Abstract
Despite the increased interest in exploring the potential of algal biomass production for food stock and renewable energy, very little work has been done in developing reliable screening protocols to enable the identification of species that are best suited to mass cultivation outdoors. Nannochloropsis is an algal genus identified as a potential source of lipids due to its ability to accumulate large quantities of these compounds, especially under nutrient-limiting conditions. The objective of the current work was to use two species of this genus, Nannochloropsis oceanica and N. oculata, as model organisms to develop a protocol that will allow the evaluation of their capacity to yield high biomass productivity under outdoor conditions. Growing the alga under different light intensities and measuring growth rate as well as a range of photosynthetic parameters based on light response curves and variable fluorescence highlighted significant differences between the two species. Our data show that N. oceanica cells have a better capacity to respond to higher light intensities, as reflected by growth measurements, photosynthetic electron transport rates, and oxygen evolution as well as their response to the very high photon flux densities expected in outdoor culture. On the other hand, N. oculata showed a higher tolerance to oxidative stress as reflected in its resistance to the reactive oxygen species generating compounds Rose Bengal (RB) and methyl viologen (MV). Based on the above evidence, we suggest that N. oceanica may perform better than N. oculata when grown under high light conditions typically found outdoors in summer, while N. oculata may perform better than N. oceanica under oxidative stress conditions usually found in outdoor cultures exposed to a combination of high light and low temperature commonly occurring in winter time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Algal biotechnology has been a topic of intensive research for the last 50 years and aims to exploit the potential of algal biomass as a source for the production of a range of commercially valuable bioproducts, as well as using algae for other applications such as wastewater treatment and environmentally friendly biofuels. However, while production of useful products is relatively well characterized at the laboratory scale, translation to large-scale cultivation of algae for biotechnology faces an array of challenges. Recently, Borowitzka and Vonshak (2017) pointed out the main limitations of, and obstacles to, the process of scaling up from laboratory scale to outdoor mass production of microalgae.
Importantly, studies dealing with up-scaling can be divided into three major topics of concern. The first topic deals with the “outdoor physiology” of the cultures and involves attempts to understand the role of the major limitations to algal growth and productivity. The second involves the development of down-stream processing implicated in the harvesting, extraction, and formulation of the desired product. The third topic is the design of suitable reactors in order to develop and make the best fit between the need to produce large amounts of biomass in an efficient way while reducing the initial investment cost and eventually achieving a sustainable large scale production facility.
There have been significant breakthroughs in the last two topics, which may be related to the fact that many technologies have been developed in the food and fermentation industries that are easily modified and adjusted to the needs of processing algal biomass at the large scale. On the other hand, in relation to the first topic, there is still a large knowledge gap between our understanding of algal physiology in the laboratory, with controlled conditions for growth, and the response of algal culture to the diverse and extreme conditions prevailing in large-scale outdoor cultures (Sandnes et al. 2005).
The two major factors, which need to be considered in up-scaling of outdoor cultures as affecting productivity, are light availability and temperature. These show diurnal as well as seasonal changes (Vonshak et al. 2014; Borowitzka and Vonshak 2017), in addition to the light/dark regime affected by irradiance changes in the depth of the mixed growth medium. Accordingly, an important role in the scaling-up process is selecting species that rapidly acclimate to these variable conditions, including surviving dilution from a concentrated inoculum culture that may cause a rapid increase in the average light intensity in the culture and thus potentially lead to photoinhibition.
The re-discovery of the potential use of microalgae for sustainable production of fuels of the future has led to a burst of publications using algal species capable of accumulating lipids at high concentrations. In this connection, green microalgae of the genus Nannochloropsis (Class Eustigmatophyceae) have emerged as leading candidates for this application from work that commenced some 30 years ago (Boussiba et al. 1987) and has been continuing to the present day (Solovchenko et al. 2014; Ma et al. 2016). According to recent data from the ISI Web of Knowledge, out of more than 2500 publications using this genus, most of which were published in the last 10 years, more than 50% related to maximizing the ability of species to accumulate lipids for their potential use as biodiesel. Despite this, very little information has been presented which deals with the justification for, or the rationalization of, the use of the selected species from among all the other species of Nannochloropsis (Simionato et al. 2013). In addition, only a very small number of publications actually deal with the photosynthetic apparatus of Nannochloropsis and its response to light or temperature changes (Fisher et al. 1996; Sandnes et al. 2005; Simionato et al. 2011; Palacios et al. 2018). Moreover, even fewer publications deal with the photoacclimation process under outdoor conditions (Sukenik et al. 2009).
All photosynthetic organisms demonstrate an ability to acclimate to varying light conditions in order to maintain a balance between capturing a sufficient number of photons to sustain the photosynthetic machinery in an active state and avoiding the possible damaging effects of over-reduction of the photosynthetic electron transport components. Generally, following exposure to stress conditions, the photosynthetic apparatus is subject to photo-oxidative stress due to generation of reactive oxygen species (ROS). In order to protect the organism, various strategies have evolved. Among these is the process known as non-photochemical quenching (NPQ) which allows rapid dissipation of excess absorbed energy as heat when the electron transport chain becomes saturated by excessive light (Weis and Berry 1987; Ruban 2014).
In a range of environmental conditions, such as the presence of photoinhibitory light or low temperature, mitochondrial respiration also plays a significant role in protecting the chloroplasts from photoinhibition. This protection mechanism is accomplished through energy dissipation involving the photorespiratory reactions (Padmasree et al. 2002) which are controlled by the activity of Rubisco and affect the rate of net photosynthetic O2 evolution, particularly in C3 vascular plants. Measurement of dark respiration can thus be an additional tool in evaluating the mechanisms and efficiency by which algal species are capable of acclimating to light stress.
Rose Bengal (4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodofluorescein, RB) interacts with oxygen in the presence of light to produce reactive oxygen species (see Methods) and thus sensitivity to RB might be used as a measure of an algal species’ sensitivity to oxidative stress. Through a different process, methyl viologen (1,1′-dimethyl-4,4′-bipyridinium, MV) catalyzes O2 photoreduction thus producing O2– and H2O2 in the chloroplast. In addition, MV has often been used as an inducer of photooxidative stress. Using both chemicals as a selection tool may therefore be used as a useful indicator to evaluate the ability of cells to deal with oxidative stress (Ben Sheleg et al. 2019).
The final effect on acclimation by these photoprotective mechanisms translates into alterations and adjustment of the photosynthesis versus irradiance (P versus I) curve parameters of cultures grown under different light conditions, including photoinhibition at high light. The achievement of photoacclimation is brought about by the modification in one or more of the parameters of the P vs I curve, namely dark respiration (Rd), the light-saturated photosynthetic rate (Pmax), and the initial slope of the P versus I curve, α, that represents the light utilization efficiency. These in turn lead to changes in other parameters such as the point of saturating irradiance (Ik) and the compensation light intensity (Ic) (Palacios et al. 2018).
The objective of the current study was to develop a tool based on a set of measurements, related to the response of growth and photosynthesis to light intensity, in order to be able to select algal species more suitable for large-scale outdoor cultivation. We have chosen two species of Nannochloropsis and evaluated their ability to respond to a variety of light conditions. We followed the changes in photosynthetic performance occurring during acclimation to high light as changes in chlorophyll fluorescence quenching and oxygen evolution measurements, which were compared to the growth rates attained by the two species.
Materials and methods
Species
Two species of the unicellular marine green alga Nannochloropsis (Eustigmatophyceae) were used in this study. Nannochloropsis oceanica strain CCALA 804 [ZMORA/1995] was obtained from the Culture Collection of Autotrophic Organisms (CCALA), Institute of Botany, and Academy of Sciences of the Czech Republic. Nannochloropsis oculata, strain CS-179, was obtained from the CSIRO Australian National Algae Culture Collection.
Growth condition
Medium
Both species were cultured in Reef Salt Enriched (RSE) medium which comprised: 34 g L−1 Reef Salt (Seachem, Madison, GA, USA), 2 g L−1 KNO3, 70 mg L−1 KH2PO4, with the addition of 1 mL L−1 A5+Co microelements solution (0.77 μM ZnSO4·7H2O, 0.31 μM CuSO4∙5H2O, 1.61 μM Na2MoO4·2H2O, 1.61 μM Na2MoO4∙2H2O, 46.3 μM H3BO3, 9.15 μM MnCl2∙4H2O, 0.172 μM Co(NO3)2∙6H2O) and 1 mL L−1 ferric citrate mixture (26.7 μM ferric citrate and 42.8 μM citric acid), and 500 μL L−1 vitamin solution (1 g L−1 vitamin B12 and 1 g L−1 biotin).
Inoculum preparation
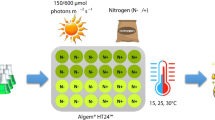
Stocks of the cultures were maintained in a rotary shaker agitated continuously at 120 rpm. Continuous light at a photon flux density of 70–75 μmol photons m−2 s−1 was provided by cool-white fluorescent lamps (Aqua Relle, TL-D 30W, Philips). Cultures were grown in flasks and bubbled with air enriched with 2% CO2. Constant temperature was maintained at the optimal value of 25 °C. The chlorophyll concentration of cultures was kept within the range of 10–20 μg mL−1 by dilution once a week with fresh medium.
Growth in a multi-cultivator (MC)
For all experiments, cultures were incubated in the 80-mL culture cylindrical vessels (i.d., 4 cm) of a Photon Systems Multi-Cultivator (MC1000-OD, Photon Systems Instruments, Drasov, Czech Republic, www.psi.cz). The cultivation vessels were immersed in a temperature-controlled water bath at the optimal temperature of 25 °C. Each cultivation vessel was monitored for growth by automatic measurement of optical density at 680 nm and 720 nm though, since the light path is 5 cm, readings become saturated at a very early stage of the growth phase and thus only a very short stage of real logarithmic growth rate can be monitored. For this reason, specific growth rate was calculated on the basis of other parameters (see below). Cultures were acclimated to the multi-cultivator (MC) incident light of 100 μmol photons m−2 s−1 for at least 1 week, along with dilution every 3 to 4 days to a chlorophyll concentration of 2 μg mL−1 to maintain growth in the exponential phase. Subsequently, each cultivation vessel was independently adjusted to the required light (Cool White LED illumination) for growth at 50, 100, 200 or 300 μmol photons m−2 s−1 and bubbled with air enriched with 2% CO2. All experiments were started with a chlorophyll concentration of 2 μg mL−1.
High light experiment
Acclimated cultures, in the 80-mL cultivation vessels of the multi-cultivator, were diluted to 2 μg chlorophyll mL−1 and maintained at the same optimal temperature of 25 °C and bubbled with 2% CO2 enriched air. The same light intensity for growth was maintained in each cultivation vessel (50, 100, 200, or 300 μmol photons m−2 s−1). After 3 days, the culture in each vessel was diluted to a chlorophyll concentration of 5 μg mL−1, and the light was increased to 1600 μmol photons m−2 s−1 for 24 h (high photon flux density, HPFD). Growth parameters (chlorophyll a and cell number), photosynthetic activity (O2 evolution), and chlorophyll fluorescence measurements were carried at the initial time (d0) and after 24 h (d1).
Growth measurements
Chlorophyll a: Samples (1–2 mL) were harvested by centrifugation for 5 min at 12,000×g. Chlorophyll was extracted with dimethyl sulfoxide (DMSO) (Burnison 1980) at 70 °C for 5 min. The absorbance of the supernatant at 665 nm was subsequently measured by spectrophotometry (Cary 50 Bio spectrophotometer, Varian, USA), and chlorophyll a concentration was determined according to Seely et al. (1972).
Cell number
Cells were counted using a Luna Dual Fluorescence Cell Counter, Logos Biosystems, Korea (www.logosbio.com). A sample of culture (10 μL) of was loaded into a Luna Cell Counting Slide (C.N L12001), and cells were counted manually.
Growth parameters
The specific growth rate was calculated using Eq. 1:
where x1 and x2 are biomass concentrations (as Chl mL−1 or cell number mL−1) at time intervals t1 and t2 within the exponential phase of growth.
Photosynthesis vs. irradiance curve (P vs I curve)
Rates of photosynthetic oxygen evolution as a function of irradiance (P vs I curve) and of dark respiration (Rd) were measured with a Clark-type oxygen electrode (DW2/2 Oxygen Electrode, Hansatech Instrument Ltd., UK) connected to a Hansatech Oxylab control box. Algal samples (2 mL) of known chlorophyll concentration (5 μg mL−1) were inserted into the water-jacketed, temperature-controlled chamber (25 °C), and mixed by magnetic stirrer. Using the Oxygraph Plus software or O2ViewXP software (Hansatech Instrument Ltd.), the oxygen evolution rate was recorded. Oxygen evolution rates (average of at least three repetitions) are reported in units of μmoles of O2 evolved per unit of time per unit of biomass, the latter expressed as cell number or chlorophyll concentration.
The irradiance was controlled and modified by intensity-controllable white LED light, which was programmed to increase in intensity according to a specific, pre-determined light regime (10–1000 μmol photons m−2 s−1), which allowed for the production of photosynthetic response curves (P vs I curves). Each P vs I curve was analyzed according to Eq. 2 (Eilers and Peeters 1988):
and allowed the determination of several photosynthetic parameters, such as the dark respiration (Rd); photosynthetic compensation point (Ic); when the photosynthetic rate is just balanced by respiration, maximum rate of light-saturated photosynthesis (Pmax); irradiance at the onset of light saturation (Ik); and the light utilization efficiency as determined by the initial slope (α). By using the equations of Eilers and Peeters (1988), the three latter parameters can be estimated as given in Eq. 3:
Measurement of specific chlorophyll optical absorption cross-section
The optical absorption cross-section (a*) of the cells (m2 mg−1 Chl a) was determined from in vivo absorption spectra (in the range from 400 to 750 nm) according to Kromkamp and Limbeek (1993) using a spectrophotometer (Varian Cary50 UV-visible). To minimize the impact of the light scattering effect from the cell surface, the sample cuvette was placed close to the detector window with standard white A4 copy paper as a light diffuser placed on the opposite face of the 1-cm cuvette.
The maximum quantum yield θmax, i.e., the ratio of the rate of oxygen evolution to that of light absorption under limiting light, was calculated according to Eq. 4 (Kromkamp and Limbeek 1993):
where α and a* are expressed per Chl a (see Table 1 for abbreviations) (Fisher et al. 1996).
Fluorescence measurements
The maximum quantum yield of PSII (Fv/Fm) was measured in dark adapted cells (10 min). Rapid light-response curves: (RLCs) of Nannochloropsis cultures were measured using a 2-mL cell sample (4 μg mL−1 Chl a) placed in a liquid-phase oxygen electrode chamber (Hansatech, DW3) thermoregulated at 25 °C cuvette using a pulse-amplitude-modulation fluorimeter (PAM-2100, H. Walz, Germany). A series of stepwise increasing irradiance intensities (LEDs, 0–636 μmol photons m−2 s−1) provided by a PAM-2100 were automatically applied at 20-s intervals to obtain the light-adapted fluorescence level F' (steady-state fluorescence yield in the light), and at the end of each step a saturating pulse (> 6000 μmol photons m−2 s−1, 0.6 s duration) was triggered to reach the maximum fluorescence level Fm' (steady-state maximum fluorescence in the light). The effective PSII photochemical quantum yield in the light, YII, was determined as (Fm′-F′)/Fm′ in the light-adapted state at the respective irradiance level. The electron transport rate was calculated according to Kromkamp and Forster (2003) (Eq. 5):
Although measurement of a* is based on the extinction coefficient of chlorophyll a and therefore cannot be considered specific for PSII (Schreiber et al. 2012; Szabó et al. 2014), it allows for a more accurate measure of ETR in microalgal suspensions compared to what directly extracted from the PAM Software which was developed to perform measurement in leaves. Moreover, the results are easier to compare with those obtained with the P/I curve measurements which are also normalized on a Chl a basis.
Analysis of RLCs was used to calculate changes in important parameters, namely the maximum relative electron transport rate through PSII, ETRmax; the initial slope, α, of the ETR vs. PFD curve; the saturation irradiance, Ik, given as the intercept between α and ETRmax; and the maximum quantum yield of photosynthesis Фmax. The curves were fitted to the non-linear least-squares regression model of Eilers and Peeters (1988) using PamWin 3 software.
Non-photochemical quenching was calculated as shown in Eq. 6:
It is inversely related to photochemistry (YII) and is considered a safety valve protecting PSII reaction centers from damage by excess irradiance (Bilger and Bjӧrkman 1990).
Light measurements
Values of photosynthetically active radiation (PAR), for both fluorescence and photosynthesis measurements, were measured as 15-s average values using a LI-250A light meter (Li-Cor, USA) equipped with a spherical sensor, model SQSA0191 (H·Walz, Germany) placed in the cuvette, or with a flat LI-190SA quantum sensor (cosine-corrected up to 80° angle of incidence) for cultures grown in the Photon Systems Multi-Cultivator PSI cultivator.
Definitions and abbreviations used are reported in Table 1.
Exposure to ROS
The rose bengal (RB) molecule (Sigma R3877) produces singlet oxygen (1O2) when it is exposed to photons in the 450–580-nm range. The reactions of singlet oxygen produced by the photosensitizer are generally limited to the subcellular region where the photosensitizer molecule is located. This is due to the fact that singlet oxygen is highly reactive with a very short half-life (Kochevar and Redmond 2000; Kovács et al. 2014). RB has been shown to localize in the chloroplast and is capable, due to the creation of 1O2, of decreasing photosystem II (PSII) electron transport and of damaging the PSII reaction center complex (Padmasree et al. 2002).
The bipyridilium herbicide paraquat (1,1-dimethyl-4,4-bipyridilium) or methyl viologen (MV) (Sigma M2254) is a highly potent herbicide. Its mode of action is to mediate the generation of superoxide by re-routing electrons from photosystem I, subsequently initiating the production of other ROS, leading to oxidative damage and cell death (Hawkes 2014). Thus, MV toxicity may mimic the mechanisms of photooxidative damage. Consequently, tolerance to photooxidation may correlate well with resistance to MV (Iturbe-Ormaetxe et al. 1998; Murgia et al. 2004).
Cultures of the two Nannochloropsis species, N. oceanica and N. oculata, at a concentration of 10 μg Chl mL−1 were dispended into 2-mL aliquots in each well of a 24-well plate, and exposed to a gradient of concentrations of RB (0-25 μM) or MV (0–400 μM). Plates were incubated under cool white fluorescent light of 70 μmol photons m−2 s−1 for 4 days, and mixed every day and before photographing the plate to provide a record of relative growth.
Statistical analysis
Sigma Plot 11.0 was used to determine significant differences between treatments. One-way ANOVA and a Holm-Sidak test were conducted for every binary combination of systems. P values less than 0.05 were considered significantly different.
Results
Growth
For any algal species that is evaluated as a potential candidate for outdoor cultivation, its ability to respond and acclimate to a wide range of light intensities should be carefully assessed. To this end, cells were acclimated to light intensities from 50 to 300 μmol photons m−2 s−1 and growth was monitored by following both the daily increase in chlorophyll a and cell number. In both species, a positive response of growth to increases in light intensity was observed. However, different trends were obtained in the response to light intensity when using either chlorophyll (Fig. 1a) or cell number (Fig. 1b) to calculate the specific growth rate (μ). Indeed, significant differences (P < 0.05) between the two methodologies of calculating growth rate were observed at 200 and 300 μmol photons m−2 s−1 for N. oceanica, and at 300 μmol photons m−2 s−1 for N. oculata. On the whole, N. oceanica appears to respond better to a wider range of light intensities than N. oculata, i.e., the growth rate of N. oculata becomes saturated at 200 μmol photons m−2 s−1 when evaluated on the basis of cell number (Fig. 1b).
Electron transport rate
Cells grown at 50, 100, 200, 300 μmol photons m−2 s−1 were used to measure the ETR vs light relationship, and the results are presented in Fig. 2. Although in both species a trend of increase in the ETR with growth light is observed, in cultures grown at higher light intensities (300 μmol photons m−2 s−1) as compared to those grown at 50 μmol photons m−2 s−1, it is worth noting that in the N. oceanica cultures (Fig. 2 (a1)) the increase is in the range of twofold, while in the N. oculata cells (Fig. 2 (a2)) (SD is provided in Table S1 in the supplementary section), this increase is no more than 30%. This different behavior supports the idea that each species has a different ability to respond to variations in light. More detailed analysis of the calculated parameters is provided in Table S1 in the supplementary data section. Calculation of the quantum requirement at the different light intensities showed only small increases with increase of the light intensity (Table S1).
The ETR Curves of both Nannochloropsis species: N. oceanica (a1) and N. oculata (a2) grown at the different light intensities: 50 (black circles), 100 (red squares), 200 (blue diamonds), and 300 μmol photons m−2 s−1 (green triangles). Data are presented for chlorophyll content per mL. Data are presented as the mean ± SD (n = 3, 4)
Maximum photochemical quantum yield, NPQ, and optical absorption cross-section changes
The maximal photochemical efficiency Fv/Fm is commonly used as an indicator of the potential for photosynthetic activity. It showed no significant changes in both species (Table 2).
Non-photochemical quenching (NPQ) is an additional parameter extracted from measurements of variable fluorescence of chlorophyll and may serve as an indicator of the ability of cells to dissipate excess energy and thus better acclimate to high light stress. As expected, measuring the NPQ of the two species grown in different light intensities indicated that the capacity of cells to dissipate light energy via NPQ increased as the growth irradiance increased (Table 2). However, as for ETR (Fig. 2), in N. oceanica cultures grown at higher light intensities (300 μmol photons m−2 s−1) as compared to those grown at 50 μmol photons m−2 s−1, the NPQ increased in the range of threefold while in N. oculata, this increase was no more than 30%, with no difference between cells grown at 200 or 300 μmol photons m−2 s−1.
Changes in the in vivo optical cross-section of cells a* (Table 2) may also serve as an indication of the ability of cells to acclimate to a variety of light intensities. These measurements were also used in converting the ETR values from relative values, as they come from the PAM fluorimeter expressed as μmol e− m−2 s−1 to ETR values expressed as μmol e− mg−1 Chl min−1. In N. oceanica, the optical absorption cross-section increased about 30% from the lowest to the highest growth irradiance tested, both normalized in terms of chlorophyll (m2 mg−1Chl) and cell (m2 cell−1), while with N. oculata changes in cross-section were not significant (5%, P > 0.05) (Table 2).
Oxygen evolution
Although measurement of ETR is a common tool in the study of photosynthesis, it does not give a direct measurement of photosynthetic activity. It uses changes in variable chlorophyll florescence and converts this to a measure of photosynthetic “activity.” Another drawback in the use of the fluorescence methodology is that it does not provide information on the dark respiration activity that may play an important role in the light acclimation process. Consequently, another parameter that is not available when using the ETR methodology is the “light compensation point” Ic, a value indicating the light required to drive a sufficient rate of light-dependent oxygen evolution to compensate for respiratory oxygen uptake. We have thus complimented the ETR measurements with a more direct measure of photosynthesis, i.e., the oxygen evolution rate. Cells grown and acclimated to different light intensities were treated as described in the “Materials and methods” and the oxygen evolution rate was measured at different light intensities (P vs I curves). The results are presented in Fig. 3, and the major parameters are summarized in the inserted table in each figure. SD is provided in Table S2 in the supplementary section. When using P-I curves to evaluate light acclimation, there is a continuing debate as to what is the best basis for normalizing the activity. MacIntyre et al. (2002) discussed the issue in detail, pointing out the limitation of each methodology. However, the use of both procedures to evaluate photosynthesis capacity should be considered when chlorophyll per cell varies between cultures. We therefore provide the results with rates calculated on the basis of (i) chlorophyll concentration (Fig. 3 (a1 and a2)) as well as (ii) cell counts (Fig. 3 (b1 and b2)). N. oceanica demonstrated, particularly when acclimated at light intensities above 50 μmol photons m−2 s−1, a much higher response to the light growth conditions than N. oculata when activities were expressed on the basis of chlorophyll (P < 0.05). However, when photosynthetic performance was expressed on the basis of cell number, an opposite pattern was observed, that is, N. oculata performed better than N oceanica between 50 and 200 μmol photons m−2 s−1 light irradiance, while at 300 μmol photons m−2 s−1, there was a reduction in Pmax and the associated parameters, indicating it may already be above the saturation level of its growth response capacity and in a region of photoinhibition. It is conceivable that the better photosynthesis performance per cell showed by N. oculata is the result of its higher chlorophyll content per cell (Table 3). As a matter of fact, the decline in Pmax per cell (Table 5) in N. oculata was observed when its chlorophyll content was comparable to that found in N. oceanica (0.33 vs 0.30 μg (106 cells)−1 (Table 3).
P-I curves of the two Nannochloropsis species: N. oceanica (a1, b1) and N. oculata (a2, b2) as a function of light intensity for growth: 50 (black circles), 100 (red squares), 200 (blue diamonds), and 300 μmol photons m−2 s−1 (green triangles). Photosynthetic activity is expressed on the basis of Chl content per mL (a1, a2) or cell number per mL (b1, b2). In each figure, the insert is a table of the parameters derived from the curves. Data are presented as the mean ± SD (n = 3)
Response to high light
Outdoor algal cultures are frequently exposed to excess light, particularly around midday. We thus decided to evaluate and compare the response of the two species to a high light stress by measuring their P-I curves before and after a 24-h exposure to 1600 μmol photons m−2 s−1. We chose a relatively lower level of light stress to avoid lethal conditions which may occur in a relatively diluted culture. In order to make sure that this was indeed the situation, we measured the changes in Fv/Fm of each culture before and after 24 h of exposure to the high light stress and found a relatively small, although significant (P < 0.05), reduction of less than 15% in both species (compare Table 2 with Table 4). In vivo optical cross-section (a*) showed the opposite behavior; it increased in both species by 60% indicating a strong effect of acclimation to high light by the cultures (compare Table 2 with Table 4). Following exposure to high light, NPQ reached 1.5 in N. oceanica and 1.24 in N. oculata (compare Table 2 with Table 4) (P < 0.05).
The responses of photosynthesis of the two species to a HPFD stress are shown in Fig. 4. In general, a significant decline in the photosynthesis parameters of the cultures after light stress was observed (see the inserted table on each figure; SD is provided in Table S3 in the supplementary section). In order to facilitate a quantitative analysis and comparison between the two species before and after exposure to the HPFD, we have summarized the data in Table 5. For many of the parameters, expressed on a cell basis, it is clear that N. oculata demonstrated a much greater sensitivity to light stress. Indeed, the maximal photosynthetic activity evaluated in terms of Pmax, and the initial slope (α), were significantly lower in N. oculata after the HPFD treatment, except in the culture previously acclimated at 300 μmol photons m−2 s−1 (Table 5). This may be a result of the higher reduction in chlorophyll per cell in N. oceanica, which dropped threefold (from 0.3 to 0.1) compared to N oculata in which values dropped by twofold (Table 3). It is important to point out that in N. oceanica, the residual activity after photoinhibition was, in general, significantly higher compared to that found in N. oculata, indicating the ability of the former species to better respond to the changes in growth and environmental stress conditions. Following exposure to high light, the average respiration rates strongly increased (by about 250 %) in both species, but without a clear trend in relation to prior light acclimation.
P-I curves of the two Nannochloropsis species: N. oceanica (left column) and N. oculata (right column) in cultures grown at different light intensities: 50 (a, e), 100 (b, f), 200(c, g), 300 μmol photons m−2 s−1 (d, e). Photosynthetic activity is presented before (black circles) and after (red squares) exposure to HPFD and expressed on the basis of cell number. Data are presented as the mean ± SD (n = 3)
Maximum quantum yield of photosynthesis and photochemical quantum yield of PSII changes
Nannochloropsis cultures exposed to high light showed a strong reduction in their maximum quantum yield of photosynthesis (Φmax) measured through chlorophyll fluorescence (compare Table 2 with Table 4). The reduction in Φmax following exposure to high light was greater in N. oculata than in N. oceanica.
Response to Rose Bengal or methyl viologen
With the aim of evaluating whether the two Nannochloropsis species have a different ability to cope with oxidative stress, they were exposed to a gradient of concentrations of RB (0–25 μM) or MV (0–400 μM) and incubated in the light for 4 days. As depicted in Fig. 5. N. oculata demonstrated a much higher ability to tolerate the oxidative stress imposed by the exposure to RB.
Discussion
Although the potential application and uses of algal biomass are well documented, only very few species have reached the state of being a commercially viable and sustainable business operation. Arthrospira and Chlorella, mainly used for total cell biomass, and Dunaliella and Haematococcus, used as sources of high value carotenoids, are the few species which have attained this status (Borowitzka and Vonshak 2017). All other species of interest are at a stage of exploration of their potential or struggling with the challenges of scaling up the production facility. Even within the commercially produced species, very little is known about the physiology of the specific species used and the rationale for choosing them. The inherent limitations of the lack of established breeding practices in most of the microalgae limit the genetic tools one can use for species improvement, especially given the current regulatory limitations imposed on GMO organisms used for food and feed. Thus, the two options left are the use of random induced mutagenesis followed by a tedious selection process that hopefully will yield improved species (Ben-Shelg et al. 2019), or relying on mother nature and selecting for existing species isolated from different habitats that may represent a variation induced by a set of environmental selection pressures that will yield improved strains, better fitted for mass culturing of a desired species. Adopting the latter approach requires the development of a set of well-defined, easy-to-perform, and easy-to-interpret measurements that will help in identifying a better fitted species as a candidate for outdoor cultivation.
The objective of the current study was to use Nannochloropsis, a promising species for lipid production, as a case study to demonstrate the relevance of the need to develop and apply such a set of criteria to evaluate and expect success in outdoor cultivation.
The term “balanced growth” refers to microbial growth conditions in which the increase in any cell component is in line with increases in cell number. In such a case, one can follow growth by measuring a single-cell component like cell number and assume that a similar change occurs when following the increase in biomass or pigment content. Such a case is relevant when all nutritional requirements are provided in excess, including the source of energy. When dealing with heterotrophic growth, the source of energy is mainly an organic carbon source that allows the above-mentioned conditions to be expressed in parallel. In phototrophic growth, where the source of energy (light) is coming from outside the cell culture, any increase in biomass concentration results in a decrease in light (energy) availability due to self-shading. Although both species used in the current study were each acclimated to the specific growth light, no balanced growth was achieved. This is reflected by the fact that at any given irradiance, the rate of increase in cell number was not similar to the rate of increase in chlorophyll concentration, indicating a fast and active mechanism of response of chlorophyll content per cell as a function of light availability in the culture.
From the data presented in the growth experiments, two major observations can be made. The first one is the unique ability of the two Nannochloropsis species to respond rapidly to the changes in the light environment, while the second one points to the difference between calculation of specific growth rate on the basis of chlorophyll or on the basis of cell number which results in different values of specific growth rate (μ) (Fig. 1), and suggests that balanced growth is not taking place in N. oculata under the high light intensity of 300 μmol photons m−2 s−1. While at this irradiance, N. oceanica maintains approximately the same Chl/cell ratio (Table 3A) and shows increased growth rate also at 300 μmol photons m−2 s−1; in the N. oculata culture, the ratio of chlorophyll per cell decreased at that light intensity for growth (Table 3B) with an influence on the growth rate (μ) calculated per cell and indicates a higher sensitivity to this level of light.
These findings correlate with the photosynthesis measurements calculated per cell number. Indeed, Pmax in N. oculata grown at 300 μmol photons m−2 s−1 decreased to a lower value compared to that in cultures grown at 200 μmol photons m−2 s−1 (Fig. 4 (B2)). Consequently, we recommend that, when evaluating species performance, growth should be measured under a wide range of light intensities in order to mimic the change in Chl/cell ratio in variable light conditions in outdoor cultivation.
Comparing the two species, N. oceanica cultures demonstrated a similar response between the photosynthesis parameters calculated per chlorophyll including Pmax (Fig. 3 (a1 and a2)), ETR measurements (Fig. 2), NPQ (Table 2), and absorption cross-section (a*) (Table 2), which may indicate a better ability of the photosynthetic apparatus of N. oceanica, compared to N. oculata to respond to a wide range of light intensities and which may be an important characteristic for species that are to be cultivated under outdoor conditions. The observed lack of significant changes in absorption cross-section at different growth intensities in N. oculata is consistent with previous studies (e.g., Kandilian et al. 2013; Szabó et al. 2014). In contrast, N. oceanica increased its optical cross-sections by about 30% when light irradiance was increased from 50 to 300 μmol photons m−2 s−1, which corresponded to an appreciable rise in NPQ, while no remarkable changes were observed with N. oculata, both in optical cross-section and NPQ. The values of the optical absorption cross-section found in Nannochloropsis cells before their exposure to high light (4.2–5.8 × 10−3 m2 mg−1 Chla) were comparable to that found by Kandilian et al. (2013) (4.32 × 10−3 m2 mg−1 Chla), while in cells exposed to high light (1600 μmol photons m−2 s−1), the values compared to that found by Kromkamp et al. (2009) (6–8 × 10−3 m2 mg−1Chla) in outdoor Nannochloropsis cultures. Increased optical absorption cross-section in cells grown under high light may be explained by a reduced effect of pigment packaging and by the synthesis of pigments such as xanthophyll which are deputed to dissipate light instead to transfer it to reaction center thus assigning the light absorbed by any pigment to chlorophyll a, thereby virtually increasing cross-sections of Chl a (Dubinsky 1992).
We found that even after exposure of cells to high light, NPQ was much lower than that found by other workers (Simionato et al. 2011; Szabó et al. 2014; Chukhutsina et al. 2017). Indeed, they found NPQ values up to 4–5 in cultures exposed to high light, while in our cultures, maximum NPQ in high light-exposed cells of N. oceanica reached 1.5 (Table 4). In contrast, other studies indicate that NPQ in Nannochloropsis was close to that found by us (max value 1.5) (Kotabová et al. 2011; Cao et al. 2013). Higher values of NPQ have been found in cultures grown without additional CO2 supply (Simionato et al. 2011; Szabó et al. 2014; Chukhutsina et al. 2017). It is conceivable that limitation of CO2 supply may have limited the dissipation of the absorbed energy via photochemical quenching and increased the complementary mechanism of dissipation of energy, via NPQ.
Under excess light, the photosynthetic electron transport chain becomes increasingly reduced as a result of limitation of reductant and ATP use by the Calvin Benson cycle. Thus, energy supply potentially becomes greater than its dissipation, leading, in the absence of protective mechanisms, to ROS formation. One of these protection mechanisms is the activation of excess energy dissipation via the xanthophyll cycle, evidenced by non-photochemical quenching of chlorophyll fluorescence (see Goss and Jakob 2010 for details of the mechanisms involved). We observed an increase in values of NPQ in both cultures following exposure to higher light (Table 4). This behavior can be explained by the synthesis of xanthophyll cycle pigments during the exposure to high light. The lower values of NPQ observed in cultures before their exposure to high light may be attributed to energy quenching promoted by ∆pH, particularly for the cultures grown under the lower light intensities of 50–100 μmol photons m−2 s−1.
An additional physiological parameter that tends to be overlooked in outdoor cultivation is dark respiration. It is clearly demonstrated that light environment significantly affects the dark respiration activity, and high respiration rates may help in the ability to deal with excess light by being a sink for extra reducing power as seen in N. oculata after exposure to high light (calculated per cell) (Table 5).
Last but not least, we would like to suggest that the ability of N. oculata to withstand increased concentrations of RB and MV, which generate free radicals, may indicate a different protective mechanism such as upregulation of enzymes like SOD and catalase to environmental stress that result in oxidative stress (Fig. 5). Thus, this may serve as an additional tool of screening and evaluating the potential of specific algal species to be grown under outdoor conditions.
Conclusion
Nannochloropsis species, grown under optimal conditions, are able to acclimate to high light by reducing their cellular pigment content, thereby increasing cellular optical cross-section which entails an increased ETR and photosynthesis rates with consequent little dissipation of energy via NPQ. An interesting question concerns the evaluation of the performance of the cultures when exposed to high light irradiance, not only for photosynthesis studies but also for outdoor mass culture and species phenotyping. Photosynthesis parameters elaborated on a chlorophyll basis showed an increase in the photosynthetic activity measured either via chlorophyll fluorescence (ETR) or oxygen evolution. Presentation of data on a cell basis showed the opposite behavior, that is, cells exposed to high light showed a strong decline in their photosynthesis rate. This is not surprising since the chlorophyll content per cell was lower, and the increase in chlorophyll optical cross section was not sufficient to balance the chlorophyll reduction. As a result, two cultures having the same chlorophyll concentration but a different acclimation state may behave very differently when inoculated outdoors under high light. High chlorophyll content per cell (low light acclimation state) may cause excessive light absorption coupled with a lower capacity to dissipate excess light via non-photochemical quenching, with a high risk of photoinhibition.
This study has demonstrated that a simple set of experiments may reveal differences between species that are systematically closely related. For this study, we applied both photosynthesis and chlorophyll fluorescence coupled to growth measurements. Obviously, for a definitive evaluation of the two species, a further validation of the data gathered in the lab is required under outdoor conditions.
References
Ben Sheleg A, Novoplansky N, Vonshak A (2019) Can Rose Bengal resilience be used as a marker for photosynthetic resilience of Nannochloropsis oceanica strains in excess light environments? Algal Res 41:101562
Bilger W, Bjӧrkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Borowitzka MA, Vonshak A (2017) Scaling up microalgal cultures to commercial scale. Eur J Phycol 52:407–418
Boussiba S, Vonshak A, Cohen Z, Avissar Y, Richmond A (1987) Lipid and biomass production by the halotolerant microalga Nannochloropsis salina. Biomass 12:37–48
Burnison BK (1980) Modified dimethyl sulfoxide (DMSO) extraction for chlorophyll analysis of phytoplankton. Can J Fish Aquat Sci 37:729–733
Cao S, Zhang X, Xu D, Fan A, Mou S, Wang Y, Ye N, Wang W (2013) A trans thylakoid proton gradient and inhibitors induce a non-photochemical fluorescence quenching in unicellular algae Nannochloropsis sp. FEBS Lett 587:1310–1315
Chukhutsina VU, Fristedt R, Morosinotto T, Croce R (2017) Photoprotection strategies of the alga Nannochloropsis gadidana. Biochem Biophys Acta 1858:544–522
Dubinsky Z (1992) The functional and optical absorption cross-sections of phytoplankton photosynthesis. In: Fakowski PJ, Woodhead AD (eds) Primary productivity and biogeochemical cycles in the sea. Aquatic photosynthesis, Plenum Press, New York, p 31–45
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215
Fisher T, Minnaard J, Dubinsky Z (1996) Photoacclimation in the marine alga Nannochloropsis sp. (Eustigmatophyte): a kinetic study. J Plankton Res 18:1797–1818
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Hawkes TR (2014) Mechanisms of resistance to paraquat in plants. Pest Manag Sci 70:1316–1323
Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M (1998) Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol 116:173–181
Kandilian R, Lee E, Pilon L (2013) Radiation and optical properties of Nannochloropsis oculata grown under different irradiances and spectra. Bioresour Technol 137:63–73
Kochevar IE, Redmond RW (2000) Photosensitized production of singlet oxygen. Methods Enzymol 319:20–28
Kotabová E, Kaňa R, Jarešová J, Prášil O (2011) Non-photochemical quenching fluorescence in Chromera velia is enabled by fast violaxanthin de-epoxidation. FEBS Lett 585:1941–1945
Kovács L, Ayaydin F, Kálai T, Tandori J, Kós PB, Hideg É (2014) Assessing the applicability of singlet oxygen photosensitizers in leaf studies, photochemistry and photobiology. Photochem Photobiol 90:129–136
Kromkamp J, Forster RM (2003) The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur J Phycol 38:103–112
Kromkamp J, Limbeek M (1993) Effect of short-term variation in irradiance on light harvesting and photosynthesis of the marine diatom Skeletonema costatum: a laboratory study simulating vertical mixing. J Gen Microbiol 139:2277–2284
Kromkamp JC, Beardall J, Sukenik A, Kopeck J, Masojidek J, van Bergeijk S, Gabai S, t Shaham E, Yamshon A (2009) Short-term variations in photosynthetic parameters of Nannochloropsis cultures grown in two types of outdoor mass cultivation systems. Aquat Microb Ecol 56:309–322
Ma X-N, Chen T-P, Yang B, Liu J, Chen F (2016) Lipid production from Nannochloropsis. Mar Drugs 14:61–79
MacIntyre HL, Kana TM, Anning T, Geider RJ (2002) Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J Phycol 38:17–38
Murgia I, Tarantino D, Vannini C, Bracale M, Carravieri S, Soave C (2004) Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to Paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J 38:940–953
Padmasree K, Padmavathi L, Raghavendra AS (2002) Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Crit Rev Biochem Mol Biol 37:71–119
Palacios YM, Vonshak A, Beardall J (2018) Photosynthetic and growth responses of Nannochloropsis oculata (Eustigmatophyceae) during batch cultures in relation to light intensity. Phycologia 57:492–502
Ruban AV (2014) Evolution under the sun: optimizing light harvesting in photosynthesis. J Exp Bot 66:7–23
Sandnes JM, Källqvist T, Wenner D, Gislerød HR (2005) Combined influence of light and temperature on growth rates of Nannochloropsis oceanica: linking cellular responses to large-scale biomass production. J Appl Phycol 17:515–525
Schreiber U, Klughammer C, Kolbowski J (2012) Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynth Res 113:127–144
Seely GR, Duncan MJ, Vidaver WE (1972) Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide. Mar Biol 12:184–188
Simionato D, Sforza E, Corteggiani-Carpinelli E, Bertucco A, Giacometti GM, Morosinotto T (2011) Acclimation of Nannochloropsis gaditana to different illumination regimes: effects on lipids accumulation. Bioresour Technol 102:6026–6032
Simionato D, Block MA, La Rocca N, Jouhet J, Maréchal E, Finazzi G, Morosinotto T (2013) The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot Cell 12:665–676
Solovchenko A, Lukyanov A, Solovchenko O, Didi-Cohen S, Boussiba S, Khozin-Goldberg I (2014) Interactive effects of salinity, high light, and nitrogen starvation on fatty acid and carotenoid profiles in Nannochloropsis oceanica CCALA 804. Eur J Lipid Sci Technol 116:635–644
Sukenik A, Beardall J, Kromkamp JC, Kopecky J, Masojídek J, van Bergeijk S, Gabai S, Shaham E, Yamshon A (2009) Photosynthetic performance of outdoor Nannochloropsis mass cultures under a wide range of environmental conditions. Aquat Microb Ecol 56:297–308
Szabó M, Parker K, Guruprasad S, Kuzhiumparambil U, Lilley RM, Tamburic B, Schliep M, Larkum AW, Schreiber U, Raven JA, Ralph PJ (2014) Photosynthetic acclimation of Nannochloropsis oculata investigated by multi-wavelength chlorophyll fluorescence analysis. Bioresour Technol 167:521–529
Vonshak A, Laorawat S, Bunnag B, Tanticharoen M (2014) The effect of light availability on the photosynthetic activity and productivity of outdoor cultures of Arthrospira platensis (Spirulina). J Appl Phycol 26:1309–1315
Weis E, Berry JA (1987) Quantum efficiency of Photosystem II in relation to “energy”-dependent quenching of chlorophyll fluorescence. Biochim Biophys Acta 894:198–208
Acknowledgements
The work by GT has been partially supported by the H2020 project “SABANA” funded from the EU Horizon 2020 research and innovation program under Grant Agreement No. 727874.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vonshak, A., Novoplansky, N., Silva Benavides, A.M. et al. Photosynthetic characterization of two Nannochloropsis species and its relevance to outdoor cultivation. J Appl Phycol 32, 909–922 (2020). https://doi.org/10.1007/s10811-019-01985-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01985-5