Abstract
This work aims to propose a pathway for the production of 2(E),4(E)-decadienal from arachidonic acid (ARA) via 11-hydroperoxide eicosanoid (11 hydroperoxyeicosatetraenoic acid, 11-HPETE) through lipoxygenase (LOX) and hydroperoxide lyase (HPL) in the brown alga, Saccharina angustata, by identifying pathway intermediates through three studies. The first study investigated the biogeneration of 2(E),4(E)-decadienal in crude homogenates of fronds (CHF), while the second and third investigated whether ARA is the precursor of the pathway and if 11-HPETE is produced from ARA as an intermediate in 2(E),4(E)-decadienal generation, respectively. The results showed that 2(E),4(E)-decadienal was formed in CHF and its concentration increased after incubation. This finding led to the hypothesis that 2(E),4(E)-decadienal is formed enzymatically via LOX-HPL and consequently its biogeneration was determined. ARA was indicated to be a precursor of the pathway since, after CHF was incubated with ARA as a substrate, the amount of aldehyde increased significantly compared with that produced by CHF without ARA and without incubation. The production of 11-HPETE from ARA was also demonstrated as an intermediate reaction in 2(E),4(E)-decadienal formation via the LOX-HPL pathway. The hydroxy isomer of 11-HPETE was produced, and ARA was incubated with homogenated fronds in combination with glutathione peroxidase and glutathione to control its stability during identification. The compound was identified as 11-HPETE because the purified isomer showed the same retention time when co-injected with the standard using an HPLC technique; moreover, the same indicated mass spectrum was obtained as that of the standard via a GC/GCMS technique. The indicated pathway and the pathway for the production of short chain aldehydes [n-hexanal, 2(E),3(Z)-nonenal and 2(E),4(E)-decadienal] from ARA and linoleic acid through theirs hydroperoxides are proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2(E),4(E)-decadienal is an aromatic aldehyde substance with fat flavour that is a natural occurring compound in various organisms, e.g. diatoms and algae (Pohnert 2002; Akakabe et al. 2003) and is as major aroma in cooked food such as chicken and beef soup (Jayasena et al. 2013; Takakura et al. 2014; Qi et al. 2017). In organisms, this compound is known as a member of the oxylipin group and is biogenerated through the lipoxygenase (LOX) and hydroperoxide lyase (HPL) pathways in which fatty acid serve as substrates of LOX, yielding its hydroperoxide that serves as a substrate of HPL to catalyse the final aldehyde product. In general, LOX is an enzyme known to catalyse the dioxygenation of polyunsaturated fatty acids that contain 1Z,4Z-pentadiene structures (conjugated diene structures) to obtain hydroperoxy unsaturated fatty acid derivatives (Kuhn 2000) that undergo subsequent reactions with various enzymes. This enzyme system is the oxylipin pathway. This pathway is distributed in both mammalian and non-mammalian organisms (Blée 1998; Andreou et al. 2009; Kuhn et al. 2015). Most of oxylipins in algae were formed by enzymatic systems; however, non-enzymatic reaction was scarce and highly unpredictable, differing between species and the surrounding of growth conditions (Mosblech et al. 2009; Barbosa et al. 2015, 2016). HPL, one of enzymes subsequent to the oxylipin pathways, carries out a unique catalysation, cleaving hydroperoxy unsaturated fatty acids derivatives to yield aldehydes (saturated and unsaturated aldehydes) and oxo-acids (Blée 1998; Noordermeer et al. 2001; Grechkin 2002; Hatanaka 2003; Matsui 2006).
An aldehyde biosynthesis pathway that be forms 11-hydroperoxyeicosatetraenoic acid from arachidonic acid (eicosatetraenoic acid) was proposed in two marine organisms, the diatom Thalassiosira rotura (Pohnert 2002) and the green macroalga Ulva conglobata (Akakabe et al. 2003). However, to date, no studies reported this pathway in the brown macroalga (Phaeophyta), Saccharina angustata (Kjellman) C.E. Lane, C. Mayes, Druehl and G.W. Saunders.
Two past studies led us to propose that this pathway exists in S. angustata. Confirming the presence of the pathway in this species will further our knowledge of the distribution of the pathway in other organisms and will strengthen our understanding of the evolutionary relationships among species of organisms.
Among marine brown algae species, S. angustata was previously reported to produce short chain aldehyde [n-hexanal, 2(E),3(Z)-nonenal] from arachidonic acid (Boonprab et al. 2003a, b, 2006). A variety of past studies reported the formation of volatile compounds in marine algae (Katayama 1958; Kajiwara et al. 1988; Gerwick et al. 1993; Gerwick 1994; Kajiwara et al. 1996; Kajiwara 1997; Fujimura and Kawai 2000). Since 11-hydroperoxyeicosatetraenoic acid (HPETE) can be formed through the arachidonic acid conversion (Boonprab et al. 2004), these past results provide the evidence that the HPL enzyme responsible for 11-HPETE cleavage could be present in algal fronds.
To prove the existence and identify the pathway in S. angustata, three studies were performed. In the first study, the biogeneration of 2(E),4(E)-decadienal in crude homogenate of fronds was investigated, while the second and third examined whether arachidonic acid is a precursor of the pathway and if 11-hydroperoxide eicosanoid is an intermediate of arachidonic acid in the generation of 2(E),4(E)-decadienal. The two important outcomes of this report include a proposed pathway for the generation of 2(E),4(E)-decadienal from arachidonic acid via 11-HPETE, and an entire pathway for producing short chain aldehydes from arachidonic acid and linoleic acid through theirs hydroperoxide in the brown alga S. angustata.
Materials and methods
Algae and chemical compounds
Saccharina angustata was harvested at Charatsunai beach, Hokkaido (northern part of Japan) facing the Pacific Ocean. Fronds were kept at 4 °C immediately after harvest, and the temperature was maintained during shipping. In the laboratory, a coarse powder was prepared from the fronds using liquid nitrogen, and it was kept at − 80 °C. The whole powder was mixed before each sample (20 g) was taken for an experiment to avoid differences in the enzymatic experiment. For simultaneous distillation extraction, bladed fresh fronds were kept at − 80 °C until use.
Arachidonic acid (ARA) 90% purity, 2(E),4(E)-decadienal, glutathione (GSH) and glutathione peroxidase (GPx) were purchased from Sigma Chemical Co. (USA). 11(R,S)-hydroxyeicosatetraenoic acid was obtained from Cayman Chemical Co. (USA). Other chemicals were obtained from Wako Chemical Co. (Japan). All solvents used in experiments were purified by distillation.
Simultaneous distillation extraction (SDE)
Fronds (100 g fresh weight) were cut into small pieces and homogenised with 100 mL of a 50-mM 2-(N-morpholino)ethanesulfonic acid-KOH (MES-KOH) buffer, pH 5.5, using a Polytron mixer (Kinematca, Switzerland). Each homogenate was incubated at 5 °C for 80 min, and then 200 mL of a saturated CaCl2 solution and 100 μg of n-undecane were added to stop the enzyme reactions. As a control, cut fronds were immediately added to a mixture of 200 mL of a saturated CaCl2 solution and 100 mL of MES-KOH buffer, and were subsequently homogenised with a Polytron mixer. After the addition of 100 μL of n-undecane (1.0 mg mL−1), each homogenate was subjected to SDE for 2 h with n-pentane:dichloromethane (2:1 v/v, 50 mL) as an extraction solvent. Each extract was dried over anhydrous Na2SO4, and then concentrated under normal pressure. The essential oil thus obtained was analysed by GC-MS (Shimadzu, GCMS-QP5050A, Japan).
Crude enzyme preparation
Saccharina angustata was crushed into a fine powder under liquid nitrogen with a mortar and pestle, and subsequently ground with a Maxim homogeniser (Nihonseiki Ltd., Japan). The powder was transferred into a glass bottle containing three volumes (v/w) of 0.1 M borate borax buffer pH 9.0, containing 2% Polyclar VT. The suspension was homogenised using a Polytron mixer to breakdown polysaccharides and then filtered through six layers of cheesecloth. The filtrate was centrifuged at 3076×g for 15 min at 4 °C to remove debris. The pH of the supernatant was readjusted to 6.9 on ice using a cold 0.1 M HCl or NaOH solution (Boonprab et al. 2003a).
The protein contents were determined with a modified Lowry method (Dulley and Grieve 1975).
Analysis
The aldehyde analysis method was performed based on Boonprab et al. (2003a). A buffer (1 mL of 0.1 M MES-KOH, pH 6.9 prepared with seawater) and 700 nmol of arachidonic acid (20 mM dissolved in ethanol) were combined in a test tube and chilled on ice for 15 min, after which crude enzyme solution (1 mL) was added. This mixture was incubated at 4 °C with gentle shaking. Preliminary experiments showed that the reaction was linear over time until 30–40 min, thus, the reaction was terminated by adding 1.4 mL of 1% 2,4-dinitrophenylhydrazine (in ethanol containing 0.5 M CH3COOH) and 8 μL of 1 mM n-heptanal (8 nmol) as an internal standard after 30 min. The hydrazone derivatives were extracted with 5 mL of n-hexane and washed with 2 mL of saturated NaCl. After the removal of n-hexane with a vacuum centrifuge, a yellow powder was yielded and re-dissolved in 50 μL of diethyl ether. The diethyl ether solution was subjected to preparative thin layer chromatography [TLC; silica gel 60 F-254 (Merck); 10 × 20 cm; ethyl acetaete: n-hexane 2:1]. Yellow bands corresponding to the authentic compounds (Rf 0.6–1.0) were scraped off and extracted with 2 mL of distilled diethyl ether at least twice. Each diethyl ether extract was filtered with hydrophobic filter unit (0.5 μm DISMIC-3JP, Advantech TOYO, Japan) and evaporated with a vacuum centrifuge at 32 °C. The resultant residues were dissolved in 50 μL of CH3CN and subjected to quantification by reversed phase high-performance liquid chromatography (HPLC). Quantification of the aldehydes was carried out using a five-points standard curve constructed with various amounts of authentic aldehydes (2(E),4(E)-decadienal) based on the area ratio of the respective peak of aldehyde to that of the internal standard (n-heptanal). The linear regression of the three standard curves was ≥ 0.995. Reversed phase HPLC was performed using a Zorbax SB C18 column (250 × 4.6 mm, 5 μm) at a flow rate of 1 mL min−1 [CH3CN:H2O:tetrahydrofuran, 66:33:1 (v/v/v)] with UV detection at 350 nm (Shimadzu, LC-9A, Japan). The ability to form aldehydes varied from fronds to fronds depending on the collection date, site, and developmental stage of the specimens (Kajiwara et al. 1993b; Sekiya et al. 1984); however, the profiles, i.e., the substrate and product specificities of the enzyme system, were practically reproducible. Representative data are shown.
For structural analyses of hydroxy-arachidonic acid, crude enzyme preparation (60 mL) were incubated with 36 nmole of arachidonic acid in the presence of 2 units mL−1 of GSH-Pxd and 6 mM GSH to produce hydroxy-arachidonic acid. The product of reaction were then extracted using a Sep-pek C18 silica cartridge (Waters Corporation, USA) (Lehmann et al. 1992). The acids were eluted with methanol, and after evaporating the methanol, the resultant yellow oil was re-dissolved in diethyl ether and washed with saturated NaCl. The diethyl ether layer was dried over Na2SO4 at − 10 °C for 12 h. After the soluble diethyl ether soluble was removed, the residue was subjected to preparative thin layer chromatography essentially as described elsewhere (Boonprab et al. 2003a). Hydroxyeicosatetraenoic acid(s) [HETE(s)] were separated using a solvent system of n-hexane:diethyl ether:CH3COOH, 1:1:0.001 (v/v/v). The spots at Rf 0.13–0.2 were scraped off, and the HETE(s) were extracted from the gel with 2 mL of diethyl ether five times. After removing the ether, each residue was re-dissolved in diethyl ether (5 mL) and washed with a saturated NaCl solution. The ether layer was dried over Na2SO4 at − 10 °C for 12 h, then ether was removed and the residue was reconstituted in ethanol (1 mL). The concentrations of the HETE(s) were estimated by measuring the absorbance at 234 nm using an extinction coefficient of 23,200 M−1 cm−1 (Graff et al. 1990). Aliquots were methylated with ethereal diazomethane for the straight phase HPLC analyses, using a Zorbax-SIL 250 × 4.6 mm column (Du Pont company) and, eluting with n-hexane:isopropanol:CH3COOH, 987:12:1 (v/v/v), at a flow rate of 0.5 mL min−1. Detection was performed with a photodiode array detector (Shimadzu, LC-10ADvp, Japan) at absorbances of 234, 210, and 270 nm (Boonprab et al. 2003a, 2004).
For mass spectrometry analyses of hydroxy-arachidonic acid, the procedure developed by Lehmann et al. (1992) was carried out. After purification of the HETE(s), they were methylated with ethereal diazomethane. Each sample was re-dissolved in ethanol (800 mL), and platinum oxide (6 mg) was added. Hydrogen gas was bubbled through each sample solution for 90 min, with platinum oxide removed by filtration, and the solvent removed under a stream of nitrogen. Subsequently, a bis-(trimethylsilyl)-trifluoroacetamide solution (200 mL, Sigma-Aldrich) was added, and the mixture was incubated for 60 min with constant stirring under argon. After cooling to room temperature, the reagents were removed under a gentle stream of nitrogen. Each sample was re-dissolved in n-hexane (40 mL) and subjected to GC-MS analysis. The GC-MS (GCMS-QP5050A, Shimadzu, Japan) was equipped with a DB-WAX fused-silica capillary column (60 m × 0.25 mm) coated with a 0.25-mm film thickness using helium as a carrier gas. The column temperature was programmed to increase from 150 to 200 °C at 5 °C min−1. Sample injection was carried out at a split ratio of 1/50. The ionisation energy was set to 70 eV.
Results
Biogeneration of 2(E),4(E)-decadienal in crude frond homogenates
To demonstrate the biogeneration of the aldehyde 2(E),4(E)-decadienal in homogenate fronds prepared via SDE, an experiment was performed in which the homogenated fronds were with incubated at 5 °C for 80 min and compared to homogenate that was not incubated (to account for aldehyde naturally occurring in the frond). The volatile aldehyde compounds in the fronds were identified and quantified using a GC-GC/MS technique. The results in Table 1 showed that, 13 aldehyde compounds naturally occurred (the control), with some of them increasing after incubation. The prominent compounds included a C-9 aldehyde [2(E)-nonenal] and a C-6 aldehyde [n-hexanal], which corresponded to previous reports in this species (Kajiwara et al. 1996; Boonprab et al. 2003a, b). A C-10 aldehyde [2(E), 4(E)-decadienal] also formed naturally occurred in the fronds and it was found to increase amount in quantity during this experiment. Thus, biogeneration of a C-10 aldehyde through enzymatic formation was indicated.
Arachidonic acid is the precursor of the pathway
Because the previous experiment (Table 1) demonstrated that a C-10 compound was formed through enzymatic generation in the homogenate fronds, we proposed that it might be formed through LOX and HPL pathway present in green algae and diatom that has 11-hydroperoxide eicosanoid as an intermediate and uses arachidonic acid as a precursor (Pohnert 2002; Akakabe et al. 2003).
To investigate if arachidonic acid is a precursor of 2(E), 4(E)-decadienal in S. angustata as it is in green algae and diatom (Akakabe et al. 2003; Pohnert 2002), three treatments were performed. The aldehyde yielded from each treatment was purified by thin layer chromatography before being quantified by HPLC using a standard curve of 2(E),4(E)-decadienal (Fig. 1). Three treatments of crude enzyme (homogenate fronds) were examined: two were incubated, one with and one without arachidonic acid [control (I)] and the third was not incubated [control (II)]. The objective of these treatments was to determine the substrate specificity of the enzyme that formed 2(E),4(E)-decadienal, i.e. to determine its arachidonic acid, is endogenous fatty acid [control (I)], and to assess the naturally formation of 2(E),4(E)-decadienal in the frond homogenate [control (II)]. The result showed that among the treatments, the amount of 2(E),4(E)-decadienal was increased in the following order: homogenated fronds without incubation < homogenated fronds with incubation < homogenated fronds with arachidonic acid. 2(E),4(E)-Decadienal was formed naturally in the homogenated fronds [control (I)] and enzymatically after being incubated using naturally occurring fatty acids in the fronds [control (II)], When arachidonic acid was added, the amount of the 2(E),4(E)-decadienal aldehyde increased to a level greater than that of either control, which led us to postulate that the fatty acid in the fronds might be arachidonic acid [control (II)]. Correspondingly, arachidonic acid might be a precursor in the pathway that forms this aldehyde.
Substrate specificity of 2(E),4(E)-decadienal formation in the frond homogenates (H-F) prepared from S. angustata. Reactions of crude enzyme (2.9 mg mL−1) with arachidonic acid (ARA; 700 nmol) were performed at 4 °C for 30 min. Under the conditions employed, aldehyde-formation proceeded almost linearly until 30–40 min. Each reaction was performed in duplicate with triplicate HPLC analyses. The means of duplicates are shown. The amounts of aldehydes formed with (black square) and without (white square) exogenous and endogenous fatty acids were determined by reversed-phase HPLC
11-Hydroperoxide eicosanoid is produced from arachidonic acid as an intermediate in the generation of 2(E),4(E)-decadienal
As in the past reports, arachidonic acid [C20:4, n-6] was shown to be a likely precursor in the pathway that forms 2(E),4(E)-decadienal, and it might be formed through LOX-HPL as it is in other algal and diatom species (Pohnert 2002; Akakabe et al. 2003), with 11-hydroperoxide eicosanoid produced from arachidonic acid as an intermediate. To confirm this hypothesis, the intermediate of this pathway should be found in the crude enzyme and arachidonic acid reaction mixture (Fig. 1). To perform this experiment, a unique technique was used in which glutathione peroxidase (GPx) was added to a reaction mixture containing 3 mM glutathione (GSH). The GSH-GPx system is known to reduce hydroperoxides to their corresponding hydroxides in mammalian cells (Bryant et al. 1982; Hatzelmann et al. 1989; Weitzel and Wendel 1993; Schnurr et al. 1996; Sutherland et al. 2001). This GSH-GPx reaction system is an effective tool for trapping fatty acid hydroperoxides in their hydroxide forms and has been reported elsewhere (Hamberg et al. 1986, 1998; Brodowsky et al. 1992; Hamberg and Gerwick 1993; Hombeck et al. 1999; Boonprab et al. 2003a, 2006). Thus, this method was used to identify 11-hydroperoxide eicosanoid in this study.
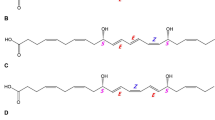
The crude products reaction yield (Fig. 2B, trace a) that was extracted by Sep-pak C18 silica cartridge and purified by thin later chromatography (detailed in the Material and Methods) was first identified using a spectrophotometric technique (Fig. 2a). The crude product obtained λmax at 243 nm, demonstrating that the prominent purified products in the reaction were 1,4-pentadiene compounds corresponding to hydroxyarachidonic acid derivatives. When this solution was then analysed by straight-phase HPLC, five isomers (I-V) were found (Fig. 2b, trace a). Each isomer had a λmax at 243 nm that was observed using the straight-phase HPLC technique. All of the isomers were identified in previous reports (Boonprab et al. 2003a, 2004, 2006). isomers I, II, III, IV, and V were 15(S)-hydroxyeicosatetraenoic acid, 12(S)-hydroxyeicosatetraenoic acid, 11-hydroxyeicosatetraenoic acid, 8-hydroxyeicosatetraenoic acid, and 9-hydroxyeicosatetraenoic acid, respectively (Fig. 2b, trace c). However, the main objective of this experiment was to determine if 11-hydroperoxide eicosanoid is the intermediate produced from arachidonic acid during 2(E),4(E)-decadienal generation; thus, standard of 11-hydroperoxide eicosanoid from arachidonic acid was used to show its retention time of 41.5 min (Fig. 2b, trace b). This retention time corresponded to that of isomer III (Fig. 2b, trace a), and a solution of the standard (Fig. 2b, trace b) was mixed (co-injection) with a crude purified reaction sample (Fig. 2b, trace a); the height of the peak for isomer III increased at the same retention as that of the standard, as shown in Fig. 2b, trace c, thus implying that isomer III is 11-hydroxyeicosanoid from arachidonic acid. Since the reaction included the GSH-GPx system, we postulated that 11-hydroxyeicosanoid was generated from 11-hydroperoxyeicosanoid, which was in turn produced from arachidonic acid. This isomer was identified to confirm its structure using its mass spectrum obtained with GC/GCMS technique (Fig. 2c). The mass spectrum had prominent peaks and a common fragment pattern at m/z 399 (M-15), 383 (M-31), and 367 (M-47). The characteristic ion peaks of methyl ester-trimethylsilyl ether derivatives were represented in its mass spectrum at m/z 229 and 287 (Boeynaems et al. 1980). This evidence also supported that compound III is 11-hydroxyeicosatetraenoic acid, which was formed from 11-hydroperoxyeicosatetraenoic acid.
Straight-phase HPLC analysis of hydroxyl-arachidonic acids (b). Reactions were composed of crude enzyme (240 mg protein), arachidonic acid (7 μ mol), glutathione peroxidase (4 units mL−1), and glutathione (3 mM). The crude purified products (C-Ps) obtained λmax at 234 nm by spectrophotometric analysis (a). The C-Ps (isomers I-V) isolated from the reaction mixture (trace a), authentic 11-hydroxy eicosatetraenoic acid (HETE; trace b) and a mixture of the products and authentic 11-HETE (trace c) were analysed by HPLC. The chromatogram in trace c represents the co-injection of the reaction (trace a) and the authentic standards (trace b). Isomer III in the C-P was identified as 11-HETE. Its structure was confirmed by mass spectrometry (c) using GC/GC-MS analysis with isomer purified from the reaction in trace a using preparative HPLC
Discussion
Biogeneration of 2(E),4(E)-decadienal in crude frond homogenate
To our knowledge, marine algae are known to produce several kinds of volatile compounds such as aldehydes, monoterpenes, and alcohols that have flavour characteristics (Katayama 1958; Kajiwara et al. 1993a, 1996). Aldehyde formation in edible marine brown algae was studied by Kajiwara et al. (1988) and Kajiwara (1997), who reported that nonenal and n-hexanal were the major and minor components, respectively. Short-chain aldehydes are widely distributed in higher plants and formed by LOX/HPL pathway (Hatanaka 1996; Blée 1998). LOX catalyses oxidative reactions with unsaturated fatty acids containing 1,4-pentadiene structures. The reactions results in hydroperoxides of the unsaturated fatty acids. LOX has been found in plants, animals, algae and fungi but not in bacteria (Kuhn 2000). The subsequent reaction with HPL cleaves the hydroperoxides to short-chain aldehydes or alcohols. In higher plants, these flavour compounds are formed from linolenic acid or linoleic acid while in animal such as fish, arachidonic acid or eicosapentaenoic acid are the typically precursors (Cadwallader 2000). Using this information, we were able to show the enzymatic formation of a C-10 aldehyde. This finding demonstrated that the compound may be formed through enzymatic generation in homogenated fronds of the brown alga Saccharina angustata. Furthermore, this C-10 aldehyde was suggested to be formed through the LOX-HPL pathway, as it is in green algae and diatoms via the intermediate 11-hydroperoxide eicosanoid (Pohnert 2002; Akakabe et al. 2003) using arachidonic acid [C20:4, n-6] as a precursor. This hypothesis was proven by subsequent experiments.
Arachidonic acid is the precursor of the pathway
The results of the first experiment indicated that arachidonic acid might be the precursor of the pathway that forms the 2(E),4(E)-decadienal aldehyde, suggesting a substrate specificity for 2(E),4(E)-decadienal formation through the LOX-HPL system in the homogenate prepared from S. angustata, as in past reports of other species (Pohnert 2002; Akakabe et al. 2003). However, evidence was needed to confirm this hypothesis, i.e. the formation of 11-hydroperoxy eicosanoid as an intermediate of the LOX-HPL pathway in the reaction system needed to be verified.
11-Hydroperoxide eicosanoid is produced from arachidonic acid as an intermediate in the generation of 2(E),4(E)-decadienal
The production of 11-hydroperoxide eicosanoid from arachidonic acid was confirmed. The compounds formed from reaction mixtures containing purified crude enzyme and arachidonic acid contained 1,4-pentadiene bonds in their structures, with five isomers as in past reports (Boonprab et al. 2003a, 2004, 2006). Isomer III was determined to be 11-hydroxyeicosatetraenoic acid as it had the same retention time as its authentic standard via a co-injection technique; in addition, structure of isomer III was confirmed through mass spectra comparisons with its authentic standard. The compound was found in the form of an 11-hydroxide eicosanoid isomer in the reaction mixture (with crude enzyme and arachidonic acid), which also contained glutathione and glutathione peroxidase; consequently, in this reaction, C10-aldehyde [2(E),4(E)-decadienal] could not be formed by HPL because the hydroxyl isomer is not its substrate. This situation provided an advantage in that 11- hydroxyeicosatetraenoic acid accumulated to a high enough amount that it could be readily identified in its hydroxyl form. Accordingly, the hydroxyl isomer form was demonstrated to be the hydroperoxy form that is catalysed by the LOX reaction, allowing us to conclude that 11-hydroperoxide eicosanoid is produced as an intermediate from arachidonic acid in the generation of 2(E),4(E)-decadienal.
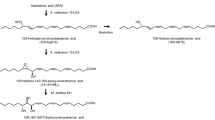
In conclusion, our results indicate that 11-hydroperoxide eicosanoid is produced from arachidonic acid as an intermediate in the generation of 2(E),4(E)-decadienal, as shown in the biosynthesis pathway for aldehyde formation that is proposed in Fig. 3. Arachidonic acid is converted by LOX to its hydroperoxide, 11-hydroperoxide eicosatetraenoic acid, before being cleaved by HPL to generate 2(E),4(E)-decadienal and its oxo-acid.
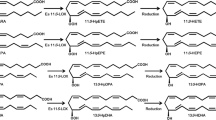
This proposed pathway (Fig. 3) was able to complete biosynthesis of a short chain bioflavour aldehyde that uses arachidonic acid and linoleic acid as the substrate in the former reports (Boonprab et al. 2003a, b, 2004, 2006) in S. angustata. The biosynthesis of all indicated short chain aldehyde bioflavour compounds [2(E) and 3(Z) nonenal, n-hexanal and 2(E),4(E)-decadienal] in this species that are converted from arachidonic acid and linoleic acid through their hydroperoxides in S. angustata are also proposed in Fig. 4. The formation of three aldehydes occurs through four branches (Fig. 4; white arrow) from two cascades (the linoleic acid [(C18:2, n-6); Fig. 4A] and arachidonic acid [(C20:4, n-6); Fig. 4B] cascades) via the LOX/HPL system. Two of the fatty acids are oxygenated by LOX, yielding hydroperoxides with specific positional and enantiomer selectivity that are then sequentially cleaved by HPL.
The proposed pathway for short chain aldehydes [2(E) and 3(Z) nonenal, n-hexanal and 2(E),4(E)-decadienal] formation in the brown alga, S. angustata from arachidonic acid and linoleic acid. [A. and partial of B. were reported by Boonprab et al. (2006), and the branch of 2(E),4(E)-decadienal formation is from the proposed pathway in Fig. 3]
The main strengths of this work were obtained by confirming the presence of the pathway and proposing the novel branch occurring that was formed from C20-fatty acid (arachidonic acid) and the completed short-chain aldehydes formation from C20-fatty acid (arachidonic acid) and C18-fatty acid (linoleic acid) in this species; however, the limitation was the expanding of the approval of the specific type of enzymatic system such as chiral structure selectivity of the enzyme and/or their substrate specificity of the enzyme systems, that should be further studied to enhance more understanding of the biogeneration developed in the species.
The directions resulting from the developments were the understanding of the occurring evolution of oxylipin system among a variety of organism, i.e. diatom (Pohnert 2002) and/or green algae (Akakabe et al. 2003) and the initiation of clarification on various topics to enhance the new knowledge, i.e. the control interactions with other organisms and with the environment, promoting algae survival by algal oxylipins, the exact mechanisms of stress tolerance that may involve in the eco-physiological role of the oxidised lipid-derivatives, and the marine drug that may develop under the understanding of the structural chemistry, biosynthesis, and pharmacological activities of these marine products as metabolites of oxylipin class also play a crucial role in both mammalian physiology and disease (Barbosa et al. 2016).
References
Akakabe Y, Matsui K, Kajiwara T (2003) 2,4-Decadienals are produced via (R)-11-HPITE from arachidonic acid in marine green alga Ulva conglobata. Bioorg Med Chem 11:3607–3609
Andreou A, Brodhun F, Feussner I (2009) Biosynthesis of oxylipins in non-mammals. Prog Lipid Res 48:148–170
Barbosa M, Collado-González J, Andrade PB, Ferreres F, Valentão P, Galano JM, Durand T, Gil-Izquierdo Á (2015) Nonenzymatic α-linolenic acid derivatives from the sea: macroalgae as novel sources of phytoprostanes. J Agric Food Chem 63:6466–6474
Barbosa M, Valentão P, Andrade PB (2016) Biologically active oxylipins from enzymatic and nonenzymatic routes in macroalgae. Mar Drugs 14:23
Blée E (1998) Phytooxylipins and plant defense reactions. Prog Lipid Res 37:33–72
Boeynaems JM, Brash AR, Oates JA, Hubbard WC (1980) Preparation and assay of monohydroxy-eicosatetraenoic acids. Anal Biochem 104:259–267
Boonprab K, Matsui K, Akakabe Y, Yotsukura N, Kajiwara T (2003a) Hydroperoxy-arachidonic acid mediated n-hexanal and (Z)-3- and (E)-2-nonenal formation in Laminaria angustata. Phytochemistry 63:669–678
Boonprab K, Matsui K, Yoshida M, Akakabe Y, Chirapart A, Kajiwara T (2003b) C6-aldehyde formation by fatty acid hydroperoxide lyase in the brown alga, Laminaria angustatata. Z Naturforsch 58c:207–214
Boonprab K, Matsui K, Akakabe Y, Yotsukura N, Kajiwara T (2004) Arachidonic conversion by lipoxygenase in the brown alga, Laminaria angustata. Kasetsart J (Nat Sci) 38:72–77
Boonprab K, Matsui K, Akkabe Y, Yoshida M, Yotsukura N, Chirapat A, Kajiwara T (2006) Formation of aldehyde flavor (n-hexanal, 3Z-nonenal and 2E-nonenal) in the brown alga, Laminaria angustata. J Appl Phycol 18:409–412
Brodowsky ID, Hamberg M, Oliw EH (1992) A linoleic acid (8R)-dioxygenase and hydroperoxide isomerase of the fungus Gaeumannomyces graminis. Biosynthesis of (8R)-hydroxylinoleic acid and (7S,8S)-dihydroxylinoleic acid from (8R)-hydroperoxylinoleic acid. J Biol Chem 267:14738–14745
Bryant RW, Simon TC, Bailey JM (1982) Role of glutathione peroxidase and hexose monophosphate shunt in the platelet lipoxygenase pathway. J Biol Chem 257:14937–14943
Cadwallader KR (2000) Enzymes and flavor biogenesis in fish. In: Haard NF, Simpson BK (eds) Seafood enzymes: utilization and influence on postharvest seafood quality. Marcel Dekker, Inc., New York, pp 365–383
Dulley JR, Grieve PA (1975) Simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem 64:136–141
Fujimura T, Kawai T (2000) Enzymes and seaweed flavor. In: Haard NF, Simpson BK (eds) Seafood enzymes: utilization and influence on postharvest seafood quality. Marcel Dekker, Inc., New York, pp 385–407
Gerwick WH (1994) Structure and biosynthesis of marine algal oxylipins. Biochim Biophys Acta 1211:243–255
Gerwick WH, Proteau PJ, Nagle DG, Wise ML, Jiang ZD, Bernart MW, Hamberg M (1993) Biologically active oxylipins from seaweeds. Hydrobiologia 260/261:653–665
Graff G, Anderson LA, Jaques LW (1990) Preparation and purification of soybean lipoxygenase-derived unsaturated hydroperoxy and hydroxy fatty acids and determination of molar absorptivities of hydroxy fatty acids. Anal Biochem 188:38–47
Grechkin AN (2002) Hydroperoxide lyase and divinyl ether synthase. Prostaglandins Other Lipid Mediat 68-69:457–470
Hamberg M, Gerwick WH (1993) Biosynthesis of vicinal dihydroxy fatty acids in the red alga Gracilariopsis lemaneiformis: identification a sodium-dependent 12-lipoxygenase and a hydroperoxide isomerase. Arch Biochem Biophys 305:115–122
Hamberg M, Herman CA, Herman RP (1986) Novel biological transformations of 15-Ls-hydroperoxy-5,8,11,13-eicosatetraenoic acid. Biochim Biophys Acta 877:447–457
Hamberg M, Su C, Oliw E (1998) Manganese lipoxygenase. Discovery of a bis-allylic hydroperoxide as product and intermediate in a lipoxygenase reaction. J Biol Chem 273:13080–13088
Hatanaka A (1996) The fresh green odor emitted by plants. Food Rev Int 12:303–350
Hatanaka A (2003) So-called “green odor” as plant origin--chemistry and biochemistry. Seikagaku 75:1414–1428
Hatzelmann A, Schatz M, Ullrich V (1989) Involvement of glutathione peroxidase activity in the stimulation of 5-lipoxygenase activity by glutathione-depleting agents in human polymorphonuclear leukocytes. Eur J Biochem 180:527–533
Hombeck M, Pohnert G, Boland W (1999) Biosynthesis of dictyopterene A: stereoselectivity of a lipoxygenase/hydroperoxide lyase from Gomphonema parvulum (Bacillariophyceae). Chem Commun 3:243–244
Jayasena DD, Ahn DU, Nam KC, Jo C (2013) Flavour chemistry of chicken meat: a review. Asian-Australas J Anim Sci 26:732–742
Kajiwara T (1997) Dynamic studies on bioflavor of seaweed. Koryo No 196:61–70. (in Japanese with English summary)
Kajiwara T, Hatanaka A, Kawai T, Ishihara M, Tsuneya T (1988) Study of flavor compounds of essential oil extracts from edible Japanese kelps. J Food Sci 53:960–962
Kajiwara T, Kodama K, Hatanaka A, Matsui K (1993a) Volatile compounds from Japanese marine brown algae. In: Teranishi R, Buttery RG, Sugisawa H (eds) Bioactive volatile compounds from plants. Am Chem Soc Symp series, vol 525, Washington, DC, pp 104–120
Kajiwara T, Matsui K, Hatanaka A, Tomoi T, Fujimura T, Kawai T (1993b) Distribution of an enzyme system producing seaweed flavor: conversion of fatty acids to long-chain aldehydes in seaweeds. J Appl Phycol 5:225–230
Kajiwara T, Matsui K, Akakabe Y (1996) Biogeneration of volatile compounds via oxylipins in edible seaweeds. In: Takeoka GR, Teranishi R, Williams PJ, Kobayashi A (eds) Biotechnology for improved foods and flavors. Am Chem Soc Symp series, vol 637, Washington, DC, pp 146–166
Katayama T (1958) Chemical studies on volatile constituents of seaweed. J Fac Fish Anim Husb Hiroshima Univ 2:67–77
Kuhn H (2000) Structural basis for the positional specificity of lipoxygenases. Prostaglandins Other Lipid Mediat 62:255–270
Kuhn H, Banthiya S, van Leyen K (2015) Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 1851:308–330
Lehmann WD, Stephan M, Fürstenberger G (1992) Profiling assay for lipoxygenase products of linoleic and arachidonic acid by gas chromatography-mass spectrometry. Anal Biochem 204:158–170
Matsui K (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9:274–280
Mosblech A, Feussner I, Heilmann I (2009) Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem 47:511–517
Noordermeer MA, Veldink GA, Vliegenthart JF (2001) Fatty acid hydroperoxide lyase: a plant cytochrome p450 enzyme involved in wound healing and pest resistance. Chembiochem 2:494–504
Pohnert G (2002) Phospholipase A2 activity triggers the wound-activated chemical defense in the diatom Thalassiosira rotula. Plant Physiol 129:103–111
Qi J, Liu DY, Zhou GH, Xu XL (2017) Characteristic flavor of traditional soup made by stewing Chinese yellow-feather chickens. J Food Sci 82:2031–2040
Schnurr K, Belkner J, Ursini F, Schewe T, Kuhn H (1996) The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J Biol Chem 271:4653–4658
Sekiya J, Kajiwara T, Hatanaka A (1984) Seasonal change in activities of enzymes responsible for the formation of C6-aldehydes and C6-alcohols in tea leaves, and the effects of environmental temperatures on the enzyme activities. Plant Cell Physiol 25:269–280
Sutherland M, Shankaranarayanan P, Schewe T, Nigam S (2001) Evidence for the presence of phospholipid hydroperoxide glutathione peroxidase in human platelets: implications for its involvement in the regulatory network of the 12-lipoxygenase pathway of arachidonic acid metabolism. Biochem J 353:91–100
Takakura Y, Mizushima M, Hayashi K, Masuzawa T, Nishimura T (2014) Characterization of the key aroma compounds in chicken soup stock using aroma extract dilution analysis. Food Sci Technol Res 20:109–113
Weitzel F, Wendel A (1993) Selenoenzymes regulate the activity of leukocyte 5-lipoxygenase via the peroxide tone. J Biol Chem 268:6288–6292
Funding
This work was performed as part of the JSPS-NRCT Core University Program on the “Development of thermotolerant microbial resources and their applications”, with the cooperation of Japanese and Thai scientists and in association with Kasetsart University in Thailand and Yamaguchi University in Japan (1999-2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boonprab, K., Matsui, K., Akakabe, Y. et al. 11-Hydroperoxide eicosanoid-mediated 2(E),4(E)-decadienal production from arachidonic acid in the brown algae, Saccharina angustata. J Appl Phycol 31, 2719–2727 (2019). https://doi.org/10.1007/s10811-019-01776-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01776-y