Abstract
In this study we report for the first time the appearance of an endophytic filamentous red alga, living associated with the edible red seaweed Chondracanthus chamissoi. Molecular identification, growth, and reproduction of this endophyte are described under different conditions: photoperiod (8:16, 12:12, and 16:08 L:D), temperature (10 and 15 °C), and photon flux density (40 and 20 μmol photons m−2 s−1). Filaments of this endophyte were isolated and cultured, and the growth was recorded according to the number of branches and its reproduction, by quantifying the emergence of monosporangia. By sequencing the COI gene and through phylogeny reconstruction using maximum likelihood, we determined that this endophyte corresponded to Colaconema daviesii. The highest growth was recorded under the treatment 16:08 (L:D), 10 °C, and 20 μmol photons m−2 s−1, reaching up to 100 branches after 18 days. On the other hand, over 80% of filaments with monosporangia were observed at 12:12 (L:D), 10 °C, and 20 μmol photons m−2 s−1. This is the first record of C. daviesii on the South-eastern Pacific as an endophyte on thalli of C. chamissoi. The results of the in vitro cultures showed that once C. daviesii is isolated, the filaments are able to grow and reproduce independently of C. chamissoi. This suggests that there may not be a strict relationship between both algae and reveals the possibility of finding C. daviesii living without a host or associated with other species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chondracanthus chamissoi (C. Agardh) Kützing is an alga in the order Gigartinales which is distributed on the coasts of Perú and Chile from 5 to 42° S, as well as in France, Korea, and Japan (Yang et al. 2015; Ramírez et al. 2018). It is exploited for extraction and commercialization of carrageenans and its biomass has also been intended for human consumption during the last 10 years (Bulboa et al. 2013), with prices that fluctuate between 23 and 32 USD$ per kg (dry weight) of raw material (Aduana 2018). Although currently C. chamissoi is not cultivated for commercial use, several studies have been carried out to develop cultivation techniques (Bulboa et al. 2005, 2013; Bulboa and Macchiavello 2006; Macchiavello et al. 2018), all of which have demonstrated the feasibility of its production, mainly through propagation strategies based on vegetative fragmentation (Bulboa et al. 2013; Hayashi et al. 2014; Macchiavello et al. 2018).
One of the most distinguishing attributes of C. chamissoi is its high morphological variability, which includes compressed or flattened thalli with axes repeatedly pinnately branched or foliaceous, often covered with papillae (Ramírez et al. 2018). This is relevant since the market for human consumption requires certain morphological traits, such as small, thin, branched, smooth surfaced thalli, free of papillae, epiphytes, and reproductive structures (Bulboa et al. 2005; Macchiavello et al. 2018). The thalli of C. chamissoi found on the coast of Chile near the town of Tumbes (36° S) presents the aforementioned desirable characteristics and has been cultivated in our laboratory for growth tests. The higher growths of these (6.8% d−1) have been achieved under long-day photoperiod conditions (16:08 light: dark), when maintained at 15 °C, with a photon flux density of 60 μmol photons m−2 s−1 (López 2017). Under these conditions, an unknown red alga of the endophyte type appeared in abundance, characterized by simple filaments and several ramifications. These ramifications grew rapidly from within the thalli of C. chamissoi, covering its surface and generating a loss of turgidity and necrosis in the middle and apical portions of the thalli. On the contrary, intermediate and short-day photoperiods, as well as conditions of low light, do not seem to favor the proliferation of this filamentous alga (López 2017).
Although C. chamissoi has the morphological characteristics required by the market for human consumption (Bulboa et al. 2010), the presence of epiphytes or endophytes is a problem for its commercialization because they reduce thallus quality by altering the appearance as well as the health condition. Indeed, infections from epiphytes as well as endophytes constitute a serious threat to seaweeds health, causing thallus malformations resulting in severe morphological alterations, injuries, and cellular damage (Correa et al. 1997; Kim et al. 2014). These negative effects are not only morphological but can also affect the fitness of the alga host affecting survival, growth, and fertility (Kim et al. 2014; Ogandaga et al. 2016), and in some cases could even destroy natural seaweed populations (Parker and Chapman 1994; Faugeron et al. 2000; Schoenrock et al. 2013). Direct negative effects of epiphytes and endophytes on biomass production and the quality of phycocolloids have been reported in commercially grown algae (Araújo et al. 2014; Vairappan et al. 2014) such as reducing their commercial value by diminishing both the yield and quality of cultivated macroalgal species (Schoenrock et al. 2013).
Previous reports show that in temperate environments, the presence of epiphytes or endophytes is markedly seasonal and it is associated with changes in environmental factors such as temperature and light, being the warmest seasons the periods when they reach greater abundance, as a result of an increase in growth and reproduction (Buschmann and Gómez 1993; Vásquez and Vega 2001). In regards of C. chamissoi, the greatest abundance and diversity of epibionts have been reported during spring and summer (Vásquez and Vega 2001; Macchiavello et al. 2018). Throughout its distribution, several epiphytic algae have been documented on C. chamissoi thalli (e.g., Vásquez and Vega 2001; Ávila et al. 2011; Bulboa et al. 2013; Macchiavello et al. 2018); however, none of them matches the red algae found previously by López (2017). Therefore, the aims of the present study were to (i) genetically identify the endophyte found in the thalli of C. chamissoi by means of DNA sequencing and phylogenetic analyses, and (ii) to determine in vitro the effects of different abiotic conditions on this endophyte.
Materials and methods
The thalli of Chondracanthus chamissoi (Fig. 1a) were obtained from Caleta Tumbes in the Region of Biobio (73° 05′ 15“ W, 36° 36’ 45” S) in April 2017. Apical tips (3 cm length) were cut and cultivated in 500 mL of sterile seawater under controlled conditions: 16:08 (light:dark), 40 ± 10 μmol photons m−2 s−1, and 15 ± 1 °C, as previously described for this species by Bulboa et al. (2008). After 2 weeks, red spots on the thallus surface were observed, and then the filaments of an endophytic algae covered the surface of C. chamissoi. Cross-sections showed that the filaments grew from the medullary region of the C. chamissoi thallus (Fig. 1b). The endophyte (2 to 5 filaments) was separated from the thalli manually with a surgical scalpel under a light stereoscope (Motic SMZ-143-N2LED). The filaments were washed with sterile sea water and then transferred into Erlenmeyer flasks containing 250 mL of filtered seawater (mesh size = 1 μm), enriched with Von Stosch’s culture medium (8 mL L−1), and maintained under the same conditions as before with weekly medium replacement. These cultures have been maintained for 2 years and provided all the biological material for the experiments.
Molecular identification
For molecular analyses, the DNA was extracted from the filaments using the protocol developed by Saunders (1993) and modified by Faugeron et al. (2001). The mitochondrial cytochrome c oxidase subunit I gene (COI) was amplified by polymerase chain reaction (PCR) using the primers GazF1 (5′-124 TCA ACA AAT CAT AAA GAT ATT GG-3′) and GazR1 (5′-ACT TCT GGA TGT CCA125 AAA AAY CA-3′) and following the Saunders (2005) amplification protocols. Amplifications were done in a GenAmp 9700 thermocycler (Perkin-Elmer, USA) with an initial denaturation step of 96 °C for 4 min, followed by 35 cycles of 1 min at 45 °C (annealing), 1.5 min at 72 °C (extension), and a final extension period of 8 min at 72 °C. PCR products were purified and sequenced using primers GazF1 and GazR1 on an ABI PRISMR 3100 Automated DNA Sequencer (Applied Biosystems, USA). The sequences were uploaded to GenBank.
Once the DNA sequence of the COI gene from the unknown endophyte was obtained, other sequences were retrieved from GenBank: from species of the Colaconematales (used as ingroup) and from the orders Nemaliales, Entwisleiales, Palmariales, Acrochaetiales, and Thoreales (as outgroup) (complete list of sequences retrieved are in Table S1, Supporting Information). The selection of outgroups was according to the multigene phylogeny by Lam et al. (2016). Multiple sequence alignment of COI sequences was performed using BioEdit v 7.2.5 (Hall 1999) with 1000 bootstrap replicates. Sequence divergences between all species employed, and within each order, were calculated using Mega v 5 (Tamura et al. 2011). A maximum likelihood (ML) phylogenetic tree reconstruction was performed using the software IQ-TREE (Trifinopoulos et al. 2016). The best model of evolution was selected using the Akaike Information Criterion (AIC) implemented in IQ-TREE. The selected model for the COI sequences was TIM2 + F + I + G4. Statistical support was estimated using 1.000 ultrafast bootstrap replicates (Nguyen et al. 2015).
Development and reproduction
To evaluate the growth and reproduction of the endophytic red alga, samples of filaments were taken from the cultures as previously described. Groups of filaments were fragmented using a hand blender, then filaments bearing few cells (3 to 5 cells), and without ramifications or reproductive cells were selected under an inverted light microscope (Nikon eclipse Ts2) and cultivated in Petri dishes with 10 mL of seawater enriched with Von Stosch’s solution. Fifteen filaments were placed in each Petri dish and exposed to combined treatments of photoperiod (8:16, 12:12 and 16:08 (L:D)), photon flux density (PFD 20 and 40 μmol photons m−2 s−1), and temperature (10 °C and 15 °C). Six independent replicates were conducted for each treatment. The experiments were carried out for 18 days, with seawater and culture medium renewed once a week. To evaluate filaments growth, the number of total branches for each filament was measured at the end of the experiments. This was used a proxy to assess growth since preliminary results showed different ramification degree using different culture conditions. Moreover, usual measurements such as weight or area could not be taken due to the small size and mass of the filaments.
To evaluate the effects of abiotic factors on reproduction performance, on each filament, the number of filaments bearing monosporangia was counted after 18 days; results were expressed as reproduction performance, measured as percentage of filaments bearing monosporangia from the total observed. All measurements were done under an inverted light microscope (Nikon eclipse Ts2).
Data analysis
The number of branches and reproduction performance were analyzed separately, using a multifactorial ANOVA to evaluate the effects of photoperiod, PFD, and temperature. Prior to the analyses, the data was tested for normality and homoscedasticity. More specific differences were then examined using Tukey’s post hoc test. The statistical analyses were performed using the R software (R core team 2019), with the ggplot2 package (Wickham 2016) for plotting the results on a Blockplot chart.
Results
The phylogenetic reconstruction using the maximum likelihood method showed that the unknown endophyte COI sequence nested within Colaconematales, which formed a monophyletic clade with a bootstrap value of 94%. More importantly, within this order, the COI sequence of our endophyte was grouped in the same clade together with specimens of the species Colaconema daviesii with strong node support (100%) (Fig. 2). This was then corroborated comparing for homologous COI sequences to our own using the NCBI BLAST tool, founding a 99.8% of similarity with the species C. daviesii (see Table S1 at supplementary material for details). Moreover, the pairwise sequence divergence between our COI sequence of Colaconema and other two C. daviesii sequences (GenBank accession numbers KT886153, and KF280895) was more than one order of magnitude lower (0.2–0.5%) in comparison to the within sequence divergence in the Colaconematales (17%) or in comparison to sequence divergences within the other orders of the Nemaliphycidae (13–24%) (see Table S2 and S3 at supplementary material for details). This intraspecific divergence within C. daviesii (0.2–0.5) is well below the barcoding gap (2–14%) for the COI in red algae (e.g., Robba et al. 2006; Sherwood 2008) and strongly suggests that our specimen corresponds to the species C. daviesii. A value of 0.5% of divergence between COI sequences is within the ranges observed for intraspecific differences in red algae (e.g., 0.66% average intraspecific variation in Gracilaria salicornia, in Yang et al. 2013); in fact, the two haplotypes of Colaconema proskaueri (KF364496 and KC130155) also showed this divergence value (Table S2) and correspond to the same species. Remarkably, the sequence divergence between our COI sequence and one haplotypes of C. daviesii from Canada (KT886153) was lower (0.3%) than compared with the second COI haplotype from Canada (KF280895) (0.5%), suggesting the existence of gene flow even between distant populations of this species. Nonetheless, this should be interpreted with caution due to the small sampling size, and the few nucleotide bases considered herein (664 bp). It is worth mentioning that in our phylogenetic reconstruction using COI, the topology at the generic and species-level resembled the results in Lam et al. (2016); nonetheless, higher taxonomic relationships were not defined properly. This is probably because the COI gene is well suited to define relationships between closely related species, but not higher levels of taxonomic relationships. Then, at higher taxonomic levels, our phylogenetic reconstruction is probably showing genetic saturation and long branch attraction; additional genes showing slower accumulation of mutations should be employed or using longer sequences (only 664 bp, in our study), as has been done previously (e.g., Scott et al. 2013; and Lam et al. 2016).
Maximum likelihood tree of the Colaconematales (ingroup), based on the alignment of DNA sequences (664 bp long) of the cytochrome oxidase1 gene (COI), and the Nemaliales, Entwisleilales, Palmariales, Acrochaetiales, and Thoreales orders (outgroups). For each node, ML bootstrap values are indicated. Only high support values (> 70) are shown. Box = Sequence generated in this study
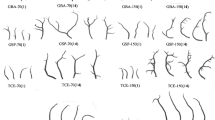
In all experimental treatments, uniseriate filaments of simple cylindrical cells were developed, showing abundant ramifications (Fig. 3a, b). In the terminal portion of the filaments, a single monosporangium was developed (Fig. 3c). At the end of the experimental period, under all conditions, were observed monospores germinating into new filaments (Fig. 3d).
a Fragments of filaments of Colaconema daviesii, with few cells and few ramifications that were used to initiate experiments (Bar = 5 μm). b uniseriate filaments of C. daviesii of simple cylindrical cells with abundant ramifications in their distal portions (Bar = 5 μm). c Apical portions of C. daviesii filaments containing monosporangia (black arrow) type (Bar = 5 μm). d Monospores germinated (Bar = 5 μm)
Photoperiod and PFD factors showed a statistically significant influence in the number of bhanches generated by C. daviesii (p < 0.05; F = 15.77). However, a third-order interaction was also recorded (p = 0.007; F = 5.34) between the three factors studied; therefore, the results cannot be explained as each causing an isolated effect (Table 1). More specifically, the Tukey’s post hoc test showed that the different combinations of photoperiod, temperature, and photon flux density were separated into three clusters (a, b, and c) (Fig. 4) regarding the number of ramifications produced. The highest number of branches (140 ± 34) was observed for cluster c, corresponding to the combination of photoperiod 16:08 (L: D), temperature 10 °C, and PFD of 20 μmol photons m−2 s−1. On the contrary, cluster a, corresponding to the combination of photoperiod treatment 12:12 (L: D), temperature of 10 °C, and PFD of 20 μmol photons m−2 s−1, was the one with lower development of C. daviesii with 23 ± 13 branches (Fig. 4). The remaining treatment combinations were grouped under cluster b and produced an intermediate number of ramifications (Fig. 4).
Effect of photoperiod (8:16, 12:12, 16:8 L:D), temperature (10 and 15 °C), and PFD (20 and 40 μmol photons m−2 s−1) on growth (as an increase of branches number) of Colaconema daviesii. Values are median and 90% quartiles shown, n = 6 per treatment combination. Letters show significant differences (Tukey’s post hoc test) among treatments combination (p < 0.05)
In all treatment combinations, the appearance of monosporangia in C. daviesii filaments was observed. The photoperiod factor showed a statistically significant influence (p = 0.004; F = 6.06) on the development of monosporangia in C. daviesii. However, the second-order interaction between photoperiod and PFD (Table 1) was also significant (p = 0.013 F = 4.61). The Tukey’s post hoc test showed that the different combinations of photoperiod, temperature, and photon flux density were separated into four clusters (a, b, ab, and c) (Fig. 5). Cluster a, corresponding to the combination of 12:12 (L:D), 10 °C, and 20 μmol photons m−2 s−1, was the one showing the highest percentage of filaments presenting monosporangia (64 ± 23%), whereas cluster c, corresponding to the experimental treatment 16:08 (L:D), 10 °C, and 20 μmol photons m−2 s−1, showed the lowest percentage of filaments bearing monosporangia (3 ± 1%) (Fig. 5). The remaining treatment combinations were grouped under cluster b (or ab) and produced an intermediate percentage of filaments with monosporangia (Fig. 5).
Effect of photoperiod (8:16, 12:12, 16:8 L:D), temperature (10 and 15 °C), and PFD (20 and 40 μmol photons m−2 s−1) on the formation of reproductive structures (%) on filaments of Colaconema daviesii. Values are median and 90% quartiles shown, n = 6 per treatment combination. Letters show significant differences (Tukey’s post hoc test) among treatments combination (p < 0.05)
Discussion
The members of the order Colaconematales have been characterized as epiphytes or endophytes, and they are exclusively marine species (Harper and Saunders 2002). In the present study, the molecular identification using the COI gene showed a strong bootstrap support, confirming that the pigmented endophyte found in thalli of C. chamissoi belongs to Colaconema daviesii (Dillwyn) Stegenga. Other studies using molecular approach have allowed before the identification of other species of this genus, such as the case of C. infestans, found in thalli of the commercial algae Kappaphycus alvarezii, cultured in Brazil (Araújo et al. 2014), but the genus so far had not been found in the southeast Pacific. Consequently, our study represents the first report of the C. daviesii species as an endophyte in the commercial algae C. chamissoi in thalli from the southeastern Pacific.
Colaconema daviesii has been found on the coast of Canada (Harper and Saunders 2002) and occasionally has been documented associated with algae as Colpomenia sinuosa (Poza et al. 2017), the seagrass Zostera marina (Garcia et al. 2017), and living as epibiont on the barnacle Lepas anatifera (Hansen et al. 2017); nonetheless, so far, the relationship with their hots has not yet been elucidated. The evidence suggests that C. daviesii does not have a specific species relationship to only one host, hinting the possibility of finding it associated with other species on the eastern Pacific coast. This is especially relevant to potential host algae with commercial importance, since it is known that intimate associations between seaweeds (epiphytes, endophytes, or parasites) could have a negative effect on their hosts (Apt 1984), including thallus thickening, degradative lesions, cellular damage, gall formation, morphological deformations, and growth rate decrease (Yoshida and Akiyama 1979; Apt 1988; Correa et al. 1988, 1997; Correa and McLachlan 1992; Preuss and Zuccarello 2014; Ogandaga et al. 2016), affecting the commercial overall market value of the algae (Lein et al. 1991). In farmed seaweeds, these interactions have recently caused problems reducing the productivity of the carragenophyte alga K. alvarezii, as a consequence of the negative effect produced by the endophyte Colaconema infestans, which promotes the fragmentation of the thalli (Araújo et al. 2014).
In regards of the infestation of C. chamissoi by (the then unknown) C. daviesii, in the preliminary studies of Montoya (2019) and López (2017), the epiphyte outgrows were followed by discoloration, loss of turgor of the thalli, and fragmentation of the host. These results are in accordance with the effects documented by Correa (1990) as signs of deterioration caused by an endophyte. New studies are needed to assess the concrete impact C. daviesii has on the host. It has been reported that some opportunistic bacteria might show similar detrimental effects on seaweeds (Weinberger et al. 1994).
It is well documented that in marine algae, there is sometimes a close relationship between hosts and colonizers, which range from mutualism through commensalism to parasitism. In the case of the latter the relationship might reach high dependency, as described for Audouinella heteroclada, an epiphytic alga of Chondrus crispus (Correa 1990) that cannot survive being isolated, since it depends on its host for the supply of nutrients, or the reports of Vertebrata lanosa, which penetrates the thallus of Ascophyllum nodosum to obtain nutrients (Rawlence and Taylor 1972; Turner and Evans 1977). Although so far it is not clear that the type of relationship between C. daviesii and its host, our results suggest that this would not be strict, since C. daviesii can live independently of C. chamissoi once isolated. Its thalli were capable of growing and reproducing, which differs with the observed for several pigmented endophytes which are ecologically obligate symbionts, which might have fragile thalli incapable of remaining attached to other organisms or to other types of substrata (e.g., Correa et al. 1988; Correa 1994). These results support the idea that C. daviesii could not only be found associated with only one host, but also freely living in the natural environment. Since the species is a pigmented endophyte, it can be considered as photosynthetically independent, such as other endophyte algae isolated in vitro, which rarely had metabolic relationships with their hosts (Nielsen 1979; Gauna et al. 2010) and have even been described as carbon independent (Correa et al. 1988; Eggert et al. 2010). For the moment, however, this cannot be assumed for C. daviesii, since it has never been observed living by itself in the wild. Nonetheless, their absence on the field could be due to the little knowledge on the species, lack of proper identification, and a deficient sampling effort.
In the present study, C. daviesii showed an antagonistic relationship between growth and reproduction, which was regulated by the photoperiod. When the temperature and PFD conditions were kept between 10 °C and 20 μmol photons m−2 s−1, a higher number of branching and a smaller presence of monosporangia were observed under the photoperiod conditions 16:08 (L:D). On the contrary, under the same temperature and PFD conditions, the decrease in daylight hours (12:12, L:D) caused the highest production of monosporangia, while the number of branching was minimal, showing a scarce development of the thalli. This differs to what was informed about different red algae for the coast of the southeastern Pacific (Otaiza et al. 2001; Vásquez and Vega 2001; Vega and Meneses 2001), in which the reproduction and growth occur in synchrony during spring-summer months. This coincides with the increase in the temperature of the sea, luminosity, and in daylight hours (Bulboa and Macchiavello 2001; Vásquez and Vega 2001). Accordingly, studies in temperate environments that have documented the abundance of epiphytes and endophytes have found a marked seasonality in their appearance, which in most cases intensifies from spring under longer photoperiod conditions and temperature increases (Buschmann and Gómez 1993; Vásquez and Vega 2001; Macchiavello et al. 2018; Bulboa et al. 2020). Based on the results of this study, C. daviesii would present an evident sensitivity to changes during photoperiod, which would regulate its growth and reproduction, suggesting a probable seasonal behavior, growing during spring-summer months (with longer photoperiod), and reproducing during fall-winter months (with neutral or shorter photoperiod). Therefore, we hypothesize that C. daviesii would be capable of infecting C. chamissoi through monospores during winter, when this species has a scarce growth and low reproduction (Bulboa et al. 2008, 2010; Ávila et al. 2011), and then increasing the development and presence of the endophyte on the host during spring-summer. This postulate is supported by the gradual infestation documented for other species, in which the epiphyte/endophyte can be present in a dormant state, and then develop and mature in better growing conditions (Potin 2012). This gradualness in the infestation has been proven in the case of the endophyte Epicladia heterotricha over Hymenena falklandica, in which there is a seasonal gradualness of the infestation, starting with a superficial presence as epiphyte and then growing as endophyte into the cortex, causing harm to the host during a late stage of infestation (Gauna and Parodi 2010).
Conclusions
We report for the first time Colaconema daviesii in the southeastern Pacific, a pigmented endophyte of the commercially important seaweed Chondracanthus chamissoi. The growth and reproduction of C. daviesii could be a problem for the cultivation attempts with C. chamissoi, a species located in a long territorial extension which covers the coast of Chile and Perú. However, further studies should be carried out to determine the negative effects on the fitness of the host alga, especially under cultivation. In addition, it is yet unknown if C. daviesii is capable of infecting other species of commercial interest, as well as the degree of specialization and parasitism of this endophyte.
References
Aduana (2018) Exportaciones de chicorea de mar en Chile, para consumo humano. Servicio Nacional de Aduana, Chile https://www.aduana.cl.
Apt K (1984) Effects of the symbiotic red alga Hypneocolax stellaris on its host Hypneamu sciformis (Hypneaceae, Gigartinales). J Phycol 20:148–150
Apt K (1988) Galls and tumor-like growth on marine macroalgae. Dis Aquat Org 3:211–217
Araújo P, Schmidt E, Kreusch M, Kano C, Guimarães S, Bouzon Z (2014) Ultrastructural, morphological, and molecular characterization of Colaconema infestans (Colaconematales, Rhodophyta) and its host Kappaphycus alvarezii (Gigartinales, Rhodophyta) cultivated in the Brazilian tropical region. J Appl Phycol 26:19–53
Ávila M, Piel M, Caceres J, Alveal K (2011) Cultivation of the red alga Chondracanthus chamissoi: sexual reproduction and seedling production in culture under controlled conditions. J Appl Phycol 23:529–536
Bulboa C, Macchiavello J (2001) The effects of light and temperature on different phases of the life cycle in the carrageenan producing alga Chondracanthus chamissoi (Rhodophyta, Gigartinales). Bot Mar 44:371–374
Bulboa C, Macchiavello J, Oliveira E, Fonck E (2005) First attempt to cultivate the carrageenan-producing seaweeds Chondracanthus chamissoi (C. Agardh) Kützing (Rhodophyta; Gigartinales) in northern Chile. Aquac Res 36:1069–1074
Bulboa C, Macchiavello J (2006) Cultivation of cystocarpic, tetrasporic and vegetative fronds of Chondracanthus chamissoi (Rhodophyta, Gigartinales) on ropes at two localities in northern Chile. Invest Mar 34:109–112
Bulboa C, Macchiavello J, Oliveira E, Véliz K (2008) Growth rate differences between four Chilean populations of edible seaweed Chondracanthus chamissoi (Rhodophyta, Gigartinales). Aquac Res 39:1550–1555
Bulboa C, Macchiavello J, Véliz K, Oliveira E (2010) Germination rate and sporeling development of Chondracanthus chamissoi (Rhodophyta, Gigartinales) varies along a latitudinal gradient on the coast of Chile. Aquat Bot 92:137–114
Bulboa C, Véliz K, Sáez F, Sepúlveda C, Vega L, Macchiavello J (2013) A new method for cultivation of the carragenophyte and edible red seaweed Chondracanthus chamissoi based on secondary attachment disc: development in outdoor tanks. Aquaculture 410-411:86–94
Bulboa C, Massad I, Contreras-Porcia L, Zapata J, Catañeda F, Ramírez M (2020) Concise review of genus Chondracanthus (Rhodophyta: Gigartinales). J Appl Phycol 32:773–785
Buschmann A, Gómez P (1993) Interaction mechanisms between Gracilaria chilensis (Rhodophyta) and epiphytes. Hidrobiologia 261:345–335
Correa J, Nielsen R, Grund D (1988) Endophytic algae of Chondrus Crispus (Rhodophyta). II. Acrochaete heteroclada sp. nov., A. operculata sp. nov, and Pharophila dendroides (Chlorophyta). J Phycol 24:528–539
Correa J (1990) Pigmented algal endophytes of Chondrus crispus Stackhouse: host-specificity, fine structure and effects on host performance in infections by Acrochaete operculata Correa & Nielsen and A. heteroclada Correa & Nielsen. PhD dissertation, Dalhousie University, Canada
Correa J, Mclachlan J (1992) Endophytic algae of Chondrus crispus (Rhodophyta). IV. Effects on the host following infections by Acrochaete operculata and A. heteroclada (Chlorophyta). Mar Ecol Prog Ser 81:73–87
Correa J (1994) Infections by pigmented algal endophytes: misuse of concepts and terminology. Rev Chil Hist Nat 67:4–8
Correa J, Buschmann A, Retamales C, Beltran J (1997) Infectious disease of Mazzaella laminarioides (Rhodophyta): changes in infection prevalence and disease expression associated with latitude, season and within site location. J Phycol 33:344–352
Eggert A, Peters A, Küpper F (2010) The potential impact of climate change on endophyte infections in kelp sporophytes. In: Seckbach J (ed) Seaweeds and their role in globally changing environments. Springer, Dordrecht, pp 139–154
Faugeron S, Martínez E, Sánchez P, Correa J (2000) Infectious diseases in Mazzaella laminarioides (Rhodophyta): estimating the effect of infections on host reproductive potential. Dis Aquat Org 42:143–148
Faugeron S, Valero M, Destombe C, Martinez E, Correa J (2001) Hierarchical spatial structure and discriminant analysis of genetic diversity in the red alga Mazzaella laminarioides (Gigartinales, Gigartinales, Rhodophyta). J Appl Phycol 37:705–716
García V, Bárbara I, Días P (2017) Las praderas de Zostera marina del Parque Nacional Marítimo Terrestre de las Islas Atlánticas de Galicia y territorios adyacentes: distribución, abundancia y flora asociada. NACC. Bioloxía 24:1–12
Gauna M, Parodi E (2010) Green epi- endophyte in Hymenena falklandica (Rhodophyta) from the Patagonia coasts of Argentina: preliminary observations. Phycol Res 56:144–1835
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hansen G, Hanyuda T, Kawai H (2017) Benthic marine algae on Japanese tsunami marine debris – a morphological documentation of the species. Part 1. The tsunami event, the project overview, and the red algae. OSU scholars archive, Corvallis, pp 1-50
Harper J, Saunders G (2002) A re-classification of the Acrochaetiales based on molecular and morphological data, and establishment of the Colaconematales Ord. Florideophyceae Rhodophyta. Eur J Phycol 37:463–476
Hayashi L, Bulboa C, Kradolfer P, Soriano G, Robledo D (2014) Cultivation of red seaweeds: a Latin American perspective. J Appl Phycol 26:719–727
Kim C, Kim Y, Choi H, Nam K (2014) New records of three endophytic green algae from Grateloupia spp. (Rhodophyta) in Korea. Algae 29:127–136
Lam D, Verbruggen H, Saunders G, Vis M (2016) Multigene phylogeny of the red algal subclass Nemaliophycidae. Mol Phylogenet Evol 94:730–736
Lein T, Sjatun K, Wakili S (1991) Mass-occurrence of a brown filamentous endophyte in the lamina of the kelp Laminaria hyperborea (Gunnerus) Foslie along the southwestern coast of Norway. Sarsia 76:187–193
López B (2017) Micropropagación y cultivo de la “Mota Lisa”, un morfotipo de Chondracanthus chamissoi (C Agardth) Kützing (Rhodophyta; Gigartinales). Dissertation, Master's thesis in marine biology, Universidad Andrés Bello, Chile
Macchiavello J, Sepúlveda C, Basaure H, Sáez F, Yañez D, Marín C, Vega L (2018) Suspended culture of Chondracanthus chamissoi (Rhodophyta: Gigartinales) in Caleta Hornos (northern Chile) via vegetative propagation with secondary attachment discs. J Appl Phycol 30:1149–1155
Montoya V (2019) Colaconema daviesii (Rhodophyta; Colaconematales) una endófita del alga comercial Chondracanthus chamissoi. Dissertation, undergraduate thesis in marine biology, Universidad Andrés bello, Chile
Nielsen R (1979) Culture studies on the type species of Acrochaete, bolbocoleon and Entodadia (Chaetophoraceae, Chlorophyceae). Bot Not 132:441–449
Nguyen L, Schmidt H, Haeseler A, Minh B (2015) IQ-THREE: a fast and effectives stochastic algorithm for estimating maximum- likelihood phylogenies. Mol Biol Evol 32:268–274
Ogandaga C, Choil H, Jang K, Wan K (2016) Growth responses of Chondrus ocellatus Holmes (Gigartinales, Rhodophyta) to two endophyte, Mikrosyphar zostera Kuckuck (Ectocarpales, Ochrophyta) and Ulvella ramosa (N. L Gardner) R. Nielsen (Ulvales, Chlorophyta) in culture. Algae 31:363–371
Otaiza R, Abades S, Brante A (2001) Seasonal changes in abundance and shifts in dominance of life history stages of the carrageenophyte Sarcothalia crispata (Rhodophyta, Gigartinales) in south-Central Chile. J Appl Phycol 13:161–171
Parker T, Chapman A (1994) Separating the grazing effects of periwinkles and amphipods on a seaweed community dominated by Fucus distichus. Ophelia 39:75–91
Potin P (2012) Intimate associations between epiphytes, endophytes, and parasites of seaweeds. In: Wiencke and Bischof (Eds) seaweed biology. Springer, Berlin pp, pp 203–234
Poza M, Gauna C, Escobar J, Parodi E (2017) Temporal dynamics of algal epiphytes on Leathesia marina and Colpomenia sinuosa macrothalli (Phaeophyceae). Mar Biol Res 14:65–75
Preuss M, Zuccarello G (2014) What’s in a name? Monophyly of genera in the red algae: Rhodophyllis parasitica sp. nov. (Gigartinales, Rhodophyta); a new red algal parasite from New Zealand. Algae 29:279–288
R Core Team (2019) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Ramírez C, Bulboa C, Contreras L, Mora A (2018) Flora Marina Bentónica de Quintay. Santiago, Ril Editores 164 pp
Rawlence D, Taylor A (1972) A light and electron microscopic study of rhizoid development in Polysiphonia lanosa (L.) Tandy. J Phycol 8:15–24
Robba L, Russell S, Barker G, Brodie J (2006) Assessing the use of the mitochondrial cox 1 marker for use in DNA barcoding of red algae (Rhodophyta). Am J Bot 2006:1101–1108
Saunders GW (1993) Gel purification of red algal genomic DNA: an inexpensive and rapid method for the isolation of polymerase chain reaction-friendly DNA. J Appl Phycol 29:251–254
Saunders GW (2005) Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Trans R Soc Lond B 360:1879–1888
Scott F, Saunders G, Kraft G (2013) Entwisleia bella, gen. Et sp. nov., a novel marine ‘batrachospermaceous’ red alga from southeastern Tasmania representing a new family and order in the Nemaliophycidae. Eur J Phycol 48:398–410
Sherwood AR (2008) Phylogeography of Asparagopsis taxiformis (Bonnemaisoniales, Rhodophyta) in the Hawaiian islands: two mtDNA markers support three separate introductions. Phycologia 47:79–88
Schoenrock K, Amsler C, McClintock J, Baker B (2013) Endophytic presence as a potential stressor on growth and survival in Antarctic macroalgal hosts. Phycologia 52:595–599
Tamura K, Peterson N, Stecher G, Neisudhir M (2011) Mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:10
Trifinopoulos J, Nguyen L, Haesele A, Quang B (2016) W-IQ-tree: a fast-online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:232–235
Turner C, Evans L (1977) Physiological studies on the relationship between Ascophyllum nodosum and Polysiphonia lanosa. New Phytol 79:363–371
Vairappan C, Chung C, Matsunaga S (2014) Effect of epiphytes infection on physical and chemical properties of carrageenan produced by Kappaphycus alvarezii Doty (Soliericeae, Gigartinales, Rhodophyta). J Appl Phycol 26:923–993
Vásquez J, Vega J (2001) Chondracanthus chamissoi (Rhodophyta, Gigartinales) in northern Chile: ecological aspects for management of wild populations. J Appl Phycol 13:267–277
Vega J, Meneses I (2001) Seasonal and spatial monitoring of productivity and of reproduction of Chondrus canaliculatus (Gigartinales, Rhodophyta) from Chile. Bot Mar 44:571–581
Weinberger F, Friedlander M, Gunkel W (1994) A bacterial facultative parasite of Gracilaria conferta. Dis Aquat Org 18:135–141
Wickham H (2016) ggplot2 elegant graphics for data analysis. Springer, New York https://ggplot2.tidyverse.org
Yang M, Geraldino P, Kim M (2013) DNA barcode assessment of Gracilaria salicornia (Gracilariaceae, Rhodophyta) from Southeast Asia. Bot Stud 54:27
Yang M, Macaya E, Kim M (2015) Molecular evidence for verifying the distribution of Chondracanthus chamissoi and C. teedei (Gigartinaceae, Rhodophyta). Bot Mar 58:103–113
Yoshida T, Akiyama K (1979) Streblonema () infection in the frond of cultivated Undaria (Phaeophyceae). In: Jensen A, Stein JR (eds) Proceedings of the IXth International Seaweed Symposium. Princeton, Science Press, pp 219–223
Acknowledgments
Montoya V. thanks to Universidad Andrés Bello for the grant 2018-2 (AAP 2018-2). CB thanks FONDEF ID15I10320 and LC FONDECYT 1170881. We appreciate the support of Quintay Marine Research Center–CIMARQ.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Montoya, V., Meynard, A., Contreras-Porcia, L. et al. Molecular identification, growth, and reproduction of Colaconema daviesii (Rhodophyta; Colaconematales) endophyte of the edible red seaweed Chondracanthus chamissoi. J Appl Phycol 32, 3533–3542 (2020). https://doi.org/10.1007/s10811-020-02176-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02176-3