Abstract
Gelidium latifolium collected monthly at Sinop Peninsula (Black Sea, Turkey) was studied during a 1-year period. Physical and gelling properties of agar extracted from G. latifolium were investigated. Results showed that agar yield was not significantly affected by seasons and ranged from 32.79 ± 0.78 to 39.26 ± 0.37%. Quality parameters such as pH, total ash, acid-insoluble ash, and moisture complied with the international standards and acceptance criteria established for agar. High melting temperature (93.86 ± 0.27 °C) and gelling temperature (47.46 ± 0.20 °C) were obtained in autumn. A relatively high viscosity, gel strength was obtained in autumn and during the second half of the year. Lowest acid-insoluble ash (0.012 ± 0.002%) and moisture (9.276 ± 0.20%) were obtained in winter, autumn, and spring, respectively. Using G. latifolium from the Sinop Peninsula, good quality of agar with a gel strength ranging from 206.83 ± 5.36 to 591.85 ± 2.42 g cm−2 was obtained, indicating its potential for commercial exploitation. The highest hysteresis temperature was obtained during summer with a maximum in June (48.63 ± 0.18 °C) and a minimum in autumn (46.20 ± 0.09 °C). Results of this study indicate that the best quality agar was obtained during the second period of the year and in autumn. FTIR analysis of agar extracted from G. latifolium showed a fairly constant spectrum that were recorded to contain high gel strengths and low sulfate residues. We conclude that the extracted agar is of good quality agar and may have the potential for commercial agar production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term “hydrocolloids” is generally used to describe a range of polysaccharides and proteins that are widely used in industrial sectors because of their unique physicochemical and gelling properties (Phillips and Williams 2009). The agar obtained from the red seaweed is one of the most important commercial hydrocolloids (Phillips and Williams 2009; Porse and Rudolph 2017). The United States Pharmacopeia (USP) and the Food Chemical Codex (FCC) describe agar as a hydrocolloid extracted from red seaweeds soluble in boiling water but insoluble in cold water (Lee et al. 2017). Agar is extracted from members of the red seaweed (Rhodophyta) genera such as Gracilaria and Gelidium spp. (Kraan 2012). It is used in food, pharmaceutical, medicinal, and biotechnological industries as a gelling agent, thickener, or stabilizing and emulsifying agent (Yaphe 1984; Li et al. 2009). Bacteriological agar with low gelling temperature is extracted only from Gelidium spp. (McHugh 2003). Agar is a polysaccharide that is found in the cellular matrix of red seaweeds and formed by a mixture of agarose and agaropectin (Naidu 2000; Lemus et al. 2008; Yarnpakdee et al. 2015). Agarose is a linear polymer and composed of the (1,4)-linked 3,6 anhydro-α-L-galactopyranose and (1,3)-linked β-D-galactopyranose units. Agaropectin, the non-gelling fraction, is a more complex structure that includes sulfate ester, pyruvate, d-galactose, and 3,6 anhydro-l-galactose (Matsuhashi 1998; Naidu 2000). High-quality agar is extracted from a seaweed belonging to the genus Gelidium but a large and growing population of the Gracilaria seaweed has caused this seaweed to be the main source of agar (Rodríguez et al. 2009; Heydari et al. 2014). Overexploitation of Gelidium natural populations led to the search for other agar-producing seaweeds. Gracilaria agar, although not as stable during storage as that of Gelidium, has adequate gelifying properties for food industry and has replaced the use of the latter in several preparations that do not require long-time storage (Armisen 1995).
Agar quality can be affected by diverse factors such as the seaweed source, extraction methods and drying conditions (Lemus et al. 2008), physiological factors, life cycle, environmental variations, and postharvest storage (Marinho-Soriano and Bourret 2003; Marinho-Soriano et al. 2006; Romero et al. 2008). Moreover, the presence of some chemical groups such as pyruvate, methyoxyl, and sulfate can affect the quality of agar (Arvizu-Higuera et al. 2008; Hurtado et al. 2011).
In the present study, the agar extracted from Gelidium latifolium was subject to several quality parameters and our results indicate that this species can be conveniently used for producing good-quality agar. Also, a suitable technique for producing the desired quality of agar was determined in this study; however, detailed studies are required to further evaluate the agar from this species for commercial use.
Materials and methods
Collection and preparation of Gelidium latifolium
Specimens of Gelidium latifolium were collected on a monthly basis from January to December 2011 from a depth of 3–5 m in the coast of the Sinop Peninsula located in the center of Black Sea, Turkey (42.11.2.85″ N, 35.4.40.88″ E). The samples were washed with tap water to remove solid debris/contaminants. The cleaned seaweeds were placed in an oven (60 °C, 48 h) immediately after harvesting. The dried seaweed was vacuum-packed and stored at room temperature.
Agar extraction
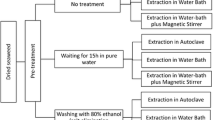
The method described by Öğretmen and Duyar (2018) was used to extract agar. First, the samples (12 g) of seaweed dried in an oven were refluxed with 900 mL of distilled water for 6 h at 95 °C (sample/distilled water ratio of about 1:75 (w/v)). Thereafter, the agar solution was filtered off and the residue was removed using a Whatman paper No. 541. The hot extracts were centrifuged at 3000 rpm for 3 min. The extracts were gelled at room temperature into a strafor plate and then frozen at − 25 °C for 20 h. The frozen gel was thawed at room temperature and oven dried at 60 °C for 20 h. Dried agar samples were ground to enhance their solubility for measurement of physical parameters and stored at room temperature.
Determination of yield and physicochemical properties of agar
FTIR analysis
The agars were analyzed using PerkinElmer Spectrum Universal ATR Sampling Accessory, over the wave number range between 4000 and 650 cm−1.
Agar yield
The agar yield (%) was calculated as the percentage of dried seaweed
Where Wa is the dry agar weight and Ws is the dry seaweed weight (g).
Gelling and melting temperatures
Gelling and melting temperatures of the agar samples were measured by the method described by Young (1974). Agar solution (1.5% w/v) was prepared in a test tube (1.7 cm diameter, 15 cm height). The gelling temperature was measured by dissolving agar in distilled water and pouring 10 mL of the agar solution in test tubes. A glass bead (3 mm diameter) was placed in each test tube and all the test tubes were immediately placed in a rack maintained at 60 °C in a water-bath. Each tube was tilted up and down in the water-bath until the glass bead ceased to move. Using a thermometer, the gelling temperature was determined to be the temperature at which the movement of the glass beads ceased. To determine the melting temperature, the test tubes were placed in a water-bath with its temperature increased gradually from 50 to 100 °C. The melting temperature was recorded with a precision thermometer when the glass beads dropped to the bottom of the test tubes.
pH value
The pH was determined according to Atay (1974). Briefly, 4 g of the agar samples were washed with tap water at 18 °C for 30 min. The samples were put in Erlenmeyer flasks and kept at room temperature for 24 h. Then, the pH of the samples was measured by using a pH meter (Metter Toledo Seven Multi mV/ORP).
Ash content
Ash content was determined by burning 1 g of the agar sample in a porcelain crucible at 600 °C for 3 h.
Viscosity
The viscosity of 1.5% agar solution may be measured by using a Haake Maers III rheometer at 85 °C and a shear rate of 100 mm−1 according to a 35-mm-diameter parallel plate/plate (α, 2°) method.
Gel strength
Agar samples were dissolved in boiling distilled water to obtain a concentration of 1.5% (w/v). The agar solutions (50 mL) were transferred to a cylindrical mold with a diameter of 5 cm and a height of 3.5 cm. The gels were equilibrated at room temperature (20 °C) for 24 h before analysis (Romero et al. 2008; Kumar and Fotedar 2009). Gel strength was determined at 20 °C using a texture analyzer (TA.XT2, UK) with a load cell of 5 kg and a cross-head speed of 1 mm/s, and equipped with a 1.27-cm diameter, flat-faced, cylindrical Teflon plunger. The maximum force (gram) taken when the plunger had penetrated 5 mm into the agar gels was recorded. Gel strength was calculated and expressed as g.cm−2.
Acid-insoluble ash
The acid-insoluble ash was determined according to the procedure of İlyas (1989). A certain amount of ash was boiled for 5 min with 25 mL of hydrochloric acid. The insoluble matter was collected in a sintered crucible or on an ashless filter paper, washed with hot distilled water, and burnt at about 400 °C to a constant weight. The weight of the acid-insoluble ash was calculated in milligram per gram of air-dried material.
Moisture
The moisture content of the agar samples was determined by the vacuum oven method (AOAC 1990). The samples were dried in a moisture dish in an oven (Nüve FN500 model) at 105 °C until they reached a constant weight.
Hysteresis
Hysteresis temperature was calculated as the difference between melting and gelling temperatures.
Statistical analysis
Monthly experiments were performed in triplicate, while a total of nine seasonal experiments were performed (three sets of three months each). A one-way analysis of variance (ANOVA) was used to evaluate seasonal variations. Seasonal data from each of the three sets were averaged. Statistically significant differences between monthly and seasonal results were tested using the JMP 5.0.1 program at 5% significance level. When a significant effect was found, Tukey-Kramer HSD was used. P values < 0.05 were considered statistically significant.
Results
The FTIR spectra of agar products extracted in different months from and its fractions are represented in Fig. 1. The most significant bands were obtained at 1646, 1372, 1258, 1042, 930, 890, 771 cm−1, typically detected as agarocolloid. Based on spectra, the raiding band was observed at 3349 cm−1 associated with OH groups. The band at 2921 cm−1 was attributed to CH2 groups of agar. The absorbance at 1646 cm−1 assigned to amide I vibrations suggests the presence proteins (Guerrero et al. 2013). The bands at 1372 cm−1 and 1258 cm−1 corresponded to ester sulfate groups. The band with wavenumber of 1042 cm−1 is equivalent to skeletal mode of the galactan (Sekkal et al. 1993). The FTIR absorbance band 930 cm−1 indicated to the C–O–C group of 3,6 anhydro-α-L-galactopyranose (3,6-AG) (Sousa et al. 2012). The band at 1152 cm−1 (C–O, axial deformation) was observed as principal band of agarose (Garcia et al. 2000). According to Garcia et al. (2000), the 890 cm−1 band is specific for agar and observed at all agar products in this study (Fig. 1).
The yields of agar from G. latifolium being the lowest in July (32.79 ± 0.78%) and highest in January (39.26 ± 0.37%) are summarized in Table 1 with different results obtained during the rest of the year (p < 0.05). Maximum agar yield from G. latifolium was obtained in winter (36.74 ± 0.77%) and minimum in summer (35.89 ± 0.78%). There was no statistical difference (p > 0.05) in the ratio of agar yield between seasonal changes (Fig. 2). A negative correlation between seasonal agar yield-gel strength (r = −0.97).
Gelling temperature varied from 47.90 ± 0.10 to 0.30 ± 0 °C. The highest gelling temperature was obtained in November and December and the lowest in April. There was a statistical difference between the results obtained on a monthly basis (p < 0.05). The generally low gelling temperature observed in the first 6 months of the year could be related to low seawater temperature. From the seasonal point of view, the highest gelling temperature was recorded in autumn (47.46 ± 0.20 °C) and the least in spring (40.98 ± 0.31 °C). While there is no statistical difference between summer and winter seasons (p > 0.05), statistical differences have been observed in the other seasons (p < 0.05) (Fig. 2) in terms of gelling temperature. Correlation analysis of seasonal gelling temperatures showed a significant positive correlation with pH (r = 0.97) and viscosity (r = 0.96).
The highest melting temperature was obtained in November whereas lower values were obtained in April. On the other hand, melting temperatures in G. latifolium were higher in autumn (93.86 ± 0.27 °C) and lower in spring (88.74 ± 0.19 °C). The monthly melting temperature values were observed higher in the second half of the year as in the gelling temperature. Statistical differences between the melting temperatures obtained in some months and in the seasons are given in Table 1 and Fig. 2. A positive correlation between seasonal melting temperatures and gelling temperatures (r = −0.94) was observed.
The maximum pH value was obtained in December (7.46 ± 0.01) whereas minimum values were obtained in March (6.25 ± 0.01). As a seasonal, pH values were higher in autumn (7.28 ± 0.02) and lower in spring (6.41 ± 0.05). pH values were obtained to be high in the second half of the year (between June–December and summer–autumn seasons) when the seawater temperature was high. Statistical differences between the pH values obtained in some months and in the seasons are given in Table 1 and Fig. 2. A strong positive correlation (r = 0.98, y = 0.1654, x = 8.216) was obtained between pH value and melting temperatures.
The maximum viscosity of agar obtained from G. latifolium was 214.67 ± 15.059 cP in November, while the minimum value of 17.28 ± 1.33 cP was determined in April. The viscosity parameters namely the melting and gelling temperatures were found to be higher in the second half of the year (Fig. 3). The viscosity of the agar reached its highest value of 153.66 + 16.51 cP in summer and its lowest value of 25.40 + 2.23 cP in spring. The agar viscosity varied significantly between months and seasons (p < 0.05). A strong positive correlation (r = 0.99) was found between viscosity and the melting temperature value and between viscosity and pH (r = 0.96).
Total ash values of extracted agar from G. latifolium were obtained highest in November (4.77 ± 0.32%) and lowest in July (2.82 ± 0.07%). The total ash values obtained in this study were found to decrease regularly toward the summer season (Fig. 2). Total ash values were found highest as 3.89 ± 0.25% (autumn) and lowest as 3.09 ± 0.07% (summer).
The highest gel strength was found in the samples obtained in September (591.85 ± 2.42 g cm−2) and lowest in those obtained in January (206.83 ± 5.36 g cm−2). Considering seasonal samples, the greatest gel strength was recorded in autumn (507.33 ± 25.21 g cm−2) and least in winter (545.92 ± 28.32 g cm−2). There were statistical differences between the results obtained on a monthly and seasonal basis (p < 0.05).
Acid-insoluble ash ranged from 0.011 ± 0.004 to 0.051 ± 0.009% in our study. The acid-insoluble ash was maximum in June and minimum in November. Considering the seasonal samples, the highest value was found in winter (0.37 ± 0.019%) and lowest in autumn (0.012 ± 0.002%). Significant statistical differences were found between acid-insoluble ash quantities in the monthly and seasonal samples (p < 0.05).
The maximum moisture content in the extracted agar was obtained in January (14.54 ± 0.11%) and minimum in June (8.54 ± 0.23%). Considering the seasonal samples, the highest moisture content was obtained in winter (12.43 ± 0.62%) and lowest in spring (9.276 ± 0.20%). Significant statistical differences were found between moisture content in the monthly and seasonal samples (p < 0.05) and are detailed in Table 1 and Fig. 2.
The hysteresis temperature of G. latifolium was highest in July (48.63 ± 0.18 °C) and lowest in October (45.70 ± 0.05 °C). Considering the seasonal samples, the values of hysteresis temperature in G. latifolium ranged from 48.01 ± 0.18 °C in summer and 46.20 ± 0.09 °C in winter. The statistical differences between hysteresis temperatures are shown in Table 1 and Fig. 2 (p < 0.05).
Discussion
The yield and gel properties of the agar extracted from G. latifolium were compared with the literature data and the standards determined by international trading company. FTIR result showed that all the agars from G. latifolium collected at different months have similar FTIR spectra suggesting that seasonal variations have no significant effect on the chemical structure of G. latifolium.
Mouradi-Givernaud et al. (1993) suggested that the amount of agar in seaweed is higher in the spring and summer months. Givernaud and El Gourji (1999) found that the agar extracted from Gelidium multipartia had the highest yield in winter, which decreased during the growth periods to a minimal in June and October. Our results are similar to those published by Givernaud and El Gourji (1999). Nil et al. (2016) reported that the yield of the agar extracted from Gelidium sesquipedale was the highest in spring and summer (32.22 ± 1.7%). Some studies performed on marine algae revealed that the yield of the agar from the algae growing in the rainy seasons was higher than those growing in the dry seasons (Roleda et al. 1997; Montano et al. 1999; Villanueva et al. 1999; Ganesan et al. 2008), except for the studies of Yenigül (1993) and Vergara-Rodarte et al. (2010), who reported a higher yield in the dry season. The inconsistency in the results is thought to be caused by the different extraction methods (alkali treatment, extraction time, and temperature), seaweed species, harvest area, water temperature, geographical location, and environmental factors. At the same time, the agar yield from G. latifolium is high in the rainy seasons, which can be attributed to hyposalinity as reported in the findings (Luhan 1992; Pondevida and Hurtado-Ponce 1996).
Armisen and Galatas (2009) reported that gelling temperature is an indicator for identifying the agarophyte used to produce agar. The gelling temperature range of agarophytes such as Gelidiella and Gracilaria has been reported to be 42–45 °C and 40–42 °C, respectively, by Armisen and Galatas (2009). Prasad et al. (2007) found that the gelling temperature of agar obtained from Gelidiella acerosa was 36 ± 0.65–42 ± 0.95 °C. Ganesan et al. (2008) extracted agar from Gelidium acerosa from four stations and obtained gelling temperatures between 39 and 51 °C. Seasonal changes in gelling temperature of G. latifolium agar are comparable with those obtained with literature data. In contrast of the present study, Freile-Pelegrín et al. (1995) found that the gelling temperatures of extracted agar from Gelidium canariensis range from 35.7–39.3 °C. The differences in gelling temperatures may be related to factors such an extraction methods and seasonal changes in environmental conditions.
The relationship between melting temperature and gel strength and both measurements are usually considered when agar quality is of interest (Selby and Wynne 1973). In the present study, the highest gelling temperature and gel strength values were obtained in autumn (gelling temperature in November, gel strength in September) and summer (gelling temperature value in July, gel strength value in June). Mouradi-Givernaud et al. (1992) found that the gel strength of extracted agar from G. latifolium increased from 400 g cm−2 in March to around 800 g cm−2 in October and then declined. In the present study, max values of gel strength were recorded between May to September. These results are in agreement with those obtained from G. latifolium by Mouradi-Givernaud et al. (1992). Melting temperature is used to judge agar quality and higher than 85 °C (Armisen and Galatas 1987; Chapter 1). All samples in this study had a high melting temperature that falls in the range of the USP standards (> 85 °C) and all melting temperature values of this study had commercial grade agar standards. The range values of melting temperatures of agar (94.86 ± 0.28–88.26 ± 0.08 °C) is comparable with those previously reported for this species (73–92 °C by Ganesan et al. (2008); 85.1–93.7 °C by Freile-Pelegrín et al. (1995); 90–98 °C by Roleda et al. (1997); above 90 °C by Villanueva et al. (1999)).
Israel et al. (1999) extracted agar from Gracilaria tenuistipitata and recorded pH values between 6.5 and 8. Scholten and Pierik (1998) extracted agar using different methods and obtained pH values between 4.2 and 7.5. In general, the pH values of extracted agar from G. latifolium were comparable to those found in the literature.
The ash content of the agar solution was in the range of 4.77 ± 0.32 to 2.82 ± 0.07%. Following its evolution according to the seasons, it was shown that it remained stable during the year. Important trading companies such as FCC, USP, ECC, and FAO have defined standard specifications and reported that the maximum ash contend for agar should not be more than 6.5% (Table 2). The total amount of ash obtained in this study ranged from 4.77 ± 0.32 to 2.82 ± 0.07%, which makes our agar suitable according to the agar standards accepted by the international trading companies.
Gelidium latifolium showed variations in the agar viscosity along seasons. In general, the highest agar viscosity was observed in autumn (maximum value in November) and summer (maximum value in August) coinciding with the highest values of temperature. The results suggest that temperature can influence the agar quality. Contrarily of the present study, Nil et al. (2016) found that the viscosity of agar extracted from G. sesquipedale had the minimum value in autumn (November, 8.53 ± 1.2 mPa.s). The difference may be ascribed to the different extraction conditions used. The viscosity values obtained from G. latifolium was superior to those obtained by other authors (Calumpong et al. 1999; Meena et al. 2007; Pereira-Pacheco et al. 2007; Prasad et al. 2007; Yarnpakdee et al. 2015; Nil et al. 2016). However, Pereira-Pacheco et al. (2007) stated that a high viscosity agar is used in the food industry as mainly in spreads and soft-texture food products.
In this study, seasonal gel strength values increased regularly throughout the year from winter season. Martín et al. (2013) studied the gel strength of agars from Gracilaria gracilis harvested in Patagonian coast (Argentina) and observed that the higher values were obtained in spring/summer and the lowest in winter. The highest gel strength values obtained from G. gracilis were obtained in summer (828 cm−2) by Marinho-Soriano and Bourret (2003). The gel strength values obtained at present study are comparable to those obtained by other authors (Ganesan et al. 2008; Vergara-Rodarte et al. 2010). In contrast, there have been studies of high values in gel strength for winter and low ones for summer for G. canariensis (Freile-Pelegrín et al. 1995). These differences may be attributed to various factors such as the presence of some chemical groups, such as pyruvate, methoxyl, and sulfate, which influence gel strength and are the main agar quality parameters as suggested by Arvizu-Higuera et al. (2008) and Hurtado et al. (2011). Eventually, our results suggested that agar from G. latifolium had gel strength equivalent to the first grade (second grade in January) food agar standards specified by Japanese Specification (see Arasaki and Arasaki 1983 for details) for Processed Agar (JSPA).
The acid-insoluble ash content of agar extracted from G. latifolium had significant seasonal variations (p < 0.05) with gradual decrease from winter to autumn. The maximum concentration of acid-insoluble ash for agar is reported not to be more than 0.5% (Commission Regulation EU No 231/2012; JECFA 2006). At the same time, important trading companies as FCC, USP, ECC, and FAO reported acid-insoluble ash contend for agar to not to be more than 0.5% (Table 2). All agar samples extracted from G. latifolium in this study had an acid-insoluble ash lower than 0.5%, which fulfills the requirement of EU commission and FCC, USP, ECC, and FAO.
The moisture content was relatively stable over the year, except a small increase in winter (14.54 ± 0.11% in January). FCC, USP, ECC, and FAO have determined the maximum moisture content to be 20% (Table 2). According to JSPA, the agar must have a moisture content of maximum 22% (Arasaki and Arasaki 1983). In this study, the results are in the acceptable range specified by the major trading companies and JSPA.
According to Stanley (2006), hysteresis is most pronounced for agars and range from 40 to 60 °C depending on the seaweed source. All agar samples in this study fall in this range. Hurtado et al. (2011) obtained hysteresis temperature ranging from 58.1 to 61.1 °C according to harvest beds of Gelidium robustum along the western coast of the Baja California Peninsula, Mexico. According to the literature results, the hysteresis temperature can differ according to the extraction method applied and the seaweed source.
Conclusion
In this study, the effect of seasonal variation on yield and physicochemical properties of agar extracted from G. latifolium was investigated. Based on our results, agar yield is not affected considerably by seasons. The quality parameters of our monthly and seasonally obtained agar samples such as moisture, ash, acid-insoluble ash met the standards specified by the FCC, USP, EEC, and FAO. The best harvest period to obtain high gel strength, high viscosity, and the high melting temperature is autumn and in the second half of the year. Armisen and Galatas (1987) propose the gelling temperature between 32 and 43 °C for agar gels used for commercial purposes. In this study, the gelling temperature values of the agar obtained in the first part of the year and in the spring season indicate that the agar extracted from G. latifolium is of commercial grade.
This study is the first study on seasonal agar production in this region and we believe that it will be the pioneer for future agar studies. Therefore, we conclude that G. latifolium from the Sinop Peninsula coast of Black Sea, Turkey, produces agar of a reasonably good quality and thus could be used as a source for commercial agar production. If commercial agar production is considered, it is necessary to determine growth parameters and investigate agriculture conditions.
References
AOAC (1990) Official methods of analysis of AOAC International. In: Association of Official Analysis Chemists International (C. II, ss. 1058–1059)
Arasaki A, Arasaki T (1983) Low calories, high nutrition. Vegetables from the sea to help you look and feel better, Japan Publications Inc, pp.39–42
Armisen R (1995) World-wide use and importance of Gracilaria. J Appl Phycol 7:231–243
Armisen R & Galatas F (1987) Production and utilization of products from commercial seaweeds. Production, properties and uses of agar. FAO Fish.Tech.Paper No. 288, 194
Armisen R, Galatas F (2009) Agar. In: Williams PA, Phillips GO (eds) Handbook of hydrocolloids. Woodhead Publishing, Cambridge, pp 82–107
Arvizu-Higuera DL, Rodriguez-Montesinos YE, Murillo-Alvarez JI, Muñoz-Ochoa M, Hernández-Carmona G (2008) Effect of alkali treatment time and extraction time on agar from Gracilaria vermiculophylla. J Appl Phycol 20:515–519
Atay D (1974) Deniz Yosunları ve Değerlendirme Olanakları. Ankara Üniversity Faculty of Agriculture, Ankara
Calumpong HP, Maypa A, Magbanua M, Suarez P (1999) Biomass and agar assessment of three species of Gracilaria from Negros Island, Central Philippines. In: Kain JM, Brown MT, Lahaye M (eds) Sixteenth International Seaweed Symposium. Developments in hydrobiology, vol 137. Springer, Dordrecht, pp 173–182
FAO/NACA (1996) Regional study and workshop on the taxonomy, ecology and processing of economically important red seaweeds. NACA Environment and Aquaculture Development Series No. 3. Network of Aquaculture Centres in Asia Pacific, Bangkok, Thailand
Freile-Pelegrín Y, Robledo DR, García-Reina G (1995) Seasonal agar yield and quality in Gelidium canariensis (Grunow) Seoane-Camba (Gelidiales, Rhodophyta) from Gran Canaria, Spain. J Appl Phycol 7:141–144
Ganesan M, Reddy CRK, Eswaran K, Jha B (2008) Seasonal variation in the biomass, quantity and quality of agar from Gelidiella acerosa (Forsskal) Feldmann et Hamel (Gelidiales, Rhodophyta) from the Gulf of Mannar Marine Biosphere Reserve, India. Phycol Res 56:93–104
Garcia RB, Vidal RRL, Rinaudo M (2000) Preparation and structural characterization of O-acetyl agarose with low degree of substitution. Polímeros 10:155–161
Givernaud T, El Gourji A (1999) Seasonal variations of growth and agar composition of Gracilaria multipartita harvested along the Atlantic coast of Morocco. Hydrobiologia 398:167–172
Guerrero P, Garrido T, Leceta I, De La Caba K (2013) Films based on proteins and polysaccharides: preparation and physical-chemical characterization. Eur Polym J 49:3713–3721
Heydari M, Nematollahi MA, Motamedzadegan A, Hashem S, Hosseini SV (2014) Optimization of the yield and quality of agar from Gracilariopsis persica. Bull Env Pharmacol Life Sci 3:289–295
Hurtado MÁ, Manzano-Sarabia M, Hernández-Garibay E, Pacheco-Ruíz I, Zertuche-González JA (2011) Latitudinal variations of the yield and quality of agar from Gelidium robustum (Gelidiales, Rhodophyta) from the main commercial harvest beds along the western coast of the Baja California peninsula, Mexico. J Appl Phycol 23:727–734
İlyas M (1989) Agar ve protein konsantresi üretiminde ham madde olarak Gracilaria verrucosa’nın (Hudson; Papenfuss) kullanım olanakları. Ege University Food Engineering Department, Doctoral thesis, İzmir
Israel A, Martinez-Goss M, Friedlander M (1999) Effect of salinity and pH on growth and agar yield of Gracilaria tenuistipitata var. liui in laboratory and outdoor cultivation. J Appl Phycol 11:543–549
JECFA (2006) JECFA (Joint FAO/WHO Expert Committee on Food Additives), E 406 Agar, (Combined Compendium of Food Additive Specifications- all Specifications. Monographs from the 1st to the 65th Meeting (1956-2005) Food Additives 1–3:1–2
Kumar V, Fotedar R (2009) Agar extraction process for Gracilaria cliftonii (Withell, Millar & Kraft, 1994). Carbohydr Polym 78:813–819
Lee WK, Lim YY, Leow ATC, Namasivayam P, Abdullah JO, Ho CL (2017) Factors affecting yield and gelling properties of agar. J Appl Phycol 29:1527–1540
Lemus RA, Pérez M, Andrés A, Roco T, Tello CM, Vega A (2008) Kinetic study of dehydration and desorption isotherms of red alga Gracilaria. LWT - Food Sci Technol 41:1592–1599
Li H, Huang J, Xin Y, Zhang B, Jin Y, Zhang W (2009) Optimization and scale-up of a new photobleaching agar extraction process from Gracilaria lemaneiformis. J Appl Phycol 21:247–254
Luhan MRJ (1992) Agar yield and gel strength of Gracilaria heteroclada collected from Iloilo, Central Philippines. Bot Mar 35:169–172
Marinho-Soriano E, Bourret E (2003) Effects of season on the yield and quality of agar from Gracilaria species (Gracilariaceae, Rhodophyta). Bioresour Technol 90:329–333
Marinho-Soriano E, Fonseca PC, Carneiro MAA, Moreira WSC (2006) Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour Technol 97:2402–2406
Martín LA, Rodríguez MC, Matulewicz MC, Fissore EN, Gerschenson LN, Leonardi PI (2013) Seasonal variation in agar composition and properties from Gracilaria gracilis (Gracilariales, Rhodophyta) of the Patagonian coast of Argentina. Phycol Res 61:163–171
McHugh D (2003) A guide to the seaweed industry: FAO Fisheries Technical Paper no.441.F
Meena R, Siddhanta AK, Prasad K, Ramavat BK, Eswaran K, Thiruppathi S, Rao PVS (2007) Preparation, characterization and benchmarking of agarose from Gracilaria dura of Indian waters. Carbohydr Polym 69:179–188
Montano NE, Villanueva RD, Romero JB (1999) Chemical characteristics and gelling properties of agar from two Philippine Gracilaria spp. (Graciales, Rhodophyta). J Appl Phycol 11:27–340
Mouradi-Givernaud A, Givernaud T, Cosson J, Morvan H (1992) Agar from Gelidium latifolium (Rhodophyceae, Gelidiales): biochemical composition and seasonal variations. Bot Mar 35:153–160
Mouradi-Givernaud A, Givernaud T, Morvan H, Cosson J (1993) Annual variations of the biochemical composition of Gelidium latifolium (Greville) Thuret et Bornet. Hydrobiologia. 260:607–612
Naidu AS (2000) Natural food antimicrobial systems. CRC Press
Öğretmen ÖY, Duyar HA (2018) The effect of different extraction methods and pre-treatments on agar yield and physico-chemical properties of Gelidium latifolium (Gelidiaceae, Rhodophyta) from Sinop Peninsula Coast of Black Sea, Turkey. J Appl Phycol 30:1355–1360
Pereira-Pacheco F, Robledo D, Rodríguez-Carvajal L, Freile-Pelegrín Y (2007) Optimization of native agar extraction from Hydropuntia cornea from Yucatán, México. Bioresour Technol 98:1278–1284
Phillips GO, Williams PA (2009) Handbook of hydrocolloids, 2nd edn. Woodhead Publishing, Cambridge
Pondevida HB, Hurtado-Ponce AQ (1996) Assessment of some agarophytes from the coastal areas of Iloilo, Philippines II. Seasonal variations in the agar quality of Gracilaria changii, Gracilaria manilaensis and Gracilariopsis bailinae (Gracilariales, Rhodophyta). Bot Mar 39:123–128
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Prasad K, Siddhanta AK, Ganesan M, Ramavat BK, Jha B, Ghosh PK (2007) Agars of Gelidiella acerosa of west and southeast coasts of India. Bioresour Technol 98:1907–1915
Rodríguez MC, Matulewicz MC, Noseda MD, Ducatti DRB, Leonardi PI (2009) Agar from Gracilaria gracilis (Gracilariales, Rhodophyta) of the Patagonic coast of Argentina - content, structure and physical properties. Bioresour Technol 100:1435–1441
Roleda MY, Ganzon-Fortes ET, Montano NE (1997) Agar from vegetative and tetrasporic Gelidiella acerosa (Gelidiales, Rhodophyta). Bot Mar 40:501–506
Romero JB, Villanueva RD, Montano MNE (2008) Stability of agar in the seaweed Gracilaria eucheumatoides (Gracilariales, Rhodophyta) during postharvest storage. Bioresour Technol 99:8151–8155
Nil S, Ali-Mehidi S, Zellal A, Abi-Ayad SMEA (2016) Effects of season on the yield and quality of agar from Gelidium sesquipedale (Rhodophyta) from Mostaganem, Algeria. Afr J Biotech 15:350–355
Scholten HJ, Pierik RLM (1998) Agar as a gelling agent: chemical and physical analysis. Plant Cell Rep 17:230–235
Sekkal M, Huvenne J-P, Legrand P, Sombret B, Mollet J-C, Mouradi-Givernaud A, Verdus M-C (1993) Direct structural identification of polysaccharides from red algae by FTIR microspectrometry I: localization of agar in Gracilaria verrucosa sections. Microchim Acta 112:1–10
Sousa AMM, Morais S, Abreu MH, Pereira R, Sousa-Pinto I, Cabrita EJ, Gonçalves MP (2012) Structural, physical, and chemical modifications induced by microwave heating on native agar-like galactans. J Agric Food Chem 60:4977–4985. 2
Stanley NF (2006) Agars. In: Stephen AM, Phillips GO, Williams PA (eds) Food polysaccharides and their applications. CRC Press, Boca Raton, pp 217–238
Vergara-Rodarte MA, Hernández-Carmona G, Rodríguez-Montesinos YE, Arvizu-Higuera DL, Riosmena-Rodríguez R, Murillo-Álvarez JI (2010) Seasonal variation of agar from Gracilaria vermiculophylla, effect of alkali treatment time, and stability of its colagar. J Appl Phycol 22:753–759
Villanueva RD, Montaño NE, Romero JB, Aliganga AKA, Enriquez EP (1999) Seasonal variations in the yield, gelling properties, and chemical composition of agars from Gracilaria eucheumoides and Gelidiella acerosa (Rhodophyta) from the Philippines. Bot Mar 42:157–182
Yaphe W (1984) Chemistry of agars and carrageenans. Hydrobiologia, 116/117:171–186
Yarnpakdee S, Benjakul S, Kingwascharapong P (2015) Physico-chemical and gel properties of agar from Gracilaria tenuistipitata from the lake of Songkhla, Thailand. Food Hydrocoll 51:217–226
Yenigül M (1993) Seasonal changes in the chemical and gelling characteristics of agar from Gracilaria verrucosa collected in Turkey. Hydrobiologia 260:627–631
Young KS (1974) An investigation of agar from Gracilaria sp. Fisheries and Marine Service Canada, Tech. Rep. No. 454
Acknowledgments
We thank Central Fisheries Research Institute for laboratory support and are also grateful to Mr. Atilla Özdemir.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Öğretmen, Ö.Y., Kaya, Y. Seasonal changes in the yield and gel properties of agar extracted from Gelidium latifolium (Rhodophyta). J Appl Phycol 31, 3091–3100 (2019). https://doi.org/10.1007/s10811-019-01786-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01786-w