Abstract
Gracilaria gracilis farming was conducted in the Bizerte lagoon (North of Tunisia) at two depths (0.5 and 2.5 m) using various initial fragment weights (5, 20, and 40 g) in order to investigate the alga growth, agar yield, and quality. IR spectra of agar were performed in comparison to the commercial one. Moreover, the proximate biochemical composition of seaweeds was investigated. The results showed that the highest daily growth rate and agar yield were recorded at 0.5 m, using 5 g as initial fragment weight (5.98 ± 1.98% day−1, 14.87 ± 1.91% dw, respectively). Maximum gel strength was 356.67 ± 15.25 g cm−2 at 2.5 m using an initial weight of 20 g. Gelling and melting temperatures varied from 34.33 ± 0.57 to 35.50 ± 0.57 °C and from 77.00 ± 1.00 to 85.00 ± 0.50 °C, respectively. IR analysis revealed that some spectra were similar, while others were different. The highest dry matter (22.68 ± 1.77%) and ash content (35.25 ± 0.02% of dw) were recorded at 2.5 m, using initial weights of 20 and 5 g, respectively. The maximum carbohydrate and the crude protein contents were obtained at 0.5 m, using 5 g as initial weight (9.52 ± 0.36 and 5.83 ± 0.7% dw, respectively). The highest R-phycoerythrin content was recorded at 0.5 m (0.050 ± 0.007 mg g−1 dw), while the maximum of total lipid content was recorded at 2.5 m with initial weight of 5 g (3.58 ± 0.63% dw). Our findings indicated that growth and biochemical composition of G. gracilis depend on the depth and initial fragment weights.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, seaweeds are increasingly used as food, feed, and fertilizers in agriculture representing a valuable source of income and a sustainable livelihood for coastal communities (Rebours et al. 2014). In industry, algae are used in various sectors such as pharmaceuticals, nutraceuticals, and cosmetics. The production of aquatic plants, mostly seaweeds, reached 27.3 million tonnes in 2014 (FAO 2016). Several interesting compounds are obtained from seaweeds. Among these, hydrocolloids, such as alginates, agars, and carrageenan, are considered the main natural substances which have a large use spectrum (Bixler and Porse 2011). The Gelidiales and Gracilariales are among the red seaweeds the most used in agar industry. Nevertheless, wild raw material is not always sufficient to supply a viable industry facing an increasing demand for seaweeds. Accordingly, seaweed cultivation is practiced in several countries, such as China, Japan, Chile, Argentina, Philippines, Indonesia, and Malaysia, to supply hydrocolloid factories with substantial biomass of agarophyte and carragenophyte macroalgae (Porse and Rudolph 2017).
Approximately 100 species of red algae, including Gracilaria spp. are cultured worldwide for the agar industry. In fact, Gracilaria is well-known for its high quality of both agar and agarose which are mainly used for food, pharmaceutical, and biotechnology applications (Hurtado et al. 2014). In addition, interest in other Gracilaria compounds, such as carbohydrates, proteins and lipids, is increasing. An increased interest in different pigments, namely the R-phycoerythrin and other secondary metabolites is also noted in several studies (Mensi et al. 2012; Francavilla et al. 2013). Indeed, three kinds of pigments are directly involved in the photosynthesis: chlorophylls, phycobiliproteins, and carotenoids (Ragan and Glombitza 1986; Rowan 1989; Dring 1990). Among the phycobiliproteins, the R-phycoerythrin is the predominant pigment in red algae. Due to its spectral properties, R-phycoerythrin is used mainly in clinical and immunological analysis (Mensi et al. 2012), fluorescence microscopy, and flow cytometry (Glazer and Stryer 1984; Kronick 1986). It is also reported that R-phycoerythrin and phycocyanin act as a part of protective mechanism in seaweeds in general (Sinha et al. 1995), including Gracilaria caudata (Araujo et al. 2014). To our best knowledge, few studies (Molloy and Bolton 1996; Mensi et al. 2009) have been published regarding the effect of both depth and initial fragment weights on growth and proximate chemical composition of Gracilaria gracilis (previously known as G. verrucosa).
Presently there was no large-scale seaweed cultivation nor an agar industry in Tunisia. Nevertheless, several attempts of Gracilaria farming were performed in the last two decades, using both spores (Ben Saïd and Aouini 2014) and cuttings/fragments (Ksouri et al. 1999, 2000; Mensi et al. 2009). Four farming methods were used: ropes, sand-filled plastic tubes, spade, and suspended system in hoop-nets. Some experimental agar extractions also have been carried out on G. gracilis (Ben Saïd and Ksouri 1999; Ben Saïd et al. 2015).
This study aimed to determine the ability of G. gracilis to be cultivated at two different water depths in the Bizerte lagoon, using net pockets. Additionally, relatively low initial fragment weights, compared with previously reported studies (Ksouri et al. 1999, 2000; Mensi et al. 2009), were also tested in order to have an appreciable biomass with a high daily growth rate (DGR) in relation to environmental factors. The biomass could be firstly used for agar extraction and secondly for other potentially interesting compounds. Hence, agar yield, gel strength, and gelling and melting temperatures were investigated because of their properties to correlate with the 3,6-anhydrogalactose and sulfate contents (Minghou et al. 1985; Oyieke 1993; Rodriguez et al. 2009). Furthermore, IR spectroscopy was used to study agar extracts (Gomez-Ordonez and Rupérez 2011). The proximate biochemical composition of farmed seaweeds was also studied. The main objective of this work is to determine which starting weight and which depth could lead to better results, mainly agar yield, quality, and biochemical characterization for various potential valorizations in the future.
Material and methods
Sampling site and culture system preparation
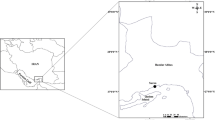
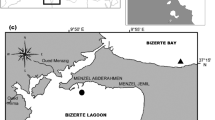
Gracilaria gracilis was collected in the Bizerte lagoon, in the North of Tunisia (37°12′N; 9°55′E) near a local shellfish farm, at Menzel Jemil, in February 2015. Young, healthy thalli were thoroughly cleaned of sand, epiphytes such as invertebrates, and other algae. They were further washed with freshly collected seawater. Seaweeds were then cut to have cuttings/fragments with different weights. Three initial fragment weights were tested: 5, 20, and 40 g, using a field scale. Fragments (5) were tied using a nylon string and then placed in netting pockets which were fixed to a nylon rope. The latter was fixed to a wooden “bridge” used by the local shellfish farmers (Fig. 1). The experiment was performed in triplicate at both the two depths: 0.5 and 2.5 m.
Environmental parameters
Water samples were collected in Bizerte lagoon at approximately three-week intervals in plastic bottles and kept in an ice cooler box and brought to the laboratory for chemical analysis. The water temperature was recorded in situ at the two depths using an electronic thermometer SA880SSXH (Huger type). Salinity, pH, and dissolved oxygen (DO) were recorded with a multiparameter apparatus (HACH, HQ40d). Nutrients, such as dissolved inorganic nitrogen (DIN) composed of nitrates (NO3−), nitrites (NO2−), and ammonium (NH4+), total nitrogen (TN), orthophosphates (PO43−), and total phosphates (TP), were determined with an auto analyzer (3 Bran Luebbe).
Growth rate
Gracilaria gracilis was cultivated for 112 days (from February 12, 2015 to June 3, 2015). The daily growth rate (DGR) was calculated every five (5) time intervals, using the following formula:
where Wt is the final wet weight (g) at t day, W0 is the initial wet weight (g), and t is the time of culture in days.
Agar extraction and quality determination
At the end of 112 days of culture, dry cleaned seaweed samples (15 g each) were first washed with tap water to remove salts, then placed in 400 mL of H2SO4 (5%) solution for 1 h at room temperature and then rinsed thoroughly with tap water. Agar extraction was performed in 500 mL of a 5% alkaline solution (NaOH) at 100 °C for 80 min. The heated solution was then filtered using a Buchner filter and a vacuum pump. The filtrate obtained was then transferred to a flat steel recipient until it was cooled at room temperature for 15–20 min and then frozen overnight at −18 °C. On the next day the filtrate was thawed at room temperature until a thin agar film was formed. The latter was bleached using a 12° sodium hypochlorite solution for 2–3 min, rinsed with tap water, and finally oven dried at 105 °C overnight. The dry agar obtained was weighed. The agar yield was calculated as follows:
where Wa is the dry agar weight and Ws is the dry seaweed weight (g).
Agar quality was determined by measuring gel strength (g cm−2) and gelling and melting temperatures (°C) as described by Lee et al. (2014) and Ben Saïd et al. (2011), with a little modification which consists of the use of a steel bead instead of a glass one to determine the melting temperature.
Fourier transform infrared spectroscopy (ATR-FTIR)
The agars obtained from farmed seaweeds at the two depths (six samples) were analyzed, using PerkinElmer Spectrum Two ATR-FTIR, over the wave number range between 4000 and 500 cm−1. Other FTIR spectra were also performed to improve resolution of overlapped bands in the original spectra (Matsuhiro and Rivas 1993; Gomez-Ordonez and Rupérez 2011). The farmed seaweed agar samples were compared to each other as well as to the commercial agar (Biokar Diagnostics, France) to detect any difference.
Proximate biochemical composition
All the following analyses were performed using the seaweeds harvested at the end of 112 days of culture.
Dry matter and ash content
The dry matter content of cleaned wet seaweeds was determined using the oven method (Mollet et al. 1998) at 50 °C until the constant dry weight was obtained. Dry matter (DM) was calculated as follows:
where Wf is the final weight and Wi is the initial weight. Ash was determined by heating seaweeds in a muffle furnace at 550 °C for 4 h and then weighing the residue (AOAC 2000). Ash contents (AC) were calculated as follows:
where Wf is the final weight and Wi is the initial dry weight.
Carbohydrate, 3,6-anhydrogalactose, and sulfate contents
The total carbohydrate content was determined according to the Phenol-Sulfuric acid method (Dubois et al. 1956) as the percentage of dry weight. Briefly, 4 mL of distilled water was added to 0.5 g of dry powdered seaweed. The seaweeds were homogenized using a vortex and ultra-turrax apparatus and then centrifuged for 15 min at 12,000 ×g. The supernatant (1 mL) was placed in an Eppendorf tube and frozen at −80 °C until analysis. A known volume (20 μL) of the extract was put in a test tube to which were added 80 μL of distilled water, 0.5 mL of a 5% phenol solution, and 2.5 mL of H2SO4. Then, the test tubes were vortexed and incubated in a water bath at 30 °C for 20 min. Finally, the absorbance was measured at 490 nm. Glucose was used to establish the standard curve.
The resorcinol reagent method of Yaphe and Arsenault (1965) was used to determine the 3,6-anhydrogalactose content. The sulfate content was determined by the turbidimetric method, using sodium sulfate (Na2SO4) after acid hydrolysis (2 M HCl at 100 °C for 2 h) of the polysaccharide (Salem et al. 2017).
Crude protein and R-phycoerythrin
Crude protein content was determined as described by Bradford (1976). Fresh alga (1 g) was first milled with a mortar and placed in 20 mL of distilled water and incubated for 10 h at 50 °C in a water bath. Samples were then filtered through a 250-μm nylon cloth filter. The filtrate was centrifuged at 4000 ×g for 15 min. The supernatant (1 mL) was then placed in a hemolytic tube to which 2 mL of Coomassie Blue reagent was added and vortexed. Absorbance was read at 595 nm after 5 min. Bovine Serum Albumin (BSA) was used to construct the calibration curve. Protein content Q (mg) in seaweed samples was calculated as follows:
where C is the protein concentration (mg g−1 fresh algae), obtained using the calibration curve; V = initial sampling volume (mL). Results are presented as percentage of dry weight (% dw), given the average dry matter/fresh matter ratio is approximately 15%.
R-phycoerythrin content was determined as described by Mensi et al. (2012), and the absorbance was measured at 565 nm, which is the maximum absorbance of R-phycoerythrin. Beer-Lambert law established the absorbance at 565 nm as follows:
where C2 = A ∗ (260 ∗ 103/2 ∗ 106) = 0.13 ∗ A, A = absorbance at 565 nm; ε is the R-phycoerythrin extinction coefficient (2.106 M−1 cm−1); L is the optic path length (= 1 cm); C1 is the molar concentration of R-phycoerythrin (M); C2 is the concentration of R-phycoerythrin (mg mL−1); and MW is the molecular weight of R-phycoerythrin (260,000 da).
Total lipid content
Total lipids were extracted according to the modified method of Floc’h et al. (1957). Dry powdered seaweed (1 g) was placed in a 50 mL conic tube to which was added 30 mL of Floc’h reagent (dichloromethane–methanol, 2:1 v/v) and vortexed, with the addition of 5 mL of a 0.73% NaCl solution. Finally, the solution was filtered using the cotton inserted in a medical syringe. The extract was then centrifuged for 10 min at 4 °C, and the lower layer was transferred to test tubes. The evaporation of the solution was performed using nitrogen gas. The lipid content was determined as follows:
where Wf is the final weight of the test tube (g), Wi is the initial weight of the test tube (g), and Ws is the dry weight of the seaweed sample (g).
Results were expressed as % of dry weight. The experiments were performed in triplicate to determine the agar yield, quality, and proximate biochemical composition.
Statistical analysis
The data are presented as the mean ± standard deviation (SD). The analysis of variance ANOVA (two-way) was performed using the SPSS (version 20.0) software to study the effect of the depth and the initial fragment weight on the DGR, agar yield, quality, and proximate biochemical composition. The homogeneity of variance was verified, and the Turkey’s test was used for the multi-comparison of means at the level confidence of 5%.
Results
Environmental parameters
The highest seawater temperature was recorded in April at 0.5 m (25.3 °C), and the lowest was 13.2 °C in February at the two depths (Table 1). The seawater salinity ranged from 33.7 to 39.3 psu. The dissolved oxygen (DO) varied from 7.19 to 10.48 mg L−1. The pH fluctuated between 6.69 and 7.82. The minimum dissolved inorganic nitrogen (DIN) content was 2.81 μM in April at 0.5 m, and the maximum was 13.64 μM in June at 2.5 m. The total nitrogen (TN) content ranged from 11.959 to 21.346 μM. The inorganic phosphate (orthophosphate PO43−) content varied from 0.190 to 0.371 μM, while the total phosphate (TP) content varied from 4.125 to 5.397 μM. The peak was observed in June 2015 at 0.5 m.
Growth rate
Daily growth rate (DGR) fluctuation is illustrated in Figs. 2 and 3. The highest DGR was always recorded when using an initial seedling weight of 5 g, followed generally by 20 g, and finally 40 g. The maximum DGR (5.98 ± 1.16% day−1) was recorded within three weeks of culture (T1 = 20 days) at 0.5 m (Fig. 2). At the end of the culture, the DGR ranged from 3.08 ± 0.74 to 3.5 ± 0.49% day−1. At 2.5 m (Fig. 3), the highest DGR was also obtained within three weeks of culture, using 5 g as initial seedling weight (5.14 ± 1.93% day−1), followed by 20 and 40 g. At the end of the experiment and after 112 days of cultivation, the DGR varied from 0.75 ± 0.63 to 2.00 ± 0.62% day−1 (Fig. 3). It was noted that at T3 (69 days), seaweeds cultured from initial weight of 40 g lost many branches, and consequently the weight drastically decreased. There was a highly significant effect of the depth and the initial weight on DGR (ANOVA p < 0.05), and there was a highly significant interaction between both factors (p < 0.05). A marked increase of average weight over the 112-day cultivation period was noted in farmed algae at 0.5 m, compared to those farmed at 2.5 m, especially in algae which had 5 g as initial weight (37.6-fold) (Table 2).
Agar yield and quality
The agar yield varied from 13.13 ± 1.03 to 14.87 ± 1.91% dw at 0.5 m and from 9.89 ± 0.34% to 14.53 ± 0.29% dw at 2.5 m. There was a highly significant effect of initial cutting weight (ANOVA p < 0.05); however, depth had no significant effect (p > 0.05) although there was a highly significant interaction between both factors (p < 0.05). The maximum was recorded at 0.5 m in seaweeds which had a starting weight of 5 g, while the minimum was recorded at 2.5 m in farmed algae with initial weight of 40 g. The highest agar yields were obtained in algae cultured from initial weight of 5 g at both 0.5 and 2.5 m (Table 3). The gel strength varied significantly from 171.66 ± 7.64 to 356.67 ± 15.25 g cm−2, and there was a highly significant effect of starting weight, depth, and the interaction of both variables (p < 0.05). The highest value was obtained at 2.5 m in the 20 g initial fragment weight algae, while the minimum was at 0.5 m in algae which had 5 g as initial weight (Table 3). The gelling temperatures ranged from 34.33 ± 0.57 to 35.50 ± 0.57 °C (Table 3). ANOVA analysis showed no significant effect of depth and initial fragment weights (p > 0.05). The melting temperature ranged from 77.00 ± 1.00 to 85.00 ± 0.50 °C, and there was significant effect of initial weights, depth, and the interaction between both variables (p < 0.05). The maximum was recorded at 0.5 m, in the algae with an initial weight of 20 g, while the minimum was obtained 2.5 m in algae having an initial fragment weight of 5 g.
Infrared spectroscopy
As presented in Fig. 4a, all the spectra (1–6) were comparable to the spectrum (7) of commercial agar via distinctive absorption bands. In fact, a broad band from 3000 to 3600 cm−1 was assigned to C-OH elongation vibration. The band at around 2910 cm−1 was probably attributed to the 6-O-methyl d-galactose stretching vibration. These results are consistent with those found, according to Mouradi-Givernaud (1992) and Mouradi-Givernaud et al. (1992) from the agar of Gelidium latifolium. In addition, we noticed that depth has an influence on the amount of the residue 6-O-meyhyl d-galactose. This was noted in spectrum 6 (2.5 m, initial weight 40 g), compared with the commercial agar and other agar extract spectra. Moreover, we noticed that agars obtained from farmed seaweeds contained probably more sulfur and nitrogen than commercial agar via the presence of a little band of N-C-S at around 2160 cm−1 (spectrum 1; 0.5 m, initial weight 5 g). Spectrum 7 does not show such a band. It means that this band is generally obtained from seaweeds belonging to the Gracilariales. In fact, the Gelidiales are well-known as source of bacteriological agars, while agars from the Gracilariales are used especially in food industry and have lower quality. The additional bands comprised between 1630 and 1643 cm−1 were referred to (N-H) absorption stretching vibrations. The band at about 1420 cm−1 indicated the presence of ester sulfate, according to Cross (1964) and Mollet et al. (1998). Also, the peak assigned to 1240 cm−1 corresponded to the asymmetric stretching S=O (Mollet et al. 1998). This was comparable with works of Christiaen and Bodard (1983) which reported that sulfate ester link was recognized at the bands 1060, 1180, and 1370 cm−1 in agar film of Gracilaria verrucosa. The sulfate ester band absorption was noted at 1250 cm−1, according to Deslandes (1988). In the current study, the bands which appeared at 1164 cm−1 were assigned to β-d-galactose (Sekkal 1990), the band at 1070 cm−1 was attributed to the skeleton mode of the galactan (Sekkal et al. 1993), and the band absorption at around 1046 cm−1 was attributed to 3,6-anhydrogalactose. For band absorption at 930 cm−1, it may be attributable to the stretching vibration of C-C of 3,6 l-galactose (Stanley 1963). According to Christiaen and Bodard (1983), the bands at 1070 cm−1 were attributed to the vibration of the C-O-C bridge of 3,6 anhydrogalactose. Also, the band comprised at 800 cm−1 was specific to the galactose skeleton (Mouradi-Givernaud 1992). The two bands at 740 and 716 cm−1 in the extracted agars were assigned to the C-O-C bending mode in glycosidic linkages (Sekkal et al. 1993). Finally, the bands at around 528 cm−1 in the spectrum 4 (2.5 m, initial fragment weight of 5 g) was attributed to the presence of C–Br absorption stretching vibrations.
a IR spectra of different agars in the region 4000–400 cm−1. 1 From farmed G. gracilis at 0.5 m and with initial weight of 5 g; 2 from farmed G. gracilis at 0.5 m and with initial weight of 20 g; 3 from farmed G. gracilis at 0.5 m and with initial weight of 40 g; 4 from farmed G. gracilis at 2.5 m and with initial weight of 5 g; 5 from farmed G. gracilis at 2.5 m and with initial weight of 20 g; 6 from farmed G. gracilis at 2.5 m and with initial weight of 40 g; 7 from commercial agar. b Spectra of different agars in the region 2000–600 cm−1. 1 From farmed G. gracilis at 0.5 m and with initial weight of 5 g; 2: from farmed G. gracilis at 0.5 m and with initial weight of 20 g; 3: from farmed G. gracilis at 0.5 m and with initial weight of 40 g; 4: from farmed G. gracilis at 2.5 m and with initial weight of 5 g; 5: from farmed G. gracilis at 2.5 m and with initial weight of 20 g; 6 from farmed G. gracilis at 2.5 m and with initial weight of 40 g; 7 from commercial agar
Figure 4b depicts agar spectra with higher resolution in the region of 2000 to 600 cm−1. In agreement with that reported by Gomez-Ordonez and Rupérez (2011) who found two characteristic agar signals at 790.9 and 715.3 cm−1, the different agar spectra (1–6) of farmed seaweeds and the commercial agar spectrum (7) showed two diagnostic bands in the region 800–700 cm−1. Matsuhiro (1996) observed two important bands at 790.8 and 717.0 cm−1 in FTIR and its second derivative spectra of the agar from Gracilaria chilensis. In the current study, the two similar bands were recorded at around 785–790 and 715–720 cm−1, respectively. These bands are usually used to distinguish between agar- and carrageenan-type galactans in red seaweeds within a few minutes (Matsuhiro 1996). The signals at 893–882 are probably due to the equatorial CH deformation as found by Neely (1957). The band at 925–936 cm−1 indicates the presence of 3,6-anhydrogalactose in agar extracts and in the commercial one. A series of bands in the region of 1166 and 1000 cm−1, especially at 1043–1053 cm−1 assigned to the sulfur–oxygen stretching vibrations for the sulfate group (Conley 1966; Nakamoto 1986; Mouradi-Givernaud et al. 1993; Matsuhiro 1996) and the presence of 3,6 anhydrogalactose, as mentioned above are observed in all the spectra.
Proximate biochemical composition
The biochemical composition of farmed seaweeds is summarized in Table 3.
Dry matter and ash content
The dry matter varied from 14.88 ± 0.08 to 22.68 ± 1.77%. ANOVA indicated that depth and initial weight had a highly significant effect on dry matter (p < 0.05), and there was a highly significant interaction between both variables (p < 0.05). The highest value was recorded in samples which had an initial weight of 20 g at 2.5 m, while the minimum was recorded in algae with an initial weight of 5 g at 0.5 m. The ash content ranged from 19.04 ± 0.16 to 35.25 ± 0.02%. ANOVA showed a significant effect of initial fragment weight, depth, and the interaction between both parameters (p < 0.05).The maximum was recorded in algae with starting fragment weight of 5 g at 2.5 m, while the minimum was recorded in algae of starting weight of 40 g at the same depth (Table 3).
Carbohydrate, 3,6-anhydrogalactose, and sulfate contents
The carbohydrate content varied significantly from 5.38 ± 0.35 to 9.52 ± 0.36% dw. ANOVA indicated a highly significant effect of depth and initial weight (p < 0.05), and there was a significant interaction between both variables (p < 0.05). The maximum was obtained at 0.5 m in algae which had an initial weight of 5 g, while the minimum was obtained at 2.5 m for algae which had a starting weight of 40 g (Table 3). The 3,6-anhydrogalactose content varied significantly from 20.12 ± 8.93 to 47.17 ± 5.07% (Table 3). The minimum and the maximum values were obtained at 0.5 m. ANOVA showed a highly significant effect of initial weight (p < 0.05), while there was no significant effect of depth (p > 0.05).The sulfate content ranged from 4.11 ± 0.19 to 5.51 ± 0.41%. The highest value was obtained with the agar samples at 0.5 m and from initial weight of 5 g, while the lowest value was recorded from the agar from seaweeds cultivated at 2.5 m with initial weight of 40 g (Table 3).
Crude protein and R-phycoerythrin content
The crude protein content varied significantly from 2.96 ± 0.28 to 5.83 ± 0.70% dw. There was a highly significant effect of depth, initial fragment weight, and the interaction between both variables (ANOVA p < 0.05). The highest value was recorded at 0.5 m in seaweeds with starting fragment weight of 5 g, while the lowest one was obtained at 2.5 m with those with a starting fragment weight of 40 g (Table 3). The R-phycoerythrin content ranged from 0.011 ± 0.006 to 0.050 ± 0.007 mg g−1, and there was a significant effect of depth, initial weight, and the interaction between both variables (p < 0.05). The maximum was recorded at 0.5 m in algae with initial weight of 5 g, whereas the minimum was recorded at 2.5 m in algae with a starting weight of 40 g.
Total lipid content
The total lipid content varied from 1.37 ± 0.11 to 3.58 ± 0.63% dw. The highest lipid content was obtained in algae cultured at 2.5 m from initial weights of 5 g, while the minimum was recorded at 0.5 m in algae cultured from initial fragment weights of 5 g (Table 3). ANOVA showed a significant effect of initial weights (p < 0.05). There was no significant effect of depth (p > 0.05), whereas there was a high interaction between the two factors (p < 0.05).
Discussion
The present study has shown that G. gracilis can be cultivated at two different depths: 0.5 and 2.5 m. The alga also can be propagated using relatively low initial fragment weights ranging from 5 to 40 g. Nevertheless, the highest DGR was recorded at 0.5 m, using a starting fragment weight of 5 g (5.98 ± 1.98% day−1, after 20 days of cultivation and 3.50 ± 0.49% day−1 after 112 days). The results obtained were higher than those reported by Ksouri et al. (1999) and Mensi et al. (2009) who found a DGR ranging from 1.3 to 3.7% day−1, respectively. In the latter study, the cultivation periods of G. verrucosa (as G. gracilis) at the Bizerte lagoon were 58 and 35 days at depths ranging from 1 to 4 m and using initial fragment weights of 200 g. However, results obtained in the current study were lower than those of Molloy and Bolton (1996) working with G. gracilis at Lüderitz, Namibia. The DGR recorded in this area ranged from 5.7 to 12.1% day−1. The optimal depth range for growth was found to be 0.5–2.5 m, which is in agreement with our results. Nevertheless, the results were in accordance with those of Molloy and Bolton (1996) regarding the decrease of DGR with an initial fragment weights increasing (10 to 70 g). Accordingly, the main differences between DGR at the two depths could be explained by the light availability for the seaweeds and the self-shading influencing seaweed light harvesting and decreasing photosynthesis. On the other hand, the water current at 2.5 m had an important impact on the farmed seaweeds which lost many branches during the study, compared to the farmed seaweeds at 0.5 m. This was reflected by negative growth in some cases. Despite of its occurrence at relatively high depth, the genus Gracilaria is well-known to prefer calm waters in estuaries, bays, and lagoons and develops generally in shallow waters. This is very likely the reason why growth at 2.5 m was lower than at 0.5 m, as the physico-chemical parameters measured in this study did not vary greatly between the two depths.
Regarding the agar yield which ranged from 9.89 ± 0.32 to 14.89 ± 1.91% dw, the results obtained were generally comparable to those reported by Mollet et al. (1998) for G. gracilis harvested in Brittany (France). The agar yield varied in this area from 11.1 to 18.7% dw. However in similar period of harvest (June), our results were higher than those found by these authors (12.7% dw). Marinho-Soriano and Bourret (2003) reported that the agar yield of G. gracilis collected in the Thau Lagoon (France) varied from 19 to 30.5% dw, and the gel strength ranged from 229.5 to 828 g cm−1. These results are generally higher than those found in this study, suggesting that geographical location and seasonal environmental factors may influence the agar synthesis and rheological properties of extracted hydrocolloids. Rebello et al. (1996) reported that G. gracilis had the highest agar yield and gel strength in early summer and spring, which is in conformity with Martin et al. (2013). These authors working with G. gracilis in the Patagonian coast of Argentina have generally recorded lower values of agar yield and gel strengths than those found in the current study. On the other hand, several studies (Bird 1988; Molloy and Bolton 1996; Arano et al. 2000) showed that light and nutrient levels, especially nitrogen level, are crucial factors affecting growth, agar yield, and quality of Gracilaria. Nutrient enrichment is known to result in a decrease in agar yield of many red seaweeds (Bird 1988; Freile-Pelegrin et al. 1996). Arano et al. (2000) reported that higher gel strength in different species of Gracilaria was observed in plants grown in culture under low light treatment and the highest nitrogen enrichment that conflicts with other studies (Bird and Ryther 1990; Penniman and Mathieson 1985). It was also reported that the growth and chemical constituents of Gracilaria tenuistipitata were affected by concentration of salts and nutrients (Israel et al. 1999). In addition, intrinsic factors such as genetic characteristics, can influence agar yield and quality. In fact, gel strength and gelling and melting temperatures are the result of the agar composition. Low gel strength is the result of the lowly methylated and high sulfated galactan. This agarocolloid is mainly composed of the galactosyl-6 sulfate residues considered as the putative precursor of the 3,6-anhydrogalcatose (Mollet et al. 1998). Whereas the methoxyl groups influence the gelling temperature (Guiseley 1970), the sulfate groups influence the gel strength and melting temperature (Duckworth and Yaphe 1971). However, Lee et al. (2014) reported that both the 3,6 anhydrogalactose and total sulfate ester content had no relationship with the agar gel strength. Roleda et al. (1997) reported that the variation of gelling temperature is related to the increase in gel strength. Furthermore, Murano et al. (1992) found a relationship between the increase of methoxyl groups and the gelling temperature as well as the molecular weight of the polymer (Kapraun et al. 1994).
It was observed that the content of the main organic components of the farmed seaweeds (proteins and the carbohydrates) varied generally in the same way and inversely to the lipid content. The relationship between R-phycoerythrin and these constituents was not very clear. This may be explained by the synergistic interaction of many factors or the inhibitory effect of some factors which orientated the algae metabolism process to produce different cellular components as a response to environmental factors. In this context, Falkowski and La Roche (1991) reported that when seaweeds are shifted from high to low irradiance, they suffer an “energy crisis,” to which they may respond by diverting macromolecule biosynthesis from lipids and carbohydrates to proteins (for light-harvesting complexes) and then back to lipids (for photosynthetic membranes). When algae are shifted to high light, their pigments decrease via dilution (through growth) and degradation. These changes are called photoacclimation (Falkowski and La Roche 1991). Floreto and Teshima (1998) reported that the exposure of Ulva, Grateloupia, and Sargassum to low irradiance resulted in an increase of poly-unsaturated fatty acids. This was explained as a result of the increased galactolipid synthesis, thylakoid membrane stacking, and chloroplast volume (Sikko-Goad et al. 1988) as the plant maximizes the capture of light. At 2.5 m light intensity is evidently lower than at 0.5 m. In these conditions the response of farmed seaweeds may be explained by the concomitant effect of light availability, water turbidity, and motion. Other factors could induce the lipid synthesis, in addition to the mineral accumulation in the cells of the farmed G. gracilis, namely in seaweeds which had a starting weight of 5 g.
On the other hand, maximum values of dry matter were recorded at 2.5 m, regardless of the starting weight. Nevertheless, the highest value was obtained in seaweeds with an initial weight of 20 g (22.68 ± 1.77%). Our results are lower than those reported by Turan et al. (2015) in other red algae harvested in Turkish coasts. The dry matter in this area ranged from 27.35 ± 0.51 to 32.68 ± 0.69%.The highest ash content was recorded at 2.5 m, using seaweeds which had a starting weight of 5 g. Nevertheless and regardless of the depth at which seaweeds were grown, the maximum ash contents were observed in seaweeds having an initial weight of 5 and 20 g. The high ash content, namely at 2.5 m, was related to the ability to absorb mineral and trace elements from the surrounding environment easily. This finding may explain in part the high percentage of dry matter in these conditions as discussed above. In this study, the ash contents were higher than those found by Zuldin et al. (2016) in Kappaphycus striatum and K. alvarezii and in three different red algae (Laurencia obtusa, L. papillosa, and Jania rubens). This finding could be explained by the differences between seaweed species, in addition to the environmental factors in each sampling location.
Concerning the carbohydrate content, our results are in accordance with those found by Mensi et al.(2009) which indicated that the maximum values were recorded at lower depth (0–1 m) and the minimum were recorded at −3 m depth. Nevertheless, our results were generally higher than those reported by these authors. The carbohydrate content of Gracilaria edulis cultured from spores in the open sea of India did not exceed 20.5% dw (Jayasankar et al. 2005). However, the depth of raft culture was not mentioned in this study. On the other hand, Turan et al. (2015) reported that the carbohydrate content varied from 199.69 ± 9.19 to 374.02 ± 7.29 mg g−1 dw in three red algae and from 506.69 ± 9.19 to 643.93 ± 4.68 mg g−1 dw in two other green seaweeds. Thus, the fluctuation of the carbohydrate content seems to be species-dependent and also probably related to several environmental factors (temperature, salinity, nutrients, etc.). The high carbohydrate content recorded at 0.5 m with seaweeds which had the lowest initial weight (5 g) was probably due higher photosynthetic carbon fixation in these conditions (Martin et al. 2013).
The 3,6-anhydrogalactose contents found in the current study are generally in accordance with the findings of Mollet et al. (1998) for three different Gracilariaceae species harvested in Britanny, France. For G. gracilis, the 3,6-anhydrogalactose content varied from 36.6 to 50.4%, according to the intertidal level. The highest value was recorded at the upper level, while the lowest one was at the lower one. The results reported by Minghou et al.(1985) showed that 3,6-anhydrogalactose of crude agars varied from 22.3 to 35.2% for different species of Gracilaria, while the sulfate content ranged from 3.2 to 5%, which is in accordance with our results. Oyieke (1993) found that the sulfate content varied markedly, with the highest amount recorded for Gracilaria corticata (3.05%) and the lowest for Gracilaria sp. (0.3%) native agars. Considerable degree of sulfation on different native agar extracts from G. gracilis, ranging from 4.6 to 16.8%, was reported by Rodriguez et al. (2009). Lee et al. (2014) observed that gel strength is not solely dependent on the amount of 3,6-anhydrogalactose as mentioned above, which is in conformity with our findings. The sulfate esterification level at various positions, molecular weight of agar, and methylation level may influence agar gel strength (Murano 1995).
The crude protein and R-phycoerythrin contents varied similarly at 0.5 m. The maximum values were obtained in samples cultured from initial weight of 5 g, followed by those of 20 and 40 g. At the two depths, R-phycoerythrin content varied inversely to the increase of the initial weight. In addition, at 2.5 m, all the values were lower than those recorded at 0.5 m. This can be explained by the availability of the light to the seaweeds at this depth. In fact, despite of the ability to grow until −5 m (FAO 1987), G. gracilis cannot reach high growth because of the decrease of photosynthesis due to the decrease of light availability and the water transparency. Martin et al. (2013) reported that high nitrogen and phosphate content in water coincided with important thylakoidal development in algal cortical cells and higher protein content in the products extracted from autumn to winter (May–August) plants of G. gracilis in Argentina coasts. In the present investigation, the period of the harvest of the farmed seaweeds was performed in early summer (June). It coincided with the highest value of total nitrogen and total phosphate, namely at 0.5 m (Table 1). In these conditions, we recorded the highest carbohydrate, protein, and R-phycoerythrin contents, especially in algae which had an initial weight of 5 g. At 2.5 m, the pigments, namely the R-phycoerythrin did not harvest sun light with high efficacy. As a result, farmed seaweeds at 2.5 m showed lower red pigmentation and were rather yellow-colored and suffered from epiphytes, like shellfishes and hydrozoa. The results obtained in the present study were higher than those reported by Mensi et al.(2009) with respect to the protein content in G. verrucosa (as G. gracilis), but were in accordance regarding the R-phycoerythrin content which ranged from 0.35 to 0.75 mg g−1 dw for farmed G. verrucosa at similar depth (0–1 m).
The lipid content varies according to the taxonomic entity, season, and location and growing conditions in both macroalgae and microalgae (Khotimchenko 2006). In this context, the lipid contents in seaweeds range from 1 to 6% dw (Fleurence et al. 1994). In the present study, the level of lipid content varied from 1.37 to 3.58% dw, which is higher than the results of Fleurence et al. (1994) for G. verrucosa from the Brittany coast (1.3–1.5% dw). On the other hand, Khotimchenko (2006) reported that the lipid content varied from 2.1 ± 0.3 to 3.1 ± 0.5% fw, according to the development stage of G. verrucosa collected in Great Peter Bay, Sea of Japan. In the current study, the highest lipid content was recorded at 2.5 m. It was similar to that obtained for Grateloupia turuturu collected from the intertidal zone at Piriac-sur Mer, in the Atlantic coast of France (Kendel et al. 2013). Yong et al.(2015), working with the carrageenophyte K. alvarezii cultured in Malaysia, registered that the lipid content varied from 2.06 ± 0.15 to 3.00 ± 0.29% dw. The results reported in this study suggest that water depth together with turbidity may stress the seaweeds affecting their lipid metabolism. It was also observed that the maximum and the minimum lipid contents were inversely related at both depths in farmed seaweeds which had 5 and 40 g as initial similar to the inverse trend of the protein contents as described above. This may be also related to the photosynthesis process and accordingly all metabolic processes.
Conclusion
The present study showed the ability of G. gracilis to be cultivated at two different depths, using relatively low starting fragment weights (ranging from 5 to 40 g). Nevertheless, the DGR was the highest at 0.5 m. It also revealed that the maximum values of agar yield and melting temperature were recorded at 0.5 m, while the gel strength and the gelation temperature were obtained at 2.5 m.
Infrared spectroscopy showed generally the same trend of the agar extract spectra when compared to each other and to the commercial one, with characteristic signals at around 790 and 715 cm−1. However, some different signals were recorded and related to the amount of some agar constituents, such as 3,6-anhydrogalactose and sulfates.
The highest carbohydrate, crude protein, and R-phycoerythrin contents were obtained in algae cultivated at 0.5 m from a starting weight of 5 g, whereas the maximum dry matter, ash, and lipid contents were recorded at 2.5 m, regardless of the initial fragment weights. The results suggested that the parameters tested (depth and initial weight) had clear effects on the growth and biochemical composition of G. gracilis.
These findings could firstly help potential seaweed farmers to choose the depth at which seaweed cultivation could lead mainly to high biomass and in parallel high agar yield with best rheological properties. Based on the results obtained in this current study, farming seaweed valorization could be extended in a second step to other constituents, such as minerals, carbohydrates, proteins, lipids, and R-phycoerythrin. On the other hand, when using a low starting weight, the seaweed farmers could preserve natural resources and in parallel have substantial farming area with numerous cuttings which give an appreciable biomass and consequently a sustainable seaweed farming activity. Further studies are needed to have more knowledge and information on amino and fatty acid composition and the different minerals of farmed seaweeds.
References
AOAC (2000) Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Gaithersburg
Araujo FO, Ursi S, Plastino EM (2014) Intraspecific variation in Gracilaria caudata (Gracilariales, Rhodophyta): growth, pigment content and photosynthesis. J Appl Phycol 26:849–858
Arano KG, Trono GC Jr, Montano NE, Hurtado AQ, Villanueva RD (2000) Growth, agar yield and quality of selected agarophyte species from Philippines. Bot Mar 3:517–524
Ben Saïd R, Ksouri J (1999) La rhodophycée Gracilaria verrucosa du lac de Bizerte (Tunisie) : Variations mensuelles de la biomasse, du rendement d’extraction et de la qualité de l’agar. Bull INSTM 26:127–136
Ben Saïd R, Romdhane MS, EL Abed A, M’rabet R (2011) Temporal variation of some biometric parameters, agar-agar and quality of Gelidium spinosum (S.G. Gmelin) P.C. Silva (Rhodophyta, Rhodophyceae, Gelidiales) from Monastir coasts (Tunisia). Cahier Biol Mar 52:71–78
Ben Saïd R, Aouini H (2014) Culture des spores de Gracilaria gracilis (Stackhouse) M. Steentoft, L.M. Irvine et W.F. Farnham, 2006 et G. bursa-pastoris (Gmelin) Silva, 1952, de la lagune de Bizerte. Bull INSTM 41:89–99
Ben Saïd R, Aouini H, Akrout F (2015) La rhodophycée Gracilaria gracilis de la lagune de Bizerte (Tunisie): Variations spatio-temporelles de quelques facteurs hydro biologiques, du rendement et de la qualité de l’agar-agar. Afrique Science 11:317–334
Bird KT (1988) Agar production and quality from Gracilaria sp. strain G-16. Effect of environmental factors. Bot Mar 31:33–39
Bird KT, Ryther JH (1990) Cultivation of Gracilaria verrucosa (Gracilariales, Rhodophyta) strain G-16 for agar. Hydrobiologia 204/205:347–351
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Christiaen D, Bodard M (1983) Spectroscopie infra-rouge de films d’agar de Gracilaria verrucosa (Hudson) Papenfuss. Bot Mar 26:425–427
Conley RT (1966) Infrared spectroscopy, Allyn and Bacon Inc., Boston, pp 179–181
Cross AD (1964) An introduction to practical infrared spectroscopy. Butterworth, London
Deslandes E (1988) Etudes biochimiques des polysaccharides extraits de quelques algues carraghénophytes des côtes de Bretagne. Thèse de Doctorat. Univ. Bretagne Occidentale, Brest, France
Dring MJ (1990) Light harvesting and pigment composition in marine phytoplankton and macroalgae. In: Hering PJ, Campbell AK, Whitfield M, Maddock L (eds) Light and life in the sea. Cambridge University Press, Cambridge, pp 89–103
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Calorimetric method of determination of sugars and related substances. Anal Chem 28:350–356
Duckworth M, Yaphe W (1971) The structure of agar. Part 1. Fractionation of complex mixture of polysaccharide. Carbohydr Res 16:189–197
FAO (1987) Fiches FAO: Identification des espèces pour les besoins de la pêche. Méditerranée et Mer Noire. Zone de pêche 37. In: Végétaux et invertébrés, vol 1
FAO (2016) La situation mondiale des pêches et de l’aquaculture 2016. Contribuer à la sécurité alimentaire et à la nutrition de tous. Rome, p 224
Falkowski PG, La Roche J (1991) Acclimation to spectral irradiance in algae. J Phycol 27:8–14
Fleurence J, Guthier G, Mabeau S, Leray C (1994) Fatty acids from 11 marine macroalgae of the French Brittany coast. J Appl Phycol 6:527–532
Floc’h J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Floreto EAT, Teshima S (1998) The fatty acid composition of seaweeds exposed to different levels of light intensity and salinity. Bot Mar 41:467–481
Francavilla M, Franchi M, Monteleone M, Caroppo C (2013) The red seaweed Gracilaria gracilis as multiple products source. Mar Drugs 11:3754–3776
Freile-Pelegrin Y, Robledo D, Armisen R (1996) Seasonal changes in agar characteristics of two populations of Pterocladia capillacea in Gran Canaria, Spain. J Appl Phycol 8:239–246
Glazer AJ, Stryer L (1984) Phycofluor probs. Trends Biochem Sci 9:423–427
Gomez-Ordonez E, Rupérez P (2011) FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll 25:1514–1520
Guiseley KB (1970) The relationship between the methoxyl content and gelling temperature of agarose. Carbohydr Res 13:247–256
Hurtado AQ, Gerung GS, Yasir S, Critchley AT (2014) Cultivation of tropical red seaweeds in the BIMP-EAGA region. J Appl Phycol 26:707–718
Israel A, Martinez-Goss M, Friedlander M (1999) Effect of salinity an pH on growth an agar yield of Gracilaria tenuistipitata var. liui in laboratory and outdoor cultivation. J Appl Phycol 11:543–549
Jayasankar R, Ramakrishnan K, Nirmala K, Seema C (2005) Biochemical constituents of Gracilaria edulis cultured from spores. Seaweed Res Utiliz 27:39–44
Kapraun DF, Ganzon-Fortes ET, Bird KT, Trono G, Breden C (1994) Karyology and agar analysis of the agarophyte Gelidiella acerosa (Forskal) Feldman et Hamel from the Philippines. J Appl Phycol 6:545–550
Kendel M, Couzinet- Mossio A, Viau M, Fleurence J, Barnathan G, Wielgosz-Collin G (2013) Seasonal composition of lipids, fatty acids and sterols in the edible red alga Grateloupia turuturu. J Appl Phycol 25:425–432
Khotimchenko SV (2006) Variations in lipid composition among different development stages of Gracilaria verrucosa (Rhodophyta). Bot Mar 49:34–38
Kronick M (1986) The use of phycobiliproteins as fluorescent labels in immunoassay. J Immunol Meth 92:1–13
Ksouri J, Ben Said R, Pellegrini M (1999) Résultats des cultures expérimentales de rhodophycée Gracilaria verrucosa dans le lac de Bizerte. Tunisie Septentrionale. Bull INSTM 26:113–125
Ksouri J, Mensi F, Ben Said R (2000) Ajustement de certains paramètres de culture par bouturage de Gracilaria verrucosa (algue rouge), dans le lac de Bizerte. Bull INSTM 27:69–74
Lee WK, Namasivayam P, Ho CL (2014) Effects of sulfate starvation on agar polysaccharides of Gracilaria species (Gracilariaceae, Rhodophyta) from Morib, Malaysia. J Appl Phycol 26:1791–1799
Marinho-Soriano E, Bourret E (2003) Effect of season on the yield and quality of agar from Gracilaria species (Gracilariacea, Rhodophyta). Bioresour Technol 90:329–333
Martin LA, Rodriguez MC, Matulewicz MC, Fissore EN, Gerschenson LN, Leonardi PI (2013) Seasonal variation in agar composition and properties from Gracilaria gracilis (Gracilariales, Rhodophyta) of the Patgonian coast of Argentina. Phycol Res 61:163–171
Matsuhiro B (1996) Vibrational spectroscopy of seaweed galactans. Hydrobiologia 326/327:481–489
Matsuhiro B, Rivas P (1993) Second-derivative Fourier transform infrared spectra of seaweed galactans. J Appl Phycol 5:45–51
Mensi F, Ksouri J, Hammami W, Romdhane MS (2009) L’algue rouge Gracilaria verrucosa (Hudson) Papenfuss de la lagune de Bizerte (Tunisie septentrionale): Essai de culture en mode suspendu et composition biochimique. Bull INSTM 36:125–137
Mensi F, Ksouri J, Seale E, Romdhane MS, Fleurence J (2012) A statistical approach for optimization of R-phycoerythrin extraction from the red alga Gracilaria verrucosa by enzymatic hydrolysis using central composite design and desirability function. J Appl Phycol 24:915–926
Minghou J, Lahaye M, Yaphe W (1985) Structure of agar from Gracilaria spp (Rhodophyta) collected in the People’s Republic of China. Bot Mar 28:521–528
Mollet JC, Rahaoui A, Lemoine Y (1998) Yield, chemical composition and gel strength of agarcolloids of Gracilaria gracilis, Graciliariopsis longissima and the newly reported Gracilaria cf. vermiculophylla from Roscoff (Brittany, France). J Appl Phycol 10:59–66
Molloy FJ, Bolton JJ (1996) The effect of season and depth on the growth of Gracilaria gracilis at Lüderitz, Namibia. Bot Mar 39:407–413
Mouradi-Givernaud A (1992) Recherches biologiques et biochimiques pour la production d’agarose chez Gelidium latifolium (Grév.) Thuret et Bornet (Rhodophycées, Gélidiales). Thèse de Doctorat d’Etat. Univ. Caen, France, p 351
Mouradi-Givernaud A, Givernaud T, Morvan H, Cosson J (1992) Agar from Gelidium latifolium (Rhodophyceae, Gelidiales). Biochemical composition and seasonal variations. Bot Mar 35:151–160
Mouradi-Givernaud A, Givernaud T, Morvan H, Cosson J (1993) Annual variations of the biochemical composition of Gelidium latifolium (Greville) Thuret et Bornet. Hydrobiologia 260:607–612
Murano E (1995) Chemical structure and quality of agars from Gracilaria. J Appl Phycol 7:245–254
Murano E, Toffanin R, Knutsen ZF, SH PS, Rizzo R (1992) Chemical and macromolecular characterization of agar polymers from Gracilaria dura (C. Agardh) J. Agardh (Gracilariaceae, Rhodophyta). Carbohydr Polym 18:171–178
Nakamoto K (1986) Infrared and Raman spectra of inorganic and coordination compounds. J. Wiley & Sons, N.Y., pp 248–250
Neely WB (1957) Infrared spectra of carbohydrates. Adv Carbohydr Chem 12:13–33
Oyieke HA (1993) The yield, physical and chemical properties of agar gel from Gracilaria species (Gracilariales, Rhodophyta) from the Kenya coast. Hydrobiologia 260:613–620
Penniman CA, Mathieson AC (1985) Photosynthesis in Gracilaria tickvahiae McLachlan (Gigartinales, Rhodophyta) from the Great Bay Estuary, New Hampshire. Bot Mar 28:427–435
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements and outlook. J Appl Phycol 29:2187–2200
Ragan A, Glombitza KW (1986) Phlorotannins, brown algal polyphenols. Prog Phycol Res 4:129–241
Rebours C, Marinho-Soriano E, Zertuche-Gonzalez JA, Ayashi L, Vasquez JA, Kradolfer P, Soriano G, Ugarte R, Abreu MH, Bay-Larsen I, Hovelsrud G, Rodven R, Robledo D (2014) Seaweeds: an opportunity for wealth and sustainable livelihood for coastal communities. J Appl Phycol 26:1939–1951
Rebello J, Ohnon M, Critchley AT, Sawamura M (1996) Growth rates and agar quality of Gracilaria gracilis (Stackhouse) Steentoft from Namibia, Southern Africa. Bot Mar 39:273–279
Rodriguez MC, Matulewicz MC, Md N, Ducatti DRB, Leonardi PI (2009) Agar from Gracilaria gracilis (Gracilariales, Rhodophyta) of the Patagonic coast of Argentina—content, structure and physical properties. Bioresour Technol 100:1435–1441
Roleda MY, Ganzon-Fortes ET, Montano NE, Reyes FN (1997) Temporal variation in the biomass, quantity and quality of agar from Gelidiella acerosa (Forskal) Feldman et Hamel (Rhodophyta, Gelidiales) from Cap Bolinao, NW Philippines. Bot Mar 40:87–495
Rowan KS (1989) Photosynthetic pigments of algae. Cambridge University Press, Cambridge
Salem YB, Amri S, Hammi KM, Abdelhamid A, Le Cerf D, Bouraoui A, Majdoub H (2017) Physico-chemical characterization and pharmacological activities of sulfated polysaccharide from sea urchin, Paracentrotus lividus. Int J Biol Macromolec 97:8–15
Sekkal M (1990) Spectrochimie: utilisation conjointe de la spectrochimie IRFT et la modélisation moléculaire à l’étude structurale des carraghénanes. Thèse d’Université. Lille, France, p 162
Sekkal M, Huvenne JP, Legrand P, Sombret B, Mollet J-C, Mouradi-Givernaud A, Verdus M-C (1993) Direct structure identification of polysaccharides from red algae by FTIR microspectrometry. Localization of agar in Gracilaria verrucosa sections. Mikrochim Acta 112:1–10
Sikko-Goad L, Simmons MS, Lazinsky D, Hall J (1988) Effect of light cycle on diatom fatty acid composition and quantitative morphology. J Phycol 24:1–7
Sinha RP, Lebert M, Kumar A, Kumar HD, Hader DP (1995) Spectroscopic and biochemical analysis of UV effects of phycobiliproteins of Anabaena sp and Nostoc carmium. Bot Acta 108:87–92
Stanley NF (1963) Process for treating a polysaccharide of seaweeds of Gigartinaceae and Solieraceae families. U.S. Patent 3094517
Turan F, Ozgun S, Saym S, Ozyilmaz G (2015) Biochemical composition of some red and green seaweeds from Iskenderun Bay, the northeastern Mediterranean coast of Turkey. J Black Sea/Mediterr Environ 21:239–249
Yaphe W, Arsenault GP (1965) Improved resorcinol reagent for the determination of fructose, and 3,6-anhydrogalactose in polysaccharides. Anal Biochem 13:143–148
Yong YS, Yong WTL, Ng SE, Anton A, Yassir S (2015) Chemical composition of farmed and micropropagated Kappaphycus alvarezzii (Rhodophyta, Gigartinales) a commercially important seaweed in Malaysia. J Appl Phycol 27:1271–1275
Zuldin WH, Yassir S, Shapawi R (2016) Growth and biochemical composition of Kappaphycus (Rhodophyta) in customized tank culture system. J Appl Phycol 28:2453–2458
Acknowledgements
This study was cofounded by the Ministry of Higher Education and Research of Tunisia and the project BIOVecQ (PS1.3_08) financed by the European Union through the Cross-border cooperation programme Italy–Tunisia in the framework of the European Neighborhood and Partnership Instrument IEVP-2007–2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Said, R., Mensi, F., Majdoub, H. et al. Effects of depth and initial fragment weights of Gracilaria gracilis on the growth, agar yield, quality, and biochemical composition. J Appl Phycol 30, 2499–2512 (2018). https://doi.org/10.1007/s10811-018-1414-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1414-5