Abstract
Consumption of microalgae, as prey, by predatory zooplankton is a major ecological process in aquatic environments. The presence of predators in large-scale cultivation, such as in open ponds, results in a devastating loss of microalgal biomass, often referred to as a “pond crash.” Reported biomass losses of 20–30% due to predator invasion in open cultivation systems is one of the bottlenecks in achieving a desired economically viable system. Many commercial scale algal cultivation setups have reported clearance of prey within 2–5 days after detection of predators. Knowledge of how to monitor and manage algal pests is limited. Research to date is largely driven towards the development of predator mitigation strategies, whereas monitoring is mainly limited to traditional (direct) methods such as microscopy- and oligonucleotide-based screening. Use of online and real-time measures for in situ estimation of microalgal grazing is sparsely reported. We suggest that more knowledge about microalgal grazing at the pond level is required for the development of indirect screening measures, based on unique features of microalgal prey and predator interactions, to enable online monitoring. This article systematically reviews the current status of available methods, both at laboratory and field level, for early detection of microalgal grazing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae have emerged as one of the most desirable clean feedstocks due to their ability to produced versatile commodity products while adapting to climate change. Commercial microalgae cultivation is estimated to be capable of fixing 513 t of carbon dioxide and producing up to 120 t of dry biomass per hectare annually (Bilanovic et al. 2009). However, the production costs of microalgal biomass are at least fivefold higher than those of plant-based feedstock. The desirable yield of microalgal biomass for economically feasible cultivation in open ponds (for biofuels) has been estimated to be 25 g m−2 day−1 to be produced at the projected minimum biomass selling price (MBSP) 330–385 US$ per tonne (Davis et al. 2016). Both productivity and MBSP are, however, mainly dependent on the type of cultivation setup. Closed photobioreactors and open raceway ponds are widely used for large-scale microalgal cultivation. Closed photobioreactor cultivation favors higher productivity, albeit at higher cost (Borowitzka 1999), due to increased capital and operational expenditures (Ruiz et al. 2016). Open pond cultivation requires relatively lower capital investment and is estimated to be less productive as compared to closed systems. At present, an ideal platform for microalgae cultivation is a debatable topic (Ruiz et al. 2016). Choice of cultivation platform needs a careful evaluation considering the type of algal strain, cultivation conditions, geographic location and, importantly, the desired end product. Closed cultivation systems are mostly preferred for production of food supplements, nutraceuticals, and high-value chemicals. In contrast, open raceway pond cultivation is preferred for biofuel production, and several studies anticipate it to be one of the commercially viable ways to produce microalgal biomass in the range of the MBSP (Borowitzka and Moheimani 2013; Davis et al. 2016).
The cultivation conditions in open ponds are generally uncontrolled, subject to harsh environmental conditions, and prone to contamination by unwanted microbes (Lammers et al. 2017). The majority of reported contaminants to date are the consumers of microalgae, i.e., predators, interchangeably referred to as grazers. The invasion and ingestion of microalgae, the prey, by predators leads to biomass loss and as a result a sudden “culture crash” is inevitable. Overall microalgal biomass losses due to predation of 20–30 % have been reported for open cultivation setups (Richardson et al. 2014). Although closed systems are less prone to culture crash, predator invasion is unavoidable as decontamination of large volumes of raw and recycled water is proven to be difficult and further adds to the cost. Many pilot- and commercial-scale cultivation systems, mainly open ponds, are reported to be infested with fungi, viruses, zooplankton such as amoebae, ciliates, copepods, rotifers, and dinoflagellates, and bacterial predators.
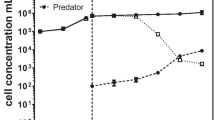
Currently, many pilot-scale commercial trials, mainly in open-raceway ponds, are underway as a collaborative effort between public and private partners. All of the open pond setups considered in this study (Table 1) have reported occasions when they have experienced a sudden culture crash due to infestation by predatory bacteria and zooplankton. For instance, a pilot-scale commercial raceway pond setup, the Algae Testbed Public-Private Partnership (ATP3), attempted to cultivate different species of microalgae across six different geographical sites. During 3 years of cultivation trials at ATP3 sites, 29 attempts out of 54 failed (~ 53%) due to predator invasion leading to the pond crash (McGowen et al. 2017; Knoshaug et al. 2018). Another pilot-scale commercial cultivation trial conducted for 6 years across different locations in the southwest of Gulf Coast USA as part of the National Alliance for Advance Biofuels and Bioproducts (NAABB) reported repetitive pond crashes due to predator invasion, notably by ciliates and rotifers (Lammers et al. 2017). The Arizona Center for Technology and Innovation, (AzCATI) reported a raceway pond crash in Chlorella cultivation and characterized the different taxonomic ranges of invaders, the majority of which were fungi, virus, flagellates, and ciliates (Wang et al. 2018). Reliance Industries Limited (RIL) India reported invasions by ciliates and dinoflagellates, that collapsed Chlorella vulgaris cultures cultivated in open raceway ponds within 2 and 4 days, respectively, after the first microscopic signs of the infection (Karuppasamy et al. 2018). Columbus Algal Biomass (CAB) farms, in New Mexico and Las Cruces Test Sites jointly operated by Sapphire Energy Inc and University of California reported infestation by Cryptomycota, a fungus-like parasite, in a Scenedesmus dimorphus culture. The Cryptomycota caused collapse of the culture 2 days after the initial symptoms of infection (McBride et al. 2014). Ganuza et al. (2016) reported collapse of four pilot commercial-scale open ponds (130,000 L) developed by Heliae Development, LLC with culture crash in less than 2 days after the first microscopic evidence of the presence of a pest which was later characterized as Vampirovibrio chlorellavorus, an obligate parasite (Park et al. 2019). These examples underline the severity of the contamination which quickly outnumbers the microalgal prey cell concentration, leaving a shorter timespan for decision making to implement any pest-deterrent treatments. Early detection of predators, before they get to levels at which they can impinge on productivity, and suitable mitigation strategies are required for cultivation of healthy algal crops. However, current research trends are more aimed at devising economical mitigation strategies rather than the development of early pest monitoring techniques. Although overlooked, early detection of grazers is of paramount importance for effective algal culture management to achieve the desired biomass productivity and avoid biomass loss due to microalgal predation. The present article systematically reviews currently reported grazer monitoring methods and associated challenges. It also focuses on the potential for on-site or in situ deployment of screening measures.
Susceptibility of open cultures to microalgal grazing
Contamination in mass-scale cultures of microalgae is one of the overlooked challenges which critically affects the sustainability and economics of the commercial cultivation. Although large scale cultivation of macroalgae (seaweeds) has been well established for centuries, mass cultivation of microalgae as a food and energy crop is a relatively new practice, about 50 years old (Borowitzka 2013), compared to the traditional farming of plant species wherein invasive weedy species and pathogens have been studied in detail (Ferrell and Sarisky-Reed 2010). Consequently, practical knowledge of contaminating microbes that infest microalgal cultures is very limited. The microalgal contaminants are primarily of two types, competitor pest species and obligate heterotrophic predators. Invaders often compete with the candidate microalgae for available nutrient resources and exist as cross-contaminant “pest species” (Smith et al. 2005). A few parasites co-exist inside microalgae and negatively affect the growth of their microalgal host. Such associations can be detrimental as their association is reported to alter the physiological behavior of microalgae. Parasite infection triggers cell clumping and further inhibits microalgal growth (Schroeder et al. 2003; Gutman et al. 2009). Fungal pathogens produce motile life stages and zoospores and can become established as parasites in microalgal cultures (Strittmatter et al. 2016; Ding et al. 2018). Chytrid species are reported to cause crashes of mass cultures of Scenedesmus (Fott 1967; Carney et al. 2014) and Haematococcus sp. (Gutman et al. 2009). However, the majority of the fungal parasites of microalgae are uncharacterized, and little is known about their mode of infection. In addition, cyst formation makes the infection persistent as it is resistant to the majority of disinfection methods during the dormant phase (Fott 1967). In contrast, the occurrence of viral infections leading to culture collapse at a commercial scale is relatively rare (Di Caprio 2020). However, phytoplankton mortality due to viral attack in the wild is extensively reported (Brussaard 2004).
As opposed to parasites, infestation with predator microbes is particularly devastating as predator species can quickly outcompete the microalgal prey (Day et al. 2012; Di Caprio 2020). Overall, 9 out of 10 contaminants are reported to be microalgal predators and pose a great threat to outdoor mass cultivation. Post et al. (1983) reported 14 different protozoa representatives of different genera that infested commercial-scale D. salina ponds. Grazing is observed to be prey species- and size-specific and, moreover, dependent on climatic conditions (Carney and Lane 2014; Day et al. 2017). Grazers of an average size < 1 mm are reported to be capable of clearing 1% of total microalgal cells every hour (Montagna 1995). At this rate, a non-dividing microalgal biomass can theoretically be completely cleared in just 4 days. In addition to the active prey ingestion grazer proliferation, increased abundance of predators (Flynn et al. 2017) can further reduce the time required for complete prey cell clearance. A recent review by Di Caprio (2020) ranks zooplankton invaders as a third highest contaminating microbe after bacteria and competitive cross-contaminating species. However, the sudden biomass loss caused by predation renders zooplankton a more imminent threat to commercial algal crops than other contaminating species. The most widely reported zooplankton predators in a commercial setting are ciliates, rotifers, copepods, amoeba, and dinoflagellates. To date, however, only a few predatory microalgal contaminants are well characterized. Moreover, their life cycle, mode of infection, genetic information, feeding behavior, and dynamics at commercial scale pond level remain largely unknown.
Infestation by zooplankton is a direct consequence of their trophic mode, heterotrophy, that is an outcome of evolution. The majority of the predators listed in Table 1 are heterotrophs and therefore must consume organic matter as sources of energy and carbon (Dagenais-Bellefeuille and Morse 2013). Sources of such organic matter include autotrophs such as microalgae. Once a suitable nutrient source in the form of microalgal prey is identified, the predator growth rate can exceed the prey doubling time (Hansen et al. 2000). An optimum ratio of C:N:P of 106:16:1, the Redfield ratio, is ideal for growth of microalgae; hence, the medium composition for mass cultivation is designed to closely match the Redfield ratio. However, the nutrient quality of microalgae cultivated by adhering to the ratio makes it an ideal prey for the predator (Sterner and Elser 2002; Flynn et al. 2017). As a result, predator species can quickly dominate the microalgal culture, leading to the crash.
The economic feasibility of microalgal mass cultivation demands a perfect balance between a year-round consistent biomass productivity at MBSP and overall cost input. Upscaling the volume of cultivation has emerged as one of the ways to achieve the desired biomass productivity. However, large input water handling makes mass cultures prone to contamination. Rogers et al. (2014) reported a water requirement of 1463 million L per day for a typical paddle wheel-driven, 2-acre raceway pond. Decontamination of large amounts of water on a daily basis is impractical and time consuming. Moreover, the step adds to increased biomass production cost. Although water filtration and hypochlorite treatments are cost effective ways to avoid contamination, the open and uncontrolled nature of the cultivation conditions makes raceway ponds more susceptible to contaminant infestation (Wang et al. 2013). Closed cultivation platforms, photobioreactors, are also prone to contamination that is probably transmitted due to the use of large volumes of recycled water or inefficient sterilization of air through membrane filters (Wang et al. 2013). Recycling of water after biomass harvesting, and an ensuing carry-over load of contaminants, is also a potential route of grazer transmission. Invaders are also likely to be present in the seed culture used as inoculum for open ponds, albeit at low concentration. At this stage, predator numbers may be only < 0.005% of total microalgal concentration and hence would remain undetected by routine microscopic inspection (Day et al. 2012; Flynn et al. 2017).
The seed cultures are generally cultivated under optimum and controlled conditions of parameters such as light, temperature, and nutrients. Therefore, an overall high growth rate of microalgae is favored. Microalgae in the outdoor setup, depending on time of the day and depth in the culture, encounter different phases of high and low light. Duration of intense light, peak sunlight, lasts up to 4–5 h in a typical day; otherwise, the culture is largely exposed to relatively low light (Richmond 2004). The availability of light, inevitably variable in high-volume cultures, is directly correlated to the amount of carbon fixation and the nutritional quality of microalga as prey. High light conditions combined with nutrient (P) limitation decouples carbon fixation from microalgal nutrient uptake. Reduced nutrient assimilation can thus lead to poor prey nutritional quality and thereby reduces grazing. In contrast, low light and low P/C conditions lead to increases in polyunsaturated fatty acids (PUFAs) content within microalgae. High PUFA levels are one of the important markers of the nutritional quality of microalgal prey for zooplankton, and predators preferentially consume prey cells with high PUFA content (Guo et al. 2016). Low light conditions, common to outdoor cultures, increase the nutritional value of the microalgal prey relative to its carbon content, thereby favoring grazing of microalgae (Urabe et al. 2002). Some predator species are known to form cysts, dormant forms, which germinate to produce viable predator progeny under suitable conditions (Post et al. 1983; Day et al. 2017). Shifts in outdoor environmental conditions (e.g., temperature, light) post seed culture inoculation favor the germination of cysts of some predator species. This leads to the vegetative growth of predator cells and overall increased predator concentration (Bravo and Figueroa 2014). Thus, changes in environmental conditions as in the case of outdoor cultures can prove to be favorable for predator proliferation, and hence increased grazing activity, as compared to laboratory conditions.
Richardson et al. (2014) reported a variability of 59% in biomass productivity in open as compared to closed cultivation. The variation in biomass productivity was mainly attributed to a culture crash frequency of 18.3% in the former. Sudden grazer outbreaks and the sheer scale of the outdoor setup make pond infestation almost unavoidable and potentially economically devastating. In practice, the rate of success of predator mitigation strategies is directly linked with the early detection of the contaminants. Early warning signs can further help to design effective dosing and length of the treatment and physical or chemical measures. Current practice for grazer screening measures relies on direct detection of predator cells. For example, microscopic counts or continuous flow cytometry involves detection of whole cells, whereas, oligonucleotide-based methods require the genomic material of contaminating microbes. As opposed to direct methods, many indirect grazer-screening measures are currently under development. Indirect grazer detection methods mainly rely on recording changes associated with prey and predator interactions. The following section provides in-depth information about reported grazer-monitoring methods, and their associated advantages and challenges (Table 2).
Monitoring methods
Direct methods
Microscopy
Microscope-assisted predator identification and enumeration are the most straightforward and cost-effective approaches. Typically, microscopic enumeration is done using hemocytometer or Sedgwick Rafter chambers which require 10–20 μL and 1000 μL samples respectively. Such small sample volumes are, however, not a good representation of the actual scale (1000–100,000 L) of the cultivation, and can lead to erroneous estimation, mostly under-representation, of predator load. Microscopic screening is unable to effectively detect algal predators, especially dinoflagellates and ciliates, at densities lower than < 103 cells mL−1 (Deore et al. 2020a). Furthermore, dense phytoplankton cultures (> 106 cells mL−1) limit grazer enumeration. Remedial dilution methods can probably lead to false negatives as under grazing conditions algal cells occasionally form clumps (leading to non-homogeneous suspensions) and highly diluted cultures can either under- or over-represent the actual predator load in instances where clumps are not properly disrupted prior to the dilution. Moreover, predators of larger body size, such as rotifers and copepods, cannot be detected using chambers such as improved Neubauer hemocytometers and Sedgwick Rafter cells which are commonly employed for microalgal counts. Predators at low concentration and of large size can be monitored using a sample fixed with Lugol’s iodine in which cells are allowed to settle in sedimentation chambers. However, this requires 10–12 h of incubation prior to the screening on an inverted microscope (Day et al. 2017). Centrifugation can be used for samples in which predators are very dilute, but runs the risk of affecting cell integrity, especially for fixed cells. Thus, centrifugation can impact the accuracy of the detection (Steedman 1976).
In addition to the technical limitations, microscopic observations are highly dependent on observer’s skill, experience, and bias for accurate estimation. A staining-based microscopy approach for contaminant detection has been developed to overcome observer bias. For example, Calcofluor White dye, which stains chitin present in fungal cell walls, is used for chytrid identification (Rasconi et al. 2009). However, the stain is non-specific and can also stain cellulose-rich cell walls of the microalga H. pluvialis, yielding false positive results (Damiani et al. 2006). SYTOX Green (Gerphagnon et al. 2013), Congo Red (Gachon et al. 2010), and Methylene Blue (Karuppasamy et al. 2018) have also been used to detect contamination.
The outlined microscopic methods are offline, time consuming, and subject to manual error. High dependence on manual microscopic counts limits the intervals of sampling for screening efforts. Detection of the signals from fluorescent dyes is often subject to errors due to interference from microalgal pigments. In commercial setups, the frequency of microscopic screening is reported to be once a day or three times a week (McGowen et al. 2017; Knoshaug et al. 2018). Such sampling regimes can fail to indicate early invasion and once spotted, significant biomass loss may have already occurred.
Continuous flow cytometer and in situ microscopy
In contrast to microscopic methods, continuous monitoring can be enabled by flow-through channels coupled with a camera recorder for particle (cells) analysis. Phytoplankton and zooplankton cells are classified and enumerated, typically involving a 50–100-mL sample volume, using cell shape, size, and fluorescence as identification criteria (Poulton and Martin 2010). Furthermore, optimization of flow rate (0.1–1 mL min−1) can enable screening of larger volumes of samples. Flow through designs can detect 1–10 grazers cells mL−1 (rotifers, ciliates, and dinoflagellates) in dense phytoplankton cultures (107 cells mL−1) of Nannochloropsis oculata (Day et al. 2012) and Chlorella sp. (Wang et al. 2017). Similarly, in situ microscopy (ISM) can be implemented as a means of online monitoring and enumeration of Chlorella and Chlamydomonas growth (Havlik et al. 2013). ISM demonstrates online and real-time monitoring potential of camera-assisted flow-through designs to detect microalgal predators. The overall operating cost of flow cytometry systems is estimated to be US$ 1–5 per sample (Poulton and Martin 2010).
The camera-based cell type classification and enumeration is based on pixel intensity (Deglint et al. 2018). Pixel processing is inherently limited in its computational ability to process overcrowded samples. Samples with higher cell concentrations and clumped cells tend to have heavy pixel load which contributes to poor identification of cell margins. Moreover, samples above 108 cells mL−1 tend to block the flow-through channel (Day et al. 2012). Furthermore, a requirement of laser-assisted microscopic cameras and the risk of flow-through channel blockage limit the implementation of flow cytometry in outdoor setups.
Various image processing algorithms combined with deep machine learning (ML) approaches are reported to improve classification and identification of microalgal species. A similar approach can be extended to enhanced automated identification of microalgal predators based on their unique morphological features such as cell shape, size, texture (regular vs. irregular or roundness vs. spiral), and density (Natchimuthu et al. 2013). Computer-assisted image processing combined with ML can further enable real-time microalgal pond diagnostics using remote sensing mobile platforms such as unmanned aerial vehicles (Samantaray et al. 2018).
Oligonucleotide markers
Advances in genomics have enabled the development of oligonucleotide-based markers, that are targeted towards nucleic acid signatures, as a means of early detection of contaminants. Polymerase chain reaction (PCR) using internal transcribed spacer (ITS), or 18S and 16S rRNA as markers, is the mostly commonly employed genomic approach for contaminant detection (Carney et al. 2016). High-resolution melting (HRM) analysis leverages differences in denaturation and melting point of nucleic acid complexes obtained from different contaminating microalgal or fungal species (Dawidziuk et al. 2017). However, further sequencing (Steichen 2016) or allele-specific fragmentation pattern analysis (Fulbright et al. 2014) is required for definitive identification of invaders. PCR-based markers provide limited information about the estimation of predator load and hence fail to serve as early warning signals. A semi-quantitative tool, qPCR, that detects the presence of contaminants in real time using a signature probe tagged with a fluorophore, is reported by McBride et al. (2014). Designing of the qPCR probe requires prior knowledge of a contaminant-specific unique nucleotide sequence for targeted detection. Although multiplex qPCR reaction is an option to detect a variety of species at once, its limited ability for estimation of uncharacterized and new invasive species, as might be expected in open ponds, is a major drawback of the technology. The techniques described above are also highly sophisticated and are limited to the laboratory use. Researchers at Sandia National Laboratory have developed a portable proof-of-concept device for the targeted identification of invasive species. The laboratory implements a two-step approach for contamination detection. First, identification of unknown pathogens is carried out using the second-generation sequencing and bioinformatics pipeline, RapTOR. Second, a FRET-based hybridization assay, SpinDx, is employed for capture and quantitation of predator load using signature molecular probes. The limit of contaminant detection by SpinDx is reported to be 1–10 cells for ciliates, chytrids, and rotifer species. The operating cost is < 2 US$ for 20 samples and portable device cost is < US$ 1000. The technological validation and implementation of this approach at the commercial raceway pond are ongoing (Lane et al. 2016). The overall success of the oligonucleotide marker-based detection is highly dependent on prior knowledge of genomic information pertaining to the contaminants. A constant probe-designing effort is required to increase the coverage of the detection. In addition, a highly skilled technician is required for reliable execution of the analysis. A recent review by Di Caprio (2020) provides a detailed explanation of the optimization, calibration requirements, and potential sources of bias for the direct measurement techniques outlined above.
Indirect methods
Spectral markers
As opposed to direct methods, outlined above, a number of indirect contamination monitoring methods have emerged in the last few years. One such method is hyperspectral reflectance-based monitoring of light backscatter, combined with numerical models. The main source of light backscatter in the case of microalgae ponds is the suspended cells. The method is based on differences in the reflectance pattern that arise due to variation in the reflective index, backscatter, of the different microalgal cell types (size and shape). Therefore, microalgal cells with different cell surface properties give rise to unique spectral features (Reichardt et al. 2014). Maes et al. (2018) reported variation in the spectral reflectance of Chlorella vulgaris culture infested with Poterioochromonas sp. and diatoms. Variation in the spectral feature at 708 nm was suggested to be associated with chlorophyll catabolism as a result of predation by Poterioochromonas sp. Similarly, changes in the photosynthetic pigments, specifically the chlorophyll to carotenoid ratio, of S. dimorphus infested with A. protococcarum was reported on the basis of hyperspectral confocal imaging by Collins et al. (2014). Multispectral imaging is also reported to serve as a non-invasive means of monitoring for cyanobacteria in Chlorella sp. cultures. The red and green color value contributed from cyanobacteria and Chlorella, respectively, acquired using an RGB (Red Green Blue) camera, is a relative indication of the respective species. The detection limit of pest invasion in terms of the concentration ratio of cyanobacteria to green algae was 0.08 (Murphy et al. 2013). However, most of the heterotrophic predators lack photosynthetic pigments, so immediate applications of technologies relying on pigment color discrimination is limited. Alternatively, Fourier transform infrared (FTIR)-based spectral markers can be leveraged to monitor the presence of predator.
FTIR in combination with chemometric methods has been used for screening of microalgal populations (Giordano et al. 2009), probing bio-macromolecular composition of a range of algal species (Kansiz et al. 1999), biomass (Sudhakar and Premalatha 2015), and lipid productivity estimation (Dean et al. 2012). However, the species discrimination potential of FTIR combined with chemometric approach has not been studied in relation to screening of contaminating microbes, including predators, in algal culture. In active grazing microalgal cultures, the nutrients ingested by predators are reallocated and repackaged into complex bio-macromolecules through predator-specific metabolic pathways. For example, phytosterol obtained from Dunaliella tertiolecta (Jeffrey 2011) acts as a precursor molecule for synthesis of cholesterol molecules by many zooplankton species (Mansour et al. 2002). Subtle changes in bio-macromolecular composition of pond culture as a result of grazer proliferation can be monitored using FTIR to track predator concentration. However, technical validation of the FTIR approach to measuring biotic contamination is required. IR-based technologies such as Near-IR are implemented for online monitoring of microalgal growth (optical density using 880 nm) and as a feedback control mechanism to maintain culture in turbidostat mode (Sandnes et al. 2006). Estimation of potential predator outbreak using IR probes coupled with an appropriate feedback control mechanism can help to develop an automated pest monitoring and management approaches.
Metabolic markers
Microalgae and zooplankton interactions involve communication using metabolic cues as means of prey attraction, grazer deterrence, and allelopathic signals. Reese et al. (2019) leveraged the elevated presence of volatile organic carbon (VOC) as an indirect marker of Brachionus pilicatilis predation in Microchloropsis salina cultures. Products of carotenoid oxidation contributed to elevated VOC and were identified as β-ionone and β-cyclocitral.
The indirect monitoring methods discussed above have a tremendous potential to be developed as an online monitoring tool. However, the spectral and metabolic features reported to-date are mainly due to the presence of the catabolic products of microalgal pigments. Chlorophyll degradation is prevalent under a variety of stress conditions and may not be an exclusive outcome of microalgal grazing. Moreover, the degradation products that arise after significant prey cell digestion by a predator are unlikely to be detected early on. As opposed to the degradation products, a metabolic prey cell response such as signals mounted against predator attack can serve as a better and early warning signal. Chlorophyll is central to the energy harvesting process, photosynthesis, of autotrophs. Therefore, changes in chlorophyll pigments are likely to affect the photosynthesis process. Tracking of chlorophyll fluorescence can provide insights into grazer-mediated alterations in photosynthetic process.
Photosynthesis-based markers
It is important to note that the majority of microalgal predators reported to infest commercial cultivation (Table 1) lack photosynthetic abilities, unlike their autotrophic prey. Therefore, monitoring photosynthetic parameters can unravel prey-specific photo-physiological signals under grazing pressure. Alterations in photo-physiological measures have been reported in the range of environmental conditions including grazing. Fundel et al. (1998) and Ratti et al. (2013) for instance reported a grazing-mediated modulation of photosynthetic pigments and process, respectively. In our previous work, we have reported a strong correlation between photosynthetic processes, particularly non-photochemical quenching (NPQ), of D. tertiolecta and ingestion rates of two predator species, Oxyrrhis marina and Euplotes sp. (Deore et al. 2020b). In laboratory conditions, NPQ levels were reported to drop by ~ 50 and 60% as compared to a control at least 1 and 2 days, respectively, prior to the culture crash. The reduction in NPQ levels was further linked to the accumulation of total ammonia-nitrogen (TAN) excreted by the predator. Ammonia is a known inhibitor of photosynthesis (Crofts 1967) and, at high concentration, can disrupt the pH gradient across the thylakoid membranes of chloroplasts. As a result, the buildup of protons on the lumenal side of the chloroplast is limited, which affects the overall electron flow through photosystem II. Moreover, the interaction of hydrogen ions with proteins is required for the formation of the quenching complex that assists in activation of NPQ (Müller et al. 2001). TAN-mediated perturbation in quenching complex formation is manifested as lowered NPQ levels, as observed by Deore et al. (2020b).
Other abiotic factors such as high light and heat can also alter NPQ and TAN levels, especially in outdoor conditions. NPQ levels are prone to high variation due to inherent fluctuation of light intensities in light:dark cycles. However, excreted TAN levels are likely to be underestimated during the day as higher ammonia uptake by green algae is observed during the light phase. Simultaneous measurement of TAN and NPQ is required to overcome respective challenges and provide reliable estimates of predator concentration (Deore et al. 2020b).
Di Caprio (2020) provides a detailed account of methodological aspects, based on cell concentration and biomass, for contamination detection in microalgal cultures regardless of species or type of cultivation setup. Furthermore, quantitative contamination detection techniques, such as ATP analysis, NanoSIMS, and single-cell elemental composition, are also discussed by Di Caprio (2020). Currently, these analytical techniques are limited to laboratory setups, and their applicability to field (outdoor ponds) is unproven. Our current review instead focusses on systematically presenting the potential of indirect methods which can be immediately extended to the field to enable on-site grazer monitoring. Overall, indirect markers can be easily recorded using currently available hand-held devices such as portable spectrophotometers. Operational cost per sample of such portable devices can be proven a relatively economical as compared to chemistry-based detection such as screening using oligonucleotide markers. Indirect methods outlined above can primarily implemented as first in-line tools for grazer monitoring in combination with direct methods which are to be treated as more reliable and confirmatory tests. Indirect markers are also potentially prone to interference from environmental factors. Therefore, sensitivity and robustness of such methods (see Table 2) require careful further evaluation.
Conclusions
Development of effective grazer monitoring measures is a twofold challenge. First, in-depth knowledge about predators of microalgal cultures is very limited. A lack of basic information regarding the proliferative, reproductive, and infection mechanisms employed by predators limits the scope of designing novel detection tools and intervention measures. In-depth understanding of changes associated with a unique microalgal prey predator process can enable development of novel indirect grazer-detection methods. The processes involved in predation that are largely the same across a range of microalgae and zooplankton are of particular importance as they can be developed as a universal marker of grazing. More work is required to identify and characterize universal processes of microalgal prey predator interactions at commercial pond level.
Furthermore, a technological advancement, ideally an automated real-time monitoring system with a high interval rate of sample screening, is required for early detection of predators. A continuous effort, in parallel to improved understanding of basic processes, is required in order to integrate suitable sensors to mass culture approaches. A relatively quick, straightforward, and easy to interpret measure of infestation is desirable so as to minimize detection time while maximizing culture exposure to pest deterrent agents. Online sensor integration would facilitate multiple online measurements, unlike currently practiced offline methods, in a short time-span, thereby increasing the likelihood of predator detection. In addition, several hand-devices such as portable FTIR and GC-MS are currently available that are used for monitoring algal growth. These devices could be further upgraded to capture predator-specific information. The detection of spectral markers can be easily up-scaled for large scale cultivation, using currently available imaging tools such as RGB cameras and remote sensing techniques for microalgal health monitoring purposes, including grazing. However, the relevance of high-throughput spectral data with the underlying biological information would require in depth knowledge of microalgal prey-predator interactions. In addition, profiling and in-detail characterization of extracellular cues, such as the stress responses of the prey against predator, involved in microalgal predation, can help to devise a quick point-of-care diagnostic test. Overall, an integrated grazer detection approach is required wherein currently employed methods are implemented in combination with markers suggested in this work. The relative success of agents, physical or chemical, used for grazer elimination would largely depend on the early warning potential of available monitoring tools. A collaborative research effort for quality control and quality assurance method development of microalgal pond diagnostics is urgently required for timely implementation of grazer mitigation strategies.
References
Bartley ML, Boeing WJ, Corcoran AA, Holguin FO, Schaub T (2013) Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenergy 54:83–88
Bilanovic D, Andargatchew A, Kroeger T, Shelef G (2009) Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations – response surface methodology analysis. Energy Convers Manag 50:262–267
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Borowitzka MA (2013) Energy from microalgae: a short history. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 1–15
Borowitzka MA, Moheimani NR (2013) Open pond culture systems. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 133–152
Bravo I, Figueroa R (2014) Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms 2:11–32
Brussaard CP (2004) Viral control of phytoplankton populations—a review. J Eukaryot Microbiol 51:125–138
Carney LT, Lane TW (2014) Parasites in algae mass culture. Front Microbiol 5:278–286
Carney LT, Reinsch SS, Lane PD, Solberg OD, Jansen LS, Williams KP, Trent JD, Lane TW (2014) Microbiome analysis of a microalgal mass culture growing in municipal wastewater in a prototype OMEGA photobioreactor. Algal Res 4:52–61
Carney LT, Wilkenfeld JS, Lane PD, Solberg OD, Fuqua ZB, Cornelius NG, Gillespie S, Williams KP, Samocha TM, Lane TW (2016) Pond crash forensics: presumptive identification of pond crash agents by next generation sequencing in replicate raceway mass cultures of Nannochloropsis salina. Algal Res 17:341–347
Collins AM, Jones HD, McBride RC, Behnke C, Timlin JA (2014) Host cell pigmentation in Scenedesmus dimorphus as a beacon for nascent parasite infection. Biotechnol Bioeng 111:1748–1757
Crofts AR (1967) Amine uncoupling of energy transfer in chloroplasts. I. Relation to ammonium ion uptake. J Biol Chem 242:3352–3359
Dagenais-Bellefeuille S, Morse D (2013) Putting the N in dinoflagellates. Front Microbiol 4:369–683
Damiani MC, Leonardi PI, Pieroni OI, Cáceres EJ (2006) Ultrastructure of the cyst wall of Haematococcus pluvialis (Chlorophyceae): wall development and behaviour during cyst germination. Phycologia 45:616–623
Davis R, Markham J, Kinchin C, Grundl N, Tan EC, Humbird D (2016) Process design and economics for the production of algal biomass: algal biomass production in open pond systems and processing through dewatering for downstream conversion. Laboratory NREL, Golden, Colorado. NREL/TP-5100-64772 pp 1–112
Dawidziuk A, Popiel D, Luboinska M, Grzebyk M, Wisniewski M, Koczyk G (2017) Assessing contamination of microalgal astaxanthin producer Haematococcus cultures with high-resolution melting curve analysis. Microb Genet 58:277–285
Day JG, Thomas NJ, Achilles-Day UE, Leakey RJ (2012) Early detection of protozoan grazers in algal biofuel cultures. Bioresour Technol 114:715–719
Day JG, Gong Y, Hu Q (2017) Microzooplanktonic grazers – a potentially devastating threat to the commercial success of microalgal mass culture. Algal Res 27:356–365
Dean AP, Nicholson JM, Sigee DC (2012) Changing patterns of carbon allocation in lake phytoplankton: an FTIR analysis. Hydrobiologia 684:109–127
Deglint JL, Jin C, Wong A (2018) Investigating the automatic classification of algae using fusion of spectral and morphological characteristics of algae via deep residual learning. https://arxiv.org/abs/1810.10889
Deore P, Beardall J, Noronha S (2020a) Non-photochemical quenching, a non-invasive probe for monitoring microalgal grazing: influence of grazing-mediated total ammonia-nitrogen. Appl Phycol 1:32–43
Deore P, Karthikaichamy A, Beardall J, Noronha S (2020b) Non-photochemical quenching, a non-invasive probe for monitoring microalgal grazing: an early indicator of predation by Oxyrrhis marina and Euplotes sp. Appl Phycol 1:20–31
Di Caprio F (2020) Methods to quantify biological contaminants in microalgae cultures. Algal Res 49:101943
Ding Y, Peng X, Wang Z, Wen X, Geng Y, Zhang D, Li Y (2018) Occurrence and characterization of an epibiotic parasite in cultures of oleaginous microalga Graesiella sp. WBG-1. J Appl Phycol 30:819–830
Ferrell J, Sarisky-Reed V (2010) National algal biofuels technology roadmap. U.S. Department of Energy Biomass program, Maryland, USA. DOE/EE-03324329 pp 1–124.
Flynn KJ, Kenny P, Mitra A (2017) Minimising losses to predation during microalgae cultivation. J Appl Phycol 29:1829–1840
Fott B (1967) Phlyctidium scenedesmi spec. nova, a new chytrid destroying mass cultures of algae. Z Allgem Mikrobiol 7:97–102
Fulbright SP, Dean MK, Wardle G, Lammers PJ, Chisholm S (2014) Molecular diagnostics for monitoring contaminants in algal cultivation. Algal Res 4:41–51
Fundel B, Stich H, Schmid H, Maier G (1998) Can phaeopigments be used as markers for Daphnia grazing in Lake Constance. J Plankton Res 20:1449–1462
Gachon CM, Sime-Ngando T, Strittmatter M, Chambouvet A, Kim GH (2010) Algal diseases: spotlight on a black box. Trends Plant Sci 15:633–640
Ganuza E, Sellers CE, Bennett BW, Lyons EM, Carney LT (2016) A novel treatment protects Chlorella at commercial scale from the predatory bacterium Vampirovibrio chlorellavorus. Front Microbiol 7:848–861
Gerphagnon M, Latour D, Colombet J, Sime-Ngando T (2013) A double staining method using SYTOX green and calcofluor white for studying fungal parasites of phytoplankton. Appl Environ Microbiol 79:3943–3951
Giordano M, Ratti S, Domenighini A, Vogt F (2009) Spectroscopic classification of 14 different microalga species: first steps towards spectroscopic measurement of phytoplankton biodiversity. Plant Ecol Divers 2:155–164
Guo F, Kainz M, Sheldon F, Bunn S (2016) The importance of high-quality algal food sources in stream food webs - current status and future perspectives. Freshw Biol 61:815–831
Gutman J, Zarka A, Boussiba S (2009) The host-range of Paraphysoderma sedebokerensis, a chytrid that infects Haematococcus pluvialis. Eur J Phycol 44:509–514
Hansen PJ, BjØrnsen PK, Hansen BW (2000) Zooplankton grazing and growth: scaling within the 2–2,000-μm body size range. Limnol Oceanogr 45:1891–1891
Havlik I, Reardon KF, Ünal M, Lindner P, Prediger A, Babitzky A, Beutel S, Scheper T (2013) Monitoring of microalgal cultivations with on-line, flow-through microscopy. Algal Res 2:253–257
Jeffrey LCW (2011) The occurrence of ergosterol and (22E,24R)-24-ethylcholesta-5,7,22-trien-3β-ol in the unicellular chlorophyte Dunaliella tertiolecta. Can J Chem 57:2569–2571
Kansiz M, Heraud P, Wood B, Burden F, Beardall J, McNaughton D (1999) Fourier transform infrared microspectroscopy and chemometrics as a tool for the discrimination of cyanobacterial strains. Phytochemistry 52:407–417
Karuppasamy S, Musale AS, Soni B, Bhadra B, Gujarathi N, Sundaram M, Sapre A, Dasgupta S, Kumar C (2018) Integrated grazer management mediated by chemicals for sustainable cultivation of algae in open ponds. Algal Res 35:439–448
Knoshaug EP, Wolfrum E, Laurens LM, Harmon VL, Dempster TA, McGowen J (2018) Unified field studies of the algae testbed public-private partnership as the benchmark for algae agronomics. Sci Data 5:180267–180277
Lammers PJ, Huesemann M, Boeing W, Anderson DB, Arnold RG, Bai X, Bhole M, Brhanavan Y, Brown L, Brown J (2017) Review of the cultivation program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res 22:166–186
Lane T, Poorey K, Geng H, Curtis DJ, Carney LT (2016) Algal crop protection strategies and technologies. Sandia National Laboratory, Livermore, United States. SAND2016-8236C
Ma M, Yuan D, He Y, Park M, Gong Y, Hu Q (2017) Effective control of Poterioochromonas malhamensis in pilot-scale culture of Chlorella sorokiniana GT-1 by maintaining CO2-mediated low culture pH. Algal Res 26:436–444
Ma M, Gong Y, Hu Q (2018) Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res 29:142–153
Maes D, Reichardt TA, Jensen TJ, Dempster TA, McGowen JA, Poorey K, Hipple T, Lane T, Timlin JA (2018) Spectroradiometric detection of competitors and predators in algal ponds. Sandia National Laboratory, Albuquerque,United States. SAND2018-6164C
Mansour MP, Volkman JK, Jackson AE, Blackburn SI (2002) The fatty acid and sterol composition of five marine dinoflagellates. J Phycol 35:710–720
McBride RC, Lopez S, Meenach S, Burnett M, Lee PA, Nohilly F, Behnke C (2014) Contamination management in low cost open algae ponds for biofuels production. Ind Biotechnol 10:221–227
McGowen J, Knoshaug EP, Laurens LM, Dempster TA, Pienkos PT, Wolfrum E, Harmon VL (2017) The Algae Testbed Public-Private Partnership (ATP3) framework; establishment of a national network of testbed sites to support sustainable algae production. Algal Res 25:168–177
Montagna PA (1995) Rates of metazoan meiofaunal microbivory: a review. Vie et Milieu 45:1–10
Moreno-Garrido I, Cañavate JP (2001) Assessing chemical compounds for controlling predator ciliates in outdoor mass cultures of the green algae Dunaliella salina. Aquac Eng 24:107–114
Müller P, Li X, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Murphy TE, Macon K, Berberoglu H (2013) Rapid algal culture diagnostics for open ponds using multispectral image analysis. Biotechnol Prog 30:233–240
Natchimuthu S, Chinnaraj P, Parthasarathy S, Senthil K (2013) Automatic identification of algal community from microscopic images. Bioinf Biol Insights 7:327–334
Park S-H, Steichen SA, Li X, Ogden K, Brown JK (2019) Association of Vampirovibrio chlorellavorus with decline and death of Chlorella sorokiniana in outdoor reactors. J Appl Phycol 31:1131–1142
Post FJ, Borowitzka LJ, Borowitzka MA, Mackay B, Moulton T (1983) The protozoa of a Western Australian hypersaline lagoon. Hydrobiologia 105:95–113
Poulton NJ, Martin JL (2010) Imaging flow cytometry for quantitative phytoplankton analysis-FlowCAM. In: Karlson B, Cusack C, Bresnan E (eds) Microscopic and molecular methods for quantitative phytoplankton analysis. UNESCO, Paris, pp 47–53
Rasconi S, Jobard M, Jouve L, Sime-Ngando T (2009) Use of calcofluor white for detection, identification, and quantification of phytoplanktonic fungal parasites. Appl Environ Microbiol 75:2545–2553
Ratti S, Knoll AH, Giordano M (2013) Grazers and phytoplankton growth in the oceans: an experimental and evolutionary perspective. PLoS One 8:e77349
Reese KL, Fisher CL, Lane PD, Jaryenneh JD, Moorman MW, Jones AD, Frank M, Lane TW (2019) Chemical profiling of volatile organic compounds in the headspace of algal cultures as early biomarkers of algal pond crashes. Sci Rep 9:1–10
Reichardt TA, Collins AM, McBride RC, Behnke CA, Timlin JA (2014) Spectroradiometric monitoring for open outdoor culturing of algae and cyanobacteria. Appl Opt 53:31–45
Reichardt TA, Maes D, Jensen T, Dempster TA, McGowen JA, Poorey K, Curtis DJ, Lane TW, Timlin J (2020) Spectroradiometric monitoring of competitor diatoms and the grazer Poteriochromonas in algal cultures. Algal Res 51:102020
Richardson JW, Johnson MD, Zhang X, Zemke P, Chen W, Hu Q (2014) A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res 4:96–104
Richmond A (ed) (2004) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Science Ltd, Oxford
Rogers JN, Rosenberg JN, Guzman BJ, Oh VH, Mimbela LE, Ghassemi A, Betenbaugh MJ, Oyler GA, Donohue MD (2014) A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res 4:76–88
Ruiz J, Olivieri G, de Vree J, Bosma R, Willems P, Reith J, Eppink M, Kleinegris D, Wijffels R, Barbosa M (2016) Towards industrial products from microalgae. Energy Environ Sci 9:3036–3043
Samantaray A, Yang B, Dietz JE, Min BC (2018) Algae detection using computer vision and deep learning. https://arxiv.org/abs/1811.10847
Sandnes JM, Ringstad T, Wenner D, Heyerdahl PH, Kallqvist T, Gislerød HR (2006) Real-time monitoring and automatic density control of large-scale microalgal cultures using near infrared (NIR) optical density sensors. J Biotechnol 122:209–215
Schroeder DC, Oke J, Hall M, Malin G, Wilson WH (2003) Virus succession observed during an Emiliania huxleyi bloom. Appl Environ Microbiol 69:2484–2490
Smith VH, Foster BL, Grover JP, Holt RD, Leibold MA, deNoyelles F (2005) Phytoplankton species richness scales consistently from laboratory microcosms to the world's oceans. Proc Natl Acad Sci 102:4393–4396
Steedman HF (ed) (1976) Zooplankton fixation and preservation. UNESCO, Paris
Steichen S (2016) Tracking an algal predator: monitoring the dynamics of Vampirovibrio chlorellavorus in outdoor culture. MSc Thesis, The University of Arizona, USA 66 pp
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, United States
Strittmatter M, Guerra T, Silva J, Gachon CMM (2016) A new flagellated dispersion stage in Paraphysoderma sedebokerense, a pathogen of Haematococcus pluvialis. J Appl Phycol 28:1553–1558
Sudhakar K, Premalatha M (2015) Characterization of micro Aalgal biomass through FTIR/TGA /CHN Aanalysis: application to Scenedesmus sp. Energy Sources A 37:2330–2337
Urabe J, Kyle M, Makino W, Yoshida T, Andersen T, Elser JJ (2002) Reduced light increases herbivore production due to stoichiometric effects of light/nutrient balance. Ecology 83:619–627
Wang H, Zhang W, Chen L, Wang J, Liu T (2013) The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour Technol 128:745–750
Wang Y, Castillo-Keller M, Eustance E, Sommerfeld M (2017) Early detection and quantification of zooplankton grazers in algal cultures by FlowCAM. Algal Res 21:98–102
Wang Y, Gong Y, Dai L, Sommerfeld M, Zhang C, Hu Q (2018) Identification of harmful protozoa in outdoor cultivation of Chlorella and the use of ultrasonication to control contamination. Algal Res 31:298–310
Funding
Research by the authors was funded by Reliance Industries Limited, Mumbai, India and IITB-Monash Research Academy, Mumbai, India. (Fund code–IMURA0303)
Author information
Authors and Affiliations
Contributions
Pranali Deore conceptualized and prepared manuscript. John Berdall corrected, improved, and critically reviewed the manuscript. Santosh Noronha critically reviewed and corrected the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deore, P., Beardall, J. & Noronha, S. A perspective on the current status of approaches for early detection of microalgal grazing. J Appl Phycol 32, 3723–3733 (2020). https://doi.org/10.1007/s10811-020-02241-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02241-x