU, Anal AK (2019) Enhanced lipid and starch productivity of microalga (Chlorococcum sp. TISTR 8583) with nitrogen limitation following effective pretreatments for biofuel production. Biotechnol Rep 21:e00298
Ryu KH, Kim B, Lee JH (2019) A model-based optimization of microalgal cultivation strategies for lipid production under photoautotrophic condition. Comput Chem Eng 121:57–66
Sajjadi B, Chen W-Y, Raman AAA, Ibrahim S (2018) Microalgae lipid and biomass for biofuel production: a comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew Sust Energ Rev 97:200–232
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg Res 1:20–43
Shah SMU, Abdullah MA (2018) Effects of macro/micronutrients on green and brown microalgal cell growth and fatty acids in photobioreactor and open-tank systems. Biocatal Agric Biotechnol 14:10–17
Shalaby EA, Shanab SMM, Singh V (2010) Salt stress enhancement of antioxidant and antiviral efficiency of Spirulina platensis. J Med Plant Res 4:2622–2632
Singh P, Kumari S, Guldhe A, Misra R, Rawat I, Bux F (2016) Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew Sust Energ Rev 55:1–16
Skjanes K, Rebours C, Lindblad P (2013) Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Crit Rev Biotechnol 33:172–215
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Su YY, Song K, Zhang P, Su YY, Cheng J, Chen X (2017) Progress of microalgae biofuels commercialization. Renew Sust Energ Rev 74:402–411
Sun X, Cao YY, Xu H, Liu Y, Sun J, Qiao D, Cao Y (2014) Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour Technol 155:204–212
Vidyashankar S, Shankara Murthy V, Venkata Swarnalatha G, Kavitha MD, Chauhan V, Ravi R, Bansal AK, Singh R, Pande A, Ravishankar GA, Sarada R (2015) Characterization of fatty acids and hydrocarbons of chlorophycean microalgae towards their use as biofuel source. Biomass Bioenergy 77:75–91
Vitova M, Bisova K, Kawano S, Zachleder V (2015) Accumulation of energy reserves in algae: from cell cycles to biotechnological applications. Biotechnol Adv 33:1204–1218
Wang S, Zhao SX, Wei CL, Yu SY, Shi JP, Zhang BG (2014) Effect of magnesium deficiency on photosynthetic physiology and triacylglyceride (TAG) accumulation of Chlorella vulgaris. Huan Jing Ke Xue 35:1462–1467
Wani TA, Wani SM, Ahmad M, Gani A, Masoodi FA (2016) Bioactive profile, health benefits and safety evaluation of sea buckthorn (Hippophae rhamnoides L.): a review. Cogent Food Agric 2:1128519
Wu G, Gao Z, Du H, Lin B, Yan Y, Li G, Guo Y, Fu S, Wei G, Wang M, Cui M, Meng C (2018) The effects of abscisic acid, salicylic acid and jasmonic acid on lipid accumulation in two freshwater Chlorella strains. J Gen Appl Microbiol 64:42–49
Xia L, Ge H, Zhou X, Zhang D, Hu C (2013) Photoautotrophic outdoor two-stage cultivation for oleaginous microalgae Scenedesmus obtusus XJ-15. Bioresour Technol 144:261–267
Xia L, Rong J, Yang H, He Q, Zhang D, Hu C (2014) NaCl as an effective inducer for lipid accumulation in freshwater microalgae Desmodesmus abundans. Bioresour Technol 161:402–409
Yang F, Long L, Sun X, Wu H, Li T, Xiang W (2014) Optimization of medium using response surface methodology for lipid production by Scenedesmus sp. Mar Drugs 12:1245–1257
Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenecity. J Agric Food Chem 43:27–37
Yoshida K, Igarashi E, Wakatsuki E, Miyamoto K, Hirata K (2004) Mitigation of osmotic and salt stresses by abscisic acid through reduction of stress-derived oxidative damage in Chlamydomonas reinhardtii. Plant Sci 167:1335–1341
Yu SJ, Shen XF, Ge HQ, Zheng H, Chu FF, Hu H, Zeng R (2016) Role of sufficient phosphorus in biodiesel production from diatom Phaeodactylum tricornutum. Appl Microbiol Biotechnol 100:6927–6934
Zappi M, Bajpai R, Hernandez R, Mikolajczyk A, Lord Fortela D, Sharp W, Revellame E (2019) Microalgae culturing to produce bio-based diesel fuels: an overview of the basics, challenges, and a look toward a true biorefinery future. Ind Eng Chem Res 58:15724–15746
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, H.E.A., El-fayoumy, E.A., Rasmy, W.E. et al. Two-stage cultivation of Chlorella vulgaris using light and salt stress conditions for simultaneous production of lipid, carotenoids, and antioxidants. J Appl Phycol 33, 227–239 (2021). https://doi.org/10.1007/s10811-020-02308-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02308-9