Abstract
Microalgae with high lipid productivity offer great promise as a potential source of biodiesel, an alternative to fossil diesel. Since a target of both high biomass yield and lipid content is difficult to attain with the classical photoautotrophic mode of nutrition, mixotrophy has evolved as a preferred mode for enhancement of growth in microalgae. In the present study, this strategy was explored extensively with six different exogenous carbon sources in eight different concentrations to investigate the effect of these carbon sources on the biomass and lipid production potential of the green alga Tetradesmus obliquus (SAG 276-3a). To further augment the effect of mixotrophy on the growth and lipid accumulation of the test microalga, selected carbon sources with their specific concentrations showing enhancement in both biomass yield and lipid accumulation in the first phase of the experiment were interacted with each other. Significant rise in biomass yield by twofold was seen in the case of 0.16% citrate + 0.08% bicarbonate interaction, whereas for lipid accumulation, maximum rise in lipid content (% dry cell wt.) by 2.3-fold and lipid yield (g L-1) by 4.5-fold was observed in the case of 0.16% acetate + 0.16% citrate-supplemented cultures. The biodiesel samples as analyzed using GC–MS were predominated with saturated and monounsaturated fatty acid methyl esters. Fuel property analysis of the biodiesel samples found them to be well suited to the Indian, American (ASTM), and European (EN) biodiesel standards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In India, the rising demand for energy is anticipated to further increase the use of fossil fuels. Such a scenario would raise the levels of greenhouse gas (GHG) emissions eventually increasing the environmental problems. Additionally, this would also lead to rising inequalities between the rural and urban populations, health problems, and various other social issues at the community level. Keeping this situation in mind, it is important to shift the focus from pollution causing conventional sources of energy to more environmentally safe options such as renewable energy resources (Ministry of Environment, Forest and Climate change (MoEFCC), 2014; International Energy Agency IEA, 2015). Microalgae are known to be the best resources for future sustainable biofuel production considering their advantage over the fuel vs food debate and the issues related to land and water footprint. One of the best ways is to utilize microalgal lipids for biodiesel production. Nutrient regime alterations and variations in the abiotic factors have been known to influence lipid productivity in algae, but their negative effect on the overall lipid productivity and cost-intensive and difficult application in large-scale systems, respectively, limits the application of such strategies (Mallick et al. 2012; Shah et al. 2014; Sharma et al., 2015; Sonkar and Mallick 2016; Shen et al. 2019). With each system having their own pros and cons, the growth and lipid accumulation potential of the microalgal strain and economics of the process makes the choice much easier. Having said that, we in this research study have chosen the chlorophycean microalga, Tetradesmus obliquus, as our test organism. Previously carried out research work in our laboratory on this microalgal strain has shown significant response by this microalga in terms of growth and lipid enhancement when subjected to different biotic and abiotic stress conditions (Mandal and Mallick, 2009, 2011, 2012; Mandal et al. 2013). Mixotrophy having evolved as an effective mode of nutrition for growth and functional component accumulation in microalgae, in the past few years, we decided to further augment the productivity of this microalga through different exogenously supplemented carbon sources.
Microalgae under mixotrophic conditions obtain chemical energy from the exogenously supplemented carbon sources which get stored in the form of lipid droplets (Chisti 2013). The lipid productivity (simultaneous increase in biomass and lipid yield) of the microalgae under mixotrophic conditions is much higher compared with that under photoautotrophic conditions. For example, Ratnapuram et al. (2018) achieved a lipid yield of 284 g kg-1 and biomass yield of 8 g L-1 in Chlorella pyrenoidosa under mixotrophic condition compared with 60 g kg-1 and 2.5 g L-1 of lipid and biomass yield, respectively, under autotrophic condition. Similarly, Ogbonna and Ogbonna (2018) found that lipid productivity in Dictyosphaerium sp. was maximum (115.8 mg L-1 day-1) when 40 g L-1 of glucose was supplemented in BG 11 medium. Likewise, other reports by Mandal and Mallick (2009), Lin and Wu (2015), Bajwaa et al. (2016), and Huang et al. (2017) also justify the use of mixotrophy for higher lipid productivity in microalgae.
However, despite such various studies which primarily concentrate on the use of any one or two forms of carbon sources, a consolidated report on the interaction of a broad range of concentrations and types of carbon sources is scarcely available to our knowledge. In the light of mixotrophic mode of cultivation being considered a promising strategy for improving the production yields of microalgal biodiesel in the future, such a research study is indeed essential to understand the dynamics of algal lipid production. Hence, this study has been carried out to observe the effect of six different carbon sources in eight different concentrations on the growth and lipid accumulation potential of the green microalga, T. obliquus. The interaction of the best carbon concentrations has also been studied followed by composition and quality analyses of the biodiesel obtained, for a complete overview of the selected strategy.
Materials and methods
Photoautotrophic cultivation of the microalga
Axenic cultures of the green microalga, Tetradesmus obliquus (Turpin) M.J. Wynne (SAG 276-3a), were grown in 100 mL of N 11 medium (Soeder and Bolze 1981) under 75 μmol photons m-2 s-1 PAR (photosynthetic active radiation) and 25 ± 2 °C. A light:dark period of 14:10 h was maintained for best growth of the test microalga. The culture flasks were shaken two to three times a day to prevent sticking of the cultures to the bottom of the flasks. This was denoted as control culture.
Mixotrophic cultivation of T. obliquus
Six different carbon sources namely, dextrose annhydrous, sodium salts of acetate, citrate and bicarbonate, glycerol and cysteine in eight different concentrations ranging from 0.04 to 2.0% were supplemented in N 11 medium for maintaining mixotrophic growth condition. During the course of the study, the individual and the combined effects of the carbon sources on the growth and lipid accumulation in T. obliquus were thoroughly and methodically investigated. To avoid any alteration in pH, the media were buffered with 2.0 mM tris(hydroxymethyl) aminomethane buffer.
Estimation of dry weight/biomass yield
Dry cell weight (dcw)/biomass yield was determined according to Rai et al. (1991). A known volume of culture after centrifugation at 5000 rpm for 10 min was harvested and transferred to a pre-weighed 30-mL glass vial followed by drying at 80 °C till constant weight. The weight of the dry biomass as obtained after subtraction of the initial weight of the vial from the final weight was considered as the biomass yield/dcw and was expressed as g L-1.
Extraction and estimation of lipid yield/ content
Extraction of lipids from the dried biomass of T. obliquus was done as per Bligh and Dyer (1959) and estimation of the extracted lipids was done as per Mandal and Mallick (2009).
Photosynthetic parameter measurement using PAM fluorometry
PAM fluorometry was done according to White et al. (2011) using a DUAL-PAM 100 chlorophyll fluorometer (Heinz Walz Gmbh, Germany). Aliquots (3 mL) of the microalgal cells were sampled for analysis of maximum photosynthetic efficiency. The maximum quantum yield of PSII (Fv/Fm) = (Fm − F0)/Fm) was determined after 15 min of dark incubation, where Fm and F0 are the maximum and minimum fluorescence of dark-adapted cells, respectively. Non-photochemical quenching (NPQ) was calculated as (Fm − Fm’)/Fm’, where Fm’ and Fm are the maximum fluorescence of light-adapted and dark-adapted cells, respectively. Photosynthetic electron transfer rate (ETR) was calculated as 0.5 × I × Y(II), where 0.5 represents the fraction of light absorbed by PSII (half of the total incident light), I is the incident light intensity, and Y(II) is the quantum yield of PSII in the light.
Transesterification of lipids and analysis of biodiesel
The protocol of Mandal et al. (2013) was followed for transesterification of microalgal lipids. Methanol: hydrochloric acid: oil in a molar ratio of 82:4:1 at 65 °C for 6.4 h reaction time was used for transesterification process. Further analysis of the fatty acid methyl ester composition of the biodiesel sample was done by using gas chromatography–mass spectrometry (GC–MS) (Autosystem XL) containing a PE-5 capillary column (phenylmethylpolysiloxane, 30 m × 0.25 mm × 0.25 μm) and a Turbomass Gold Mass Spectrometer (Perkin- Elmer, USA). An internal standard was prepared using methylpentadecanoate.
Analysis of fuel characteristics of T. obliquus biodiesel
Density
The density of the microalgal biodiesel was calculated as per the protocol suggested by the American Standards for Testing of Materials ASTM D 4052-96 (2003).
The density of the biodiesel was expressed as kg m-3 and calculated as:
where M1 and M2 are the mass of the empty relative density bottle and the mass of the bottle along with the sample, respectively, and V is the volume of the relative density bottle.
Viscosity
The protocol of the Indian standard (IS, 15607) (Indian Standard IS: 15607, 2005) was used for the measurement of the viscosity (expressed as cSt) of the algal biodiesel sample using the formula:
Viscosity (ν) = C × t, where C and t represent the calibration constant of viscometer (0.0343 cSt s-1) and the flow time in seconds, respectively.
Acid value
The amount (mg) of KOH required for neutralizing the organic acids found in 1 g of algal oil is defined as the acid value. The titrimetry method mentioned in the European standards for biodiesel (European Standard EN 14214, 2003) is used for calculation of acid value.
where N represents the normality of KOH solution (0.1 N) and V and W represent titrate volume (mL) and weight of the sample (g), respectively.
Saponification value
The titrimetry method of Vicente et al. (2004) was used for the measurement of the saponification value. Saponification is the process of hydrolyzing the fatty acids present in the triglycerides to glycerol and potassium salts of fatty acids.
About 1 g of oil was transferred to a round bottom flask and dissolved in 5 mL of distilled ethyl alcohol. Twenty five milliliters of 0.5 N alcoholic KOH was then added into it and mixed well. This was referred to as the test (T). A blank flask (B) was simultaneously prepared in the same way without containing any oil. After the saponification reaction, 2 drops of phenolphthalein were added and titrated against a standard solution of 0.5 N hydrochloric acid until the pink color disappeared. This procedure was done to determine the amount of unused KOH in the respective flasks does determine the amount of KOH required to saponify 1 g of oil. As the quantity of unused KOH was higher in the flask referred to as blank, the formula for determining the saponification number/value was (blank–test).
where B and T refer to blank (mL) and test (mL) and W is the weight of the sample (g).
Molecular weight
According to Xu et al. (2006), the molecular weight (MW) of T. obliquus biodiesel can be calculated from the acid and saponification values as per the formula:
where SV and AV are the saponification value and acid value, respectively.
Calorific value
The calculation in the ASTM D 240-02 standard was used for measuring the calorific value (MJ kg-1) of the algal biodiesel sample.
where WC is the heat capacity of the calorimeter (2250 cal °C-1); ΔT the temperature rise (°C); l1 and l2 are the lengths of the thread (8 cm) and the wire (3 cm); CVT and CVW indicate the calorific values of the thread and the wire which are 1.9 and 2.1 cal cm-1, respectively; and m denotes the mass of the algal biodiesel sample (g).
Iodine value
The oxidative stability of the algal biodiesel is best indicated by the iodine value (expressed as g I2 (100 g)-1 of biodiesel). The titrimetry method of calculation of the American Oil Chemists’ Society (AOCS) (1998) was used:
where B, T, and W denote the blank (mL), titrate (mL), and weight of the sample (g), respectively, and N is the normality of Na2S2O3 solution.
Cetane index
The combustion efficiency of algal biodiesel can be checked by the cetane index. The formula of Krisnangkura (1986) was used for cetane index calculation.
where SV and IV are the saponification and iodine values, respectively.
Ash content
The ash content was calculated as per ASTM D 482-74. The equation for the calculation is:
where Wa and Ws are the ash weight and sample weight, respectively.
Water content
A microprocessor-based Karl Fisher Titrator (model, S-970; Systonic Instruments, Panchkula, Haryana, India) was used for measuring the water content in the biodiesel sample. An audible alarm and front panel LCD display in the microprocessor was used for indicating the total water content (%).
Statistical analysis
All the experiments were conducted in triplicate independent cultures. A window-based software MSTAT-C was used to perform Duncan’s new multiple range test to analyze the difference between the yields of various products in control and selected treatments.
Results
Effect of individual carbon sources on growth and lipid accumulation potential of a chlorophycean microalga, Tetradesmus obliquus
The effect of the different concentrations of dextrose, glycerol, acetate, citrate, bicarbonate, and cysteine supplementation in N 11 medium on the growth and lipid yield of T. obliquus is shown in Table 1 along with the control values (without supplementation of exogenous carbon). Of the eight different concentrations used, supplementation of 1.6% dextrose was found to stimulate growth/biomass yield by 2.6-fold to 3.16 g L-1 and lipid yield by 2.4-fold to 0.36 g L-1 compared with 1.22 and 0.15 g L-1, respectively, under control condition. Glycerol supplementation in N 11 medium also significantly stimulated growth and lipid accumulation in T. obliquus. 1.6% glycerol stimulated maximum growth up to 2.35 g L-1 (twofold higher), compared with 1.15 g L-1 in the control. The lipid yield under the same condition rose by 2.8-fold to 0.40 g L-1 compared with 0.14 g L-1 in the control culture. Although 1.6% of glycerol supplementation was found to produce the best results, 1.2 and 2% glycerol supplementation had values for growth and lipid accumulation marginally different from the best concentration and hence were used for combined studies later. With acetate, a maximum biomass yield of 3.13 g L-1 was obtained with supplementation of 0.3% sodium acetate which is 2.5-fold higher than the biomass yield of the photoautotrophically (control) grown culture. Acetate supplementation of 0.3% also increased lipid yield to 0.69 g L-1, i.e., 4.6-fold higher than the control culture. Raising the concentration of acetate beyond 0.3% was not found to be useful for increasing the biomass yield, lipid yield, and lipid content. Citrate supplementation of 0.3% significantly raised the biomass yield by 2.6-fold and lipid yield by 4.8-fold. Concentrations higher than 0.6% did not induce growth and lipid accumulation in T. obliquus. Although lipid content was higher than the control culture, the rise was marginal. Maximum biomass yield was 2.2-fold higher under 0.16% bicarbonate supplementation compared with 1.26 g L-1 under control condition and maximum lipid yield was 2.6-fold higher than the control. Cysteine did not significantly stimulate growth and lipid accumulation in T. obliquus although a rise in the biomass yield, lipid yield, and lipid content was observed in cysteine supplementations up to 0.3% concentration in comparison with the control culture, beyond which values lower than that for the control culture were recorded. The maximum growth in all the mixotrophic (exogenously carbon-supplemented) conditions was attained on the 15th day of incubation after a lag period of 3 days whereas under control condition, the maximum growth was found after 21 days.
Effect of combined carbon sources
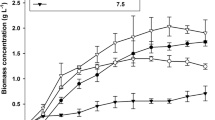
After carrying out a detailed study to test the effect of individual carbon sources on the growth and lipid accumulation potential of T. obliquus, a study with combined carbon sources was carried. The results of this combined study have been detailed in the paragraphs below but the graphical or tabular representation of all the combinations have not been shown, rather a detailed graphical representation of the best combination and concentration of carbon sources is shown in (Fig. 1).
Biomass yield (a), lipid yield (b), and lipid content (c) of T. obliquus under combined conditions between acetate and citrate. The values in the graph legend in the top show the different concentrations (%) of the carbon sources used. The letters ‘A’ and ‘C’ in the graph legends denote the carbon sources, acetate and citrate, respectively
Bicarbonate and acetate
From the results of the supplementation of individual carbon sources in N 11 medium, it was observed that some concentrations of bicarbonate and acetate used showed marginal difference in the biomass yields of T. obliquus grown in those supplemented media. As it is clearly evident from the above results that exogenous supplementation of carbon sources led to higher growth eventually leading to higher lipid accumulation, the interaction study between bicarbonate and acetate was carried out in two parts. First, two concentrations of bicarbonate (one concentration showing the maximum biomass yield i.e. 0.16% and another concentration penultimate to the best one i.e. 0.08%) and four concentrations of acetate (one concentration showing the maximum biomass yield i.e. 0.3% and other three concentrations penultimate to the best one i.e. 0.16, 0.6, and 1.2%) were combined. The results showed that 0.08% of bicarbonate + 0.16% of acetate produced the best results in terms of 3.5-fold rise in lipid yield with the biomass yield and lipid content being 2.06 g L-1 and 25.7% dcw, respectively, compared with 1.24 g L-1 and 11.8% dcw of biomass yield and lipid content, respectively, under control conditions (data not shown).
Second, two concentrations of acetate (one concentration showing the maximum biomass yield i.e. 0.3% and another concentration penultimate to the best one i.e. 0.16%) and four concentrations of bicarbonate (one concentration showing the maximum biomass yield i.e. 0.16% and other three concentrations penultimate to the best one i.e. 0.08, 0.3, and 0.6%) were combined. The results showed that 0.16% of acetate + 0.08% bicarbonate produced the best results in terms of 3.8-fold rise in lipid yield, with the biomass yield and lipid content being 2.31 g L-1 and 25.2% dcw, respectively, compared with 1.18 g L-1 and 12.7% dcw of biomass yield and lipid content, respectively, in the control (data not shown).
Bicarbonate and citrate
With the same strategy as described above, the interaction between bicarbonate and citrate was also studied in two parts. First, two concentrations of bicarbonate (one concentration showing the maximum biomass yield i.e. 0.16% and another concentration penultimate to the best one i.e. 0.08%) and four concentrations of citrate (one concentration showing the maximum biomass yield i.e. 0.3% and other three concentrations penultimate to the best one i.e. 0.16, 0.6, and 0.08%) were combined. The results showed that 0.08% of bicarbonate + 0.16% of citrate produced the best results with a 2.9-fold rise in lipid yield with the biomass yield and lipid content being 1.87 g L-1 and 22.4% dcw, respectively, compared with 1.21 g L-1 and 12.3% dcw of biomass yield and lipid content, respectively, under control conditions (data not shown).
Second, two concentrations of citrate (one concentration showing the maximum biomass yield i.e. 0.3% and another concentration penultimate to the best one i.e. 0.16%) and four concentrations of bicarbonate (one concentration showing the maximum biomass yield i.e. 0.16% and other three concentrations penultimate to the best one i.e. 0.08, 0.3, and 0.6%) were combined. The results showed that 0.16% citrate + 0.08% bicarbonate produced the best results with a 3.4-fold rise in lipid yield, with the biomass yield and lipid content being 2.41 g L-1 and 21% dcw, respectively, compared with 1.27 g L-1 and 11.7% dcw of biomass yield and lipid content, respectively, under control conditions (data not shown).
Glycerol and acetate
The combination of glycerol and acetate was first studied by taking two concentrations of glycerol (one concentration showing the maximum biomass yield i.e. 1.6% and another concentration penultimate to the best one i.e. 1.2%) and four concentrations of acetate (one concentration showing the maximum biomass yield i.e. 0.3% and other three concentrations penultimate to the best one i.e. 0.16, 0.6, and 1.2%). It was found that 1.2% glycerol + 0.16% acetate raised the lipid yield and lipid content by > 2-fold to 0.41 g L-1 and 26% dcw, respectively, compared with 0.16 g L-1 and 12.8% dcw of lipid yield and lipid content, respectively, in the control. The biomass yield under the same condition only increased slightly marginally to 1.57 g L-1 in comparison with 1.25 g L-1 for the control culture (data not shown).
Second, two concentrations of acetate (one concentration showing the maximum biomass yield i.e. 0.3% and another concentration penultimate to the best one i.e. 0.16%) and four concentrations of glycerol (one concentration showing the maximum biomass yield i.e. 1.6% and other three concentrations penultimate to the best one i.e. 1.2, 2, and 0.6%) were combined. The results showed that 0.16% acetate + 1.2% glycerol increased the lipid yield by 2.6-fold to 0.39 g L-1 and the lipid content by twofold to 26.7 % dcw, with the biomass yield rising to 1.49 g L-1 (data not shown).
Glycerol and citrate
Applying the same strategy, the combination of glycerol and citrate was studied first by taking two concentrations of glycerol (one concentration showing the maximum biomass yield i.e. 1.6% and another concentration penultimate to the best one i.e. 1.2%) and four concentrations of citrate (one concentration showing the maximum biomass yield i.e. 0.3% and other three concentrations penultimate to the best one i.e. 0.16, 0.6, and 0.08%). The combination of 1.6% glycerol + 0.16% citrate significantly increased the lipid yield by 2.5-fold to 0.35 g L-1, with the biomass yield and lipid content being 1.42 g L-1 and 24.7% dcw, respectively, compared with 1.17 g L-1 and 12.1% dcw of biomass yield and lipid content, respectively, under control conditions (data not shown).
Second, two concentrations of citrate (one concentration showing the maximum biomass yield i.e. 0.3% and another concentration penultimate to the best one i.e. 0.16%) and four concentrations of glycerol (one concentration showing the maximum biomass yield i.e. 1.6% and other three concentrations penultimate to the best one i.e. 1.2, 2, and 0.6%) were combined. The results showed that 0.16% citrate + 1.6% glycerol raised the lipid yield to 0.31 g L-1 and the lipid content to 24.2% dcw with the biomass yield being 1.31 g L-1 compared with 1.15 g L-1 biomass yield under control condition (data not shown).
Acetate and citrate
Of all the combinations studied, the best results were obtained by combining acetate and citrate. First, two concentrations of acetate (one concentration showing the maximum biomass yield i.e. 0.3% and another concentration penultimate to the best one i.e. 0.16%) and four concentrations of citrate (one concentration showing the maximum biomass yield i.e. 0.3% and other three concentrations penultimate to the best one i.e. 0.16, 0.6, and 0.08%) were studied. A 4.8-fold rise in the lipid yield from 0.14 to 0.67 g L-1 was observed after supplementation of 0.16% acetate + 0.16% citrate, with the lipid content increasing by 2.3-fold. A significant increase in the biomass yield to 2.42 g L-1 was also observed (Fig. 1).
Second, two concentrations of citrate (one concentration showing the maximum biomass yield i.e. 0.3% and another concentration penultimate to the best one i.e. 0.16%) and four concentrations of acetate (one concentration showing the maximum biomass yield i.e. 0.3% and other three concentrations penultimate to the best one i.e. 0.16, 0.6, and 1.2%) were combined. Similar to the results above, the combination of 0.16% citrate + 0.16% acetate also increased lipid yield by 4.6-fold to 0.65 g L-1 and lipid content by 2.5-fold to 29.6% dcw. A significant rise in biomass yield under the same condition was also observed. The maximum yields in all the above mentioned mixotrophic conditions were attained on the 15th day of incubation.
From the results of the abovementioned experiments, it is evident that the maximum biomass yield was obtained under 1.6% dextrose supplementation, but it was only marginally higher than the maximum biomass yields obtained with 0.3% acetate and 0.3% citrate supplementation. On the contrary, 0.3% acetate and 0.3% citrate yielded the highest amount of lipid, a value which was significantly higher than the lipid yield obtained with 1.6% dextrose supplementation. Furthermore, the lipid content observed under acetate and citrate supplementation was not significantly enhanced. However, combined supplementation with 0.16% acetate + 0.16% citrate resulted in a significant rise in both the lipid yield and the lipid content. With maximum biodiesel production being the primary objective of this research study, exogenous supplementation of acetate and citrate showing promising possibilities for enhancing the growth and lipid accumulation in T. obliquus was selected for future studies.
PAM Fluorometry
After observing the individual and combined effect of different exogenous carbon sources on the growth and lipid accumulation potential of T. obliquus, their effect on different photosynthetic parameters was studied by using a PAM fluorometer. Mixotrophic/exogenously carbon-supplemented conditions did not show any improvements in the maximum quantum efficiency or the electron transport rate of the algal cells compared with photoautotrophic condition although significant improvements in the non-photochemical quenching values was observed (Table 2).
Values are mean ± SE (n = 3). Values within a column followed by different letters are significantly different from each other (p < 0.05; Duncan’s new multiple-range tests). A separate analysis was done for each column.
Characterization of the transesterified lipids
After selection of the best mixotrophic combination for maximum lipid accumulation in T. obliquus, the extracted lipids were transesterified for biodiesel and the transesterified lipids/biodiesel was then characterized using GC-MS. The fatty acid methyl ester (FAME) composition is shown in Table 3. The FAME composition of the biodiesel samples showed a predominance of five major fatty acid methyl esters, namely, palmitic acid, stearic acid, oleic acid, linoleic acid, and linolenic acid along with few traces of omega-3 fatty acids. About 80% of the FAMEs in the biodiesel samples obtained under selected mixotrophic conditions consisted of saturated fatty acids (SFA) + monounsaturated fatty acids (MUFA) compared with 75% in the control samples (Table 3). Although the percentage of oleic acid methyl ester in all samples was found to vary marginally, the presence of stearic acid in the biodiesel samples grown under few mixotrophic conditions was found to be responsible for raising the percentage of SFAs in the said samples. However, maximum SFA + MUFA content of 82.7% was observed under 0.16 + 0.16% acetate + citrate supplementation, with a linoleic + linolenic acid content of 14.7% being the lowest among all the samples tested.
Values are mean ± SE (n = 3). Values within a column followed by different letters are significantly different from each other (p < 0.05; Duncan’s new multiple-range tests). A separate analysis was done for each column
EPA, Eicosapentaenoic acid; DHA, docosahexaenoic acid. Peak areas with less than 0.1% were considered to be negligible.
Analysis of fuel characteristics of T. obliquus biodiesel
Fuel property analysis was done for the biodiesel sample obtained from the best mixotrophic condition, i.e., 0.16 + 0.16% of acetate + citrate supplementation in N 11 medium. The mean value of all the tests after three replications was calculated (Table 4). Compared with petrodiesel, the density, viscosity, acid value, and cetane index were slightly higher in the algal biodiesel sample. Contrarily, the calorific value of the algal biodiesel (40.0 MJ kg-1) was lower than that of petrodiesel. The ash and moisture contents were similar to petrodiesel.
Discussion
A number of metabolic interactions between organic and inorganic compounds in the microalgal cells are responsible for their higher biomass production during the mixotrophic mode of cultivation (Cid et al. 1992; Nelson and Cox, 2000; Van Wagenen et al., 2015). With an intention to apply this strategy for improvement of growth and lipid accumulation in the test microalga, T. obliquus, six different carbon sources, namely, dextrose, glycerol, acetate, citrate, bicarbonate, and cysteine were supplemented in eight different concentrations ranging from 0.04 to 2.0%, each for a period of 36 days to observe their effects on the said parameters. The results obtained demonstrated a > 2-fold rise in biomass yield of T. obliquus in 1.6% dextrose-, 0.3% acetate-, 0.3% citrate-, and 0.16% bicarbonate-supplemented cultures compared with the control photoautotrophic culture. Increased production of ATP resulting in increased glucose anabolism results in higher production of biomolecules for higher growth of the microalga under glucose-supplemented conditions (Martinez-Blanco et al., 1990); oxidation of acetate to form malate in glyoxysomes and citrate in mitochondria to provide carbon skeletons for biosynthesis under acetate and citrate available conditions (Perez-Garcia et al., 2011), shift in the photoinhibitory light intensity (Estévez-Landazábal et al., 2013), and the compensation of photorespiratory loss by active uptake of bicarbonate (Subramanian et al., 2013) could be possible reasons for such higher biomass yields under the above conditions (Mokashi et al. 2016; Rai and Gupta 2016; Ogbonna and Ogbonna 2018; Ratnapuram et al. 2018). Despite the significant rise in the biomass yield of T. obliquus, cellular lipid content in the above mixotrophic conditions showed a marginal rise compared with the control, except in case of dextrose supplementation where a reduction in the lipid content was observed. However, the lipid yield was found to be significantly enhanced in all the exogenously carbon-supplemented conditions except under cysteine supplementation. The lipid yield being a product of the biomass yield and lipid content of the microalga, a rise or fall in either of the two or both the factors influences the yield of the extracted lipid eventually influencing the total lipid production rate. A highly positive correlation (r = 0.95, p < 0.01) between the biomass and lipid yield in T. obliquus further testifies the simultaneous rise in the biomass and lipid yield under the abovementioned conditions. However, the contradictory observation in case of cysteine supplementation could probably have been due to the insignificant contribution of cysteine degradation to the acetyl-CoA pool for enhanced energy production through the TCA cycle in the microalgal cells (Nelson and Cox, 2000). Any further increase in the concentrations of the exogenous carbon sources did not result in the increase of the measured parameters which might probably have been due to substrate inhibition (Estévez-Landazábal et al., 2013). Nevertheless, species-specific dependence of microalgal lipid accumulation on the concentrations and interaction of carbon sources used cannot be ignored (Degrenne et al. 2010).
After analyzing the results obtained under supplementations of individual carbon sources in N 11 medium, the carbon sources capable of simultaneously stimulating biomass yield, lipid content, and lipid yield in T. obliquus compared with the control cultures were studied in various combinations so as to find an optimal concentration of carbon sources capable of enhancing maximum growth and lipid accumulation in T. obliquus. A significant rise in biomass yield by twofold was seen in case of 0.16% citrate + 0.08% bicarbonate supplementation. A rise in the rate of photosynthesis and respiration for production of biomolecules and energy by additional input of bicarbonate and citrate which act as substrates for the respective metabolic pathways justifies the higher biomass yield under citrate + bicarbonate–supplemented condition. The interactive concentrations of citrate and bicarbonate showing maximum growth were found to be reduced to half compared with the concentration of each carbon source used individually for inducing maximum growth in the test microalga, thus confirming their additive effect on the discussed parameter. The lipid content under this interactive condition was, however, not significantly affected possibly due to the inability of citrate and bicarbonate in acting as direct substrates for lipid production (Subramanian et al., 2013; Lowrey et al. 2015).
In the case of lipid content, > 2-fold rise was observed in case of 0.16% acetate + 0.08% bicarbonate–, 1.2% glycerol + 0.16% acetate–, 1.6% glycerol + 0.16% citrate–, and 0.16% acetate + 0.16% citrate–supplemented conditions. In the case of 1.2% glycerol + 0.16% acetate, the lipid content of 26% dcw compared with that of 12.5% dcw under control condition was observed due to the direct role of both the carbon sources used in fatty acid synthesis in the microalgal cells as glycerol-3-phosphate, a phosphoric ester of glycerol, acts as the starting material for de novo synthesis of glycerolipids (Radakovits et al., 2010; Andruleviciute et al., 2014). Similarly, the rise in lipid content in the case of 0.16% acetate + 0.08% bicarbonate and 1.6% glycerol + 0.16% citrate, by comparatively lesser quantity compared with the above two mixotrophic conditions, was justified by the role of the interacting carbon sources on the lipid accumulation in T. obliquus.
Maximum rise in lipid accumulation (4.8-fold rise in lipid yield and 2.3-fold rise in lipid content) without compromising with the biomass yield (~ 2-fold rise) was observed in the case of 0.16% acetate + 0.16% citrate. Acetate acts as a direct substrate for acetyl-CoA formation by the action of the acetyl coenzyme A synthetase (ACS) (Jogl and Tong, 2004). This acetyl-CoA then acts as a precursor molecule for the formation of lipids. Citrate on the other hand is used in the TCA cycle for respiration and energy generation. Additionally, the citrate formed during cell respiration is cleaved into acetyl-CoA in the cytosol by ATP-citrate lyase (Nelson and Rinne, 1977). The acetyl-CoA formed by both, the activity of ACS and pyruvate dehydrogenase (PDH) in the chloroplast, is also fed into the TCA cycle. On excessive accumulation of acetyl-CoA, citrate formation is inhibited as such a metabolic state indicates excessive accumulation of energy in the microalgal cells, thus diverting the acetyl-CoA reserve towards lipid production through malonyl-CoA formation by acetyl-CoA carboxylase (ACCase) (Estévez-Landazábal et al., 2013; Menegol et al. 2019). The ACS1 is activated in the presence of acetate but is deactivated in the presence of glucose (De Cima et al., 2005).
Although evidence in support of combined strategy can be found in few reports such as Kong et al. (2013), contradictory findings have been reported by Sun et al. (2014), Silva et al. (2016), and Bajwaa et al. (2016), where significant enhancement in biomass yield of Chlorella vulgaris, Neochloris oleoabundans, and Chlorella pyrenoidosa, respectively, was observed, but the lipid contents in the microalgae were not significantly stimulated upon addition of 1% glycerol + 1% glucose, 0.75% glucose + 0.75% acetate, and 1% glycerol + 0.2% glucose, respectively. Contrary to these examples, in our study, we successfully selected organic carbon compounds which in combination can significantly stimulate lipid accumulation in T. obliquus along with a relative rise in the biomass yield.
As mentioned above, the presence of exogenous carbon sources in the growth medium can effectively enhance growth and lipid accumulation in T. obliquus. A detailed description of the possible biochemical interactions within the microalgal cells, under the different carbon-supplemented conditions, has been provided above. Yet, monitoring the role of the photosynthetic machinery in the test microalga can give deeper insights into the interactions of different energy generating and consuming pathways in the algal cells under the abovementioned conditions. The results obtained from the PAM fluorometric studies indicate a reduction in the maximum quantum yield of PSII (FV/FM) and electron transport rate under mixotrophic conditions compared with the autotrophic condition. An analogous observation has been reported in Phaeodactylum tricornutum by Liu et al. (2009) and in Ochromonas danica by Wilken et al. (2014). The reasons cited by them for such an observation imply a downregulation of carbon fixation by the Calvin-Benson cycle, reduction in the need for linear electron transport to provide NADPH as reducing equivalents for inorganic carbon fixation, and a reduction in RUBISCO activity under mixotrophic conditions. Additionally, repression of photosynthetic oxygen evolution, modulation of the levels of PSII reaction center protein D1 (Oesterhelt et al. 2007), reduction of carbonic anhydrase activity, inhibition of chlorophyll a/b binding gene, and an increase in PSI:PSII ratio due to the need to sustain ATP consumption during acetate metabolism in the glyoxylate cycle (Steinbiß and Zetsche 1986; Kroymann et al. 1995); and reduction of the degree of thylakoid packing, number of thylakoids cell−1, and PS II particle size (Lewitus et al. 1991) are also possible reasons for the said inferences.
NPQ reflects the energy dissipated as heat following energization of the thylakoid membrane due to accumulation of protons in the lumen. It occurs due to rapid downregulation of light-harvesting ability and is induced by ΔpH across the thylakoid membranes. Its extent and capacity are correlated with the accumulation of carotenoids. Zeaxanthin, a carotenoid, is formed in thylakoids by the reversible de-epoxidation of violaxanthin in a process called the xanthophyll cycle, which in turn is activated by low luminal pH (Chu et al. 1995; Latowski et al., 2011). Luminal acidification is required not only for the de-epoxidation reaction but also for the activation of another key factor (PsbS) for the induction of NPQ. This protein with which the quencher is associated has been a subject of intense research. Few reports by Niyogi (1999) and Mozzo et al. (2008) suggest a possible role of up-/downregulation of this protein for increase/decrease of NPQ, while few others (Cecchin et al. 2018) report no effect of this protein on the quenching capacity of algae. However, our study demonstrates an increase in NPQ of T. obliquus cells in the presence of exogenous carbon sources compared with autotrophic conditions. This could be either due to higher carotenoid production and/or higher expression of PsbS which shall be experimentally elucidated in the future.
Metabolic pathways related to the synthesis and degradation of carbon and nitrogen storage compounds in microalgal cells are interlinked via their substrate-product dependency. To improve lipid biosynthesis in microalgae, a redirection of carbon flux towards fatty acid biosynthetic pathways accompanied with inhibition/blocking of more energy-consuming pathways is indispensable. Gene expression studies on microalgae relating to the enhancement of fatty acid biosynthesis have been carried out (Dunahay et al. 1995; Dehesh et al. 2001; Zhang et al. 2007), but despite the availability of the research reports, changes in response patterns in different microalgal species to different nutrient and abiotic conditions cannot be ruled out. As detailed in the paragraphs above, supplementation of 0.16% acetate + 0.16% citrate led to a significant rise in lipid accumulation in T. obliquus, possibly because of higher accumulation of acetyl-CoA or inhibition of citrate synthase or overexpression of acetyl-CoA synthetase and/or acetyl-CoA carboxylase and/or ATP-citrate lyase (Nelson and Rinne 1977; Deng et al. 2013; Beld et al. 2014; Villanova et al., 2017). This requires a detailed expression analysis of the genes involved in the different metabolic pathways (especially gluconeogenesis and Krebs cycle) for an in-depth understanding of the fundamental interactions at the genetic level under the treated condition. Research work on this aspect of the effect of exogenous carbon sources on growth and lipid accumulation of T. obliquus is under progress.
The properties of biodiesel are mainly determined by its fatty acid esters (Knothe, 2005). The fatty acid composition of algae can vary both quantitatively and qualitatively with their physiological status and culture conditions. The role of the source and sink of precursors for fatty acid biosynthesis is of considerable significance in deciding the FAME composition of algae. To understand the underlying mechanism behind the alterations in the FAME composition shown in this manuscript, it is important to know the process of formation of different fatty acid esters. Fatty acid biosynthesis occurs mainly in the chloroplast where acetyl-CoA carboxylase (ACCase) acts upon acetyl-CoA to form malonyl-CoA. This is further transferred to malonyl-ACP (acyl carrier protein) which acts as a carbon donor for subsequent chain elongation. The formation of 16 or 18 carbon fatty acids requires nearly 6 or 7 cycles of condensation reactions of the malonyl group with acyl-ACP catalyzed by multiple isoforms of ketoacyl-ACP synthase (KAS). The elongation of the carbon chain in the chloroplast is terminated when the acyl group is removed from ACP by acyl-ACP thioesterase enzymes, oleoyl ACP hydrolase (OAT), or when acyl-ACP thioesterase A (FatA) hydrolyzes the acyl-ACP and releases the free fatty acid, or when acyl transferases in the chloroplast transfer the fatty acid directly from ACP to glycerol-3-phosphate or monoacylglycerol-3-phosphate. The final fatty acid chain length/FAME composition is therefore determined by the activities of enzymes that use these acyl-ACPs at the termination phase of fatty acid synthesis (Rismani-Yazdi et al. 2011).
Further to this point, it is important to know that palmitic acid is the first and the commonest saturated fatty acid found in algae, but it generally forms only 30–40% of the total FAME because palmitic acid undergoes rapid conversion to either form stearic acid or gets desaturated to form palmitoleic acid (the precursor of omega-7 fatty acids). There is an enzyme called ∆9 desaturase, a very active lipid enzyme present in algae, plants, and animals. This enzyme either acts on the 9–10 position of stearic acid to form oleic acid or it acts upon 7–8 position of palmitic acid to form palmitoleic acid (Bigogno 2002). FAME composition in T. obliquus grown under glycerol and acetate supplementations showed the absence of stearic acid which could probably have been due to the upregulation of ∆9 and 12 desaturases leading to rapid conversion of the accumulated stearic acid to oleic acid esters. Similarly, a higher accumulation of palmitic acid under dextrose and acetate supplemented conditions could be due to higher availability of chloroplastic acetyl-CoA from acetate and glucose metabolism eventually leading to higher ACCase activity. The oleic acid content under all the mixotrophic conditions was observed to be more or less regulated in balanced proportions but comparatively higher accumulation under glycerol-, acetate + bicarbonate–, and acetate + glycerol–supplemented conditions could be due to the activation of pyruvate dehydrogenase by higher accumulation of glyceraldehyde-3-phosphate (a major intermediate of glycolysis), higher accumulation of malonyl-CoA from acetyl-CoA and bicarbonate, and additive effect of acetate and glycerol towards the acetyl-CoA pool under the respective culture conditions. However, an overall analysis of the FAME composition of algae in different cultivation conditions demonstrates a higher accumulation of SFA + MUFA compared with other fatty acid esters (Estévez-Landazábal et al., 2013; Piasecka et al. 2017). Analysis of the FAME composition of the transesterified lipids/biodiesel obtained under different mixotrophic conditions in this research study showed a presence of ~ 80% of the FAMEs as SFA + MUFA in the biodiesel samples obtained under selected mixotrophic conditions. This observation was found to be well in agreement with the report by Higgins et al. (2015) in which a higher proportion of SFA + MUFA i.e. > 70% was found in C. minutissima when grown under mixotrophic condition. The increased accumulation of palmitoleic and oleic acid was found to be responsible for such an observation. The higher levels of SFA + MUFA and the level of linolenic acid well below 12% as per the European (EN) and American (ASTM) biodiesel standards were found to increase the oxidative stability of the biodiesel samples (Knothe, 2005), thus certifying them to be good quality biodiesel.
The elongation of the carbon chain to form long-chain PUFAs takes place by the action of different elongases, reductases, and desaturases which act upon either palmitoleic or oleic acids. The action of ∆12 and 15 desaturases on the oleic acid first leads to the formation of linoleic acid (the precursor of omega-6 fatty acids) and then to α-linolenic acid (the precursor of omega-3 fatty acids). The subsequent actions of the ∆5 and ∆4 desaturases further lead to the formation of long-chain PUFAs such as EPA and DHA (Certik and Shimizu, 1999; Ratledge et al. 2001). The role of acetate in upregulating or overexpressing these desaturases and/or the acyl-thioesterases and/or acyl transferases could be a possible reason behind the presence of the omega-3 fatty acids in the microalga although a denial of any such overexpression mechanism of elongases and desaturases in algal cells under acetate-supplemented condition has been highlighted by Cecchin et al. (2018). They rather suggest the role of a higher pool of fatty acid synthesis precursors (mostly acetyl-CoA) under acetate-supplemented conditions for acting as a signaling mechanism in pushing the fatty acid chain elongation to the maximum possible extent for accumulation of PUFAs in TAG. These PUFAs are then known to enrich the acyl-CoA pool where they serve as acyl donors for continued TAG synthesis via the Kennedy pathway. An attestation of this mechanism has been provided by Kitano et al. (1998), Vazhappilly and Chen (1998), Ratledge et al. (2001), Guihéneuf et al. (2009), Wan et al. (2011), and Estévez-Landazábal et al. (2013). Furthermore, the presence of EPA and DHA in acetate-supplemented conditions may be due to the reorganization of membrane-based fatty acids from chloroplast to mitochondria and cytoplasmic lipid bodies (LBs) (Cohen and Khozin-Goldberg, 2005; Hu et al., 2008; Zili et al. 2017; Lari et al. 2019).
Fuel property analysis of the biodiesel sample obtained under the best mixotrophic condition of 0.16 + 0.16% acetate + citrate showed that the parameters of the microalgal biodiesel were well within the specified limits of the Indian and International Biodiesel Standards. Compared with petrodiesel, the density, viscosity, acid value, and cetane index were slightly higher in the algal biodiesel sample, but as reported by Venkatesan et al. (2017), such values do not harm the engine parts and indicate proper atomization and combustion of the algal biodiesel inside the engine. Contrarily, the calorific value of the algal biodiesel (40.0 MJ kg-1) was found to be lower than that of the petrodiesel but was sufficiently higher compared with those of coal, palm, and jatropha oil (Karmakar et al. 2018), indicating higher power generation of algal diesel to run an engine. The ash and moisture contents were similar to those of petrodiesel indicating lower deposition on the engine parts and better engine life of the vehicles.
The research strategy proposed in this manuscript, using mixotrophy as a mode of nutrition, is a widely practised strategy for enhancing the accumulation of lipids in algae without any negative impact on the growth/biomass yield. Yet, certain knowledge gaps in the existing research based on mixotrophic nutrition hinder the progress of the strategy to new levels. For sustainability to prevail and prosper, the use of natural product substitutes of the commercially available carbon sources is strongly recommended, but for choosing the right natural substitute, an understanding and initial validation of the effect of a wide range of carbon sources on the growth and lipid accumulation in microalgae are indispensable. Here, we have given a detailed overview of the correlation between a wide range of carbon sources and the growth and lipid accumulation potential in T. obliquus and the quality of the biodiesel produced from it. In conclusion, 0.16 + 0.16% of acetate + citrate is proposed for maximum growth and lipid accumulation in the green microalga, T. obliquus. This strategy can be applied on a pilot scale in the future by using natural product substitutes of acetate and citrate, in turn, minimizing the costs and environmental impact of the production process.
References
American Oil Chemists’ Society (AOCS) (1998) Iodine value of fats and oils. Wijs method. In: Firestone D (ed) Official methods and recommended practices of the American Oil Chemists’ Society, 5th edn. AOCS Press, Champaign, USA, pp 1–25
American Standards for Testing of Materials ASTM (2003) D 4052-96. D 240-02:482–474
Andruleviciute V, Makareviciene V, Skorupskaite V, Gumbyte M (2014) Biomass and oil content of Chlorella sp., Haematococcus sp., Nannochloris sp. and Scenedesmus sp. under mixotrophic growth conditions in the presence of technical glycerol. J Appl Phycol 26:83–90
Bajwaa K, Silambarasan T, Smita BNR (2016) Effect of glucose supplementation and mixotrophic effects of glycerol and glucose on the production of biomass, lipid yield and different physiological, biochemical attributes of Chlorella pyrenoidosa. J Algal Biomass Util 7:93–103
Beld J, Finzel K, Burkart MD (2014) Versatility of acyl-acyl carrier protein synthetases. Chem Biol 21:1293–1299
Bigogno C (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60:497–503
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Cecchin M, Benfatto S, Griggio F, Mori A, Cazzaniga S, Vitulo N, Delledonne M, Ballottari M (2018) Molecular basis of autotrophic versus mixotrophic growth in Chlorella sorokiniana. Sci Rep 8:1–13
Certik M, Shimizu S (1999) Biosynthesis and regulation of microbial polyunsaturated fatty acid production. J Biosci Bioeng 87:1–14
Chisti Y (2013) Constraints to commercialization of algal fuels. J Biotechnol 167:201–214
Chu W-L, Phang S-M, Goh S-H (1995) Influence of carbon source on growth, biochemical composition and pigmentation of Ankistrodesmus convolutus. J Appl Phycol 7:59–64
Cid A, Abalde J, Herrero C (1992) High yield mixotrophic cultures of the marine microalga Tetraselmis suecica (Kylin) Butcher (Prasinophyceae). J Appl Phycol 4:31–37
Cohen Z, Khozin-Goldberg I (2005) Searching for PUFA-rich microalgae, In Single Cell Oils. In: Cohen Z, Ratledge C, Champaign IL (eds) American Oil Chemists’ Society, pp 53–72
De Cima S, Rua J, Perdiguero E, del Valle P, Busto F, Baroja-Mazo A, de Arriaga D (2005) An acetyl-CoA synthetase not encoded by the facA gene is expressed under carbon starvation in Phycomyces blakesleeanus. Res Microbiol 156:663–669
Degrenne B, Pruvost S, Christophe G, Cornet J F, Cogne G, Legrand J (2010) Investigation of the combined effects of acetate and photobioreactor illuminated fraction in the induction of anoxia for hydrogen production by Chlamydomonas reinhardtii. Int J Hydrogen Energy 35:10741–10749
Dehesh K, Tai H, Edwards P, Byrne J, Jaworski JG (2001) Overexpression of 3-ketoacyl-acyl-carrier protein synthase IIIs in plants reduces the rate of lipid synthesis. Plant Physiol 125:1103–1114
Deng X, Cai J, Fei X (2013) Effect of the expression and knockdown of citrate synthase gene on carbon flux during triacylglycerol biosynthesis by green algae Chlamydomonas reinhardtii. BMC Biochem 38:1–11
Dunahay TG, Jarvis EE, Roessler PG (1995) Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J Phycol 31:1004–1012
Estévez-Landazábal LL, Barajas-Solano AF, Barajas-Ferreira C, Kafarov V (2013) Improvement of lipid productivity on Chlorella vulgaris using waste glycerol and sodium acetate. Ciencia, Tecnología Futuro 5:113–126
European Standard EN 14214 (2003) European standards for biodiesel. http://www.din.de. Accessed on 8.07.2016.
Guihéneuf F, Mimouni V, Ulmann L, Tremblin G (2009) Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalga Pavlova lutheri commonly used in mariculture. J Exp Mar Biol Ecol 369:136–143
Higgins BT, Labavitch JM, Vander Gheynst JS (2015) Co-culturing Chlorella minutissima with Escherichia coli can increase neutral lipid production and improve biodiesel quality. Biotechnol Bioeng 112:1801–1809
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huang A, Sun L, Wu S, Liu C, Zhao P, Xie X, Wang G (2017) Utilization of glucose and acetate by Chlorella and the effect of multiple factors on cell composition. J Appl Phycol 29:23–33
Indian Standard IS: 15607 (2005) Biodiesel specification. Bureau of Indian Standards. http://www.bis.org.in. Accessed on 5.05.2016.
International Energy Agency IEA (2015) Energy and climate change. World Energy Outlook Special Report. OECD/IEA, Paris, pp 17–31
Jogl G, Tong L (2004) Crystal structure of yeast acetyl-coenzyme A synthetase in complex with AMP. Biochemistry 43:1425–1431
Karmakar R, Kundu K, Rajor A (2018) Fuel properties and emission characteristics of biodiesel produced from unused algae grown in India. Pet Sci 15:385–395
Kitano M, Matsukawa R, Karube I (1998) Enhanced eicosapentaenoic acid production by Navicula saprophila. J Appl Phycol 10:101–105
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070
Kong WB, Yang H, Cao YT, Song H, Hua SF, Xia CG (2013) Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotrophic culture. Food Technol Biotechnol 51:62–69
Krisnangkura KA (1986) Simple method for estimation of cetane index of vegetable oil methyl esters. J Am Oil Chem Soc 63:552–553
Kroymann J, Schneider W, Zetsche K (1995) Opposite regulation of the copy number and the expression of plastid and mitochondrial genes by light and acetate in the green flagellate Chlorogonium. Plant Physiol 108:1641–1646
Lari Z, Abrishamchi P, Ahmadzadeh H, Soltani N (2019) Differential carbon partitioning and fatty acid composition in mixotrophic and autotrophic cultures of a new marine isolate Tetraselmis sp. KY114885. J Appl Phycol 31:201–210
Latowski D, Kuczyn´ska P, Strzałka K (2011) Xanthophyll cycle – a mechanism protecting plants against oxidative stress. Redox Rep 16: 1-14.
Lewitus AJ, Caron DA, Miller KR (1991) Effects of light and glycerol on the organization of the photosynthetic apparatus in the facultative heterotroph Pyrenomonas salina (Cryptophyceae). J Phycol 27:578–587
Lin TS, Wu JY (2015) Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour Technol 184:100–107
Liu X, Duan S, Li A, Xu N, Cai Z, Hu Z (2009) Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J Appl Phycol 21:239–246
Lowrey J, Brooks MS, McGinn PJ (2015) Heterotrophic and mixotrophic cultivation of microalgae for biodiesel production in agricultural wastewaters and associated challenges—a critical review. J Appl Phycol 27:1485–1498
Mallick N, Mandal S, Singh AK, Bishai M, Dash A (2012) Green microalga Chlorella vulgaris as a potential feedstock for biodiesel. J Chem Technol Biotechnol 87:137–145
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291
Mandal S, Mallick N (2011) Waste utilization and biodiesel production by the green microalga Scenedesmus obliquus. Appl Environ Microbiol 77:374–377
Mandal S, Mallick N (2012) Biodiesel production by the green microalga Scenedesmus obliquus in a recirculatory aquaculture system. Appl Environ Microbiol 78:5929–5933
Mandal S, Patnaik R, Singh AK, Mallick N (2013) Comparative assessment of various lipid extraction protocols and optimization of transesterification process for microalgal biodiesel production. Environ Technol 34:2009–2018
Martinez-Blanco H, Reglero A, Luengo JM (1990) Carbon catabolite regulation of phenylacetyl-CoA ligase from Pseudomonas putida. Biochem Biophys Res Commun 167:891–897
Menegol T, Romero-Villegas GI, López-Rodríguez M, Navarro-López E, López-Rosales L, Chisti Y, Cerón-García MC, Molina-Grima E (2019) Mixotrophic production of polyunsaturated fatty acids and carotenoids by the microalga Nannochloropsis gaditana. J Appl Phycol 31:2823–2832
Ministry of Environment, Forest and Climate change (MoEFCC) (2014) Statement by the Hon’ble Minister at the high level segment of UNFCC COP-20. http://www.moef.nic.in/content/statement-hon%E2%80%99ble-minister-high-level-segment-unfccc-cop-20-december-9-2014.
Mokashi K, Shetty V, George SA, Sibi G (2016) Sodium bicarbonate as inorganic carbon source for higher biomass and lipid production integrated carbon capture in Chlorella vulgaris. Arch Life Sci 10:111–117
Mozzo M, Passarini F, Bassi R, van Amerongen H, Croce R (2008) Photoprotection in higher plants: the putative quenching site is conserved in all outer light-harvesting complexes of photosystem II. Biochim Biophys Acta 1777:1263–1267
Nelson DL, Cox M (2000) Amino acid oxidation and the production of urea. In: Ryan M, Strange L, Neal V(eds.) Lehninger principles of biochemistry. Worth Publishers, New York, p 643
Nelson DR, Rinne RW (1977) The role of citrate in lipid synthesis in developing soybean cotyledons. Plant Cell Physiol 18:1021–1027
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359
Oesterhelt C, Schmälzlin E, Schmitt JM, Lokstein H (2007) Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J 51:500–511
Ogbonna OI, Ogbonna CJ (2018) Effects of carbon source on growth characteristics and lipid accumulation by microalga Dictyosphaerium sp. with potential for biodiesel production. Energy Power Eng 10:29–42
Perez-Garcia O, Escalante FME, de-Bashan YLE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45: 11-36.
Piasecka A, Krzemińska I, Tys J (2017) Enrichment of Parachlorella kessleri biomass with bioproducts: oil and protein by utilization of beet molasses. J Appl Phycol 29:1735–1743
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Rai MP, Gupta S (2016) Growth and lipid production from Scenedesmus sp. under mixotrophic condition for bioenergy application. In: Kumar S, Khanal SK, Yadav YK (eds) Proceedings of the first international conference on recent advances in bioenergy research. Springer Nature, India, pp 159–168
Rai LC, Mallick N, Singh JB, Kumar HD (1991) Physiological and biochemical characteristics of a copper tolerant and a wild type strain of Anabaena doliolum under copper stress. J Plant Physiol 138:68–74
Ratledge C, Kanagachandran K, Anderson AJ, Grantham DJ, Stephenson JC (2001) Production of docosahexaenoic acid by Crypthecodinium cohnii grown in a pH-auxostat culture with acetic acid as principal carbon source. Lipids 36:1241–1246
Ratnapuram HP, Vutukuru SS, Yadavalli R (2018) Mixotrophic transition induced lipid productivity in Chlorella pyrenoidosa under stress conditions for biodiesel production. Heliyon 4:1–20
Rismani-Yazdi H, Haznedaroglu BZ, Bibby K, Peccia J (2011) Transcriptome sequencing and annotation of the microalgae Dunaliella tertiolecta: pathway description and gene discovery for production of nextgeneration biofuels. BMC Genomics 12:1–18
Shah SMU, Radziah CC, Ibrahim S, Latiff F, Othman MF, Abdullah MA (2014) Effects of photoperiod, salinity and pH on cell growth and lipid content of Pavlova lutheri. Ann Microbiol 64:157–164
Sharma KK, Li Y, Schenk PM (2015) Rapid lipid induction in Chlorella sp.by UV-C radiation. Bioenergy Res 8:1824–1830
Shen X-F, Qin Q-W, Yan S-K, Huang J-L, Liu K, Zhou S-B (2019) Biodiesel production from Chlorella vulgaris under nitrogen starvation in autotrophic, heterotrophic, and mixotrophic cultures. J Appl Phycol 31:1589–1596
Silva HR, Prete CEC, Zambrano F, de Mello VH, Tischer CA, Andrade DS (2016) Combining glucose and sodium acetate improves the growth of Neochloris oleoabundans under mixotrophic conditions. AMB Express 6:1–11
Soeder CJ, Bolze A (1981) Sulphate deficiency stimulates the release of dissolved organic matter in synchronus culture of Scenedesmus obliquus. Plant Physiol 52:233–238
Sonkar S, Mallick N (2016) Development of a single phase nitrate feeding strategy for enhanced lipid productivity from green microalgae for biodiesel production. Environ Prog Sustain Energy 36:222–231
Steinbiß HJ, Zetsche K (1986) Light and metabolite regulation of the synthesis of ribulose-1,5-bisphosphate carboxylase/oxygenase and the corresponding mRNAs in the unicellular alga Chlorogonium. Planta 167:575–581
Subramanian S, Barry AN, Pieris S, Sayre RT (2013) Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: implications for biomass and biofuel production. Biotechnol Biofuels 150:1–12
Sun Y, Liu J, Xie T, Xiong X, Liu W, Liang B, Zhang Y (2014) Enhanced lipid accumulation by Chlorella vulgaris in a two-stage fed-batch culture with glycerol. Energy Fuel 28:3172–3177
Van Wagenen J, De Francisci D, Angelidaki I (2015) Comparison of mixotrophic to cyclic autotrophic/heterotrophic growth strategies to optimize productivity of Chlorella sorokiniana. J Appl Phycol 27:1775–1782
Vazhappilly R, Chen F (1998) Eicosapentaenoic acid and docosahexaenoic acid production potential of microalgae and their heterotrophic growth. JAOCS 75:393–397
Venkatesan H, Sivamani S, Sampath S, Gopi V, Dinesh Kumar M (2017) A comprehensive review on the effect of nano metallic additives on fuel properties, engine performance and emission characteristics. Int J Renew Energy Res 7:825–843
Vicente G, Martınez M, Aracil J (2004) Integrated biodiesel production: a comparison of different homogeneous catalysts systems. Bioresour Technol 92:297–305
Villanova V, Fortunato AE, Singh D, Bo DD, Conte M, Obata T, Jouhet J, Fernie AR, Marechal E, Falciatore A, Pagliardini J, Monnier AL, Poolman M, Curien G, Petroutsos D, Finazzi G (2017) Investigating mixotrophic metabolism in the model diatom Phaeodactylum tricornutum. Philos Trans R Soc B 372:1–14
Wan M, Liu P, Xia J, Rosenberg JN, Oyler GA, Betenbaugh MJ, Nie Z, Qiu G (2011) The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl Microbiol Biotechnol 91:835–844
White S, Anandraj A, Bux F (2011) PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresour Technol 102:1675–1682
Wilken S, Schuurmans JM, Matthijs HCP (2014) Do mixotrophs grow as photoheterotrophs? Photophysiological acclimation of the chrysophyte Ochromonas danica after feeding. New Phytol 204:882–889
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalgae Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Zhang Y, Adams IP, Ratledge C (2007) Malic enzyme: the controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiol 153:2013–2025
Zili F, Bouzidi N, Ammar J, Zakhama W, Ghoul M, Sayadi S, Ben Ouada H (2017) Mixotrophic cultivation promotes growth, lipid productivity, and PUFA production of a thermophilic Chlorophyta strain related to the genus Graesiella. J Appl Phycol 29:35–43
Funding
Financial support from Indian Institute of Technology Kharagpur, West Bengal, and NASF, Indian Council of Agricultural Research, New Delhi, India, are thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patnaik, R., Mallick, N. Individual and combined supplementation of carbon sources for growth augmentation and enrichment of lipids in the green microalga Tetradesmus obliquus. J Appl Phycol 32, 205–219 (2020). https://doi.org/10.1007/s10811-019-01979-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01979-3