Abstract
Sargassum polycystum C. Agardh, a brown alga that is widely distributed in tropical areas, has significant economic value by virtue of its use as food, traditional medicine, animal fodder, and chemical products in Asian countries. In order to explore suitable conditions for the aquaculture of this alga, we used 9–10 cm long branches of S. polycystum to assess the effects of various environmental factors, including temperature, salinity, and irradiance upon the relative growth rate (RGR), photosynthetic pigment content, and chlorophyll fluorescence characteristics of this species. Data showed that S. polycystum exhibited a relatively broad tolerance to various environmental conditions, and grew well in different conditions of temperature, salinity, and irradiance, ranging from 15 to 25 °C, 20–40 PSU, and 10–80 μmol photons·m−2·s−1. The maximum RGR of S. polycystum occurred at 23 °C, 32 PSU, and 20–80 μmol photons·m−2·s−1 (P < 0.05). Temperature had a significant effect (P < 0.05) upon RGR and the chlorophyll fluorescence characteristics of S. polycystum, and lower temperature (10 °C) led to rapid reductions in the maximal photochemical quantum yield of photosystem II (F v/F m) and non-photochemical quenching (NPQ) values. Salinity exhibited a relatively weak effect upon the growth of S. polycystum, and the plant was able to survive at salinities from 16 to 40 PSU; during a two-week cultivation period, significant differences in RGR were not observed among different salinity conditions until 2 weeks of cultivation. Chlorophyll a and carotenoid content of S. polycystum decreased as irradiance increased (P < 0.05), and photo-inhibition occurred under higher levels of irradiance (> 80 μmol photons·m−2·s−1). These physiological data provide valuable data related to the successful cultivation of S. polycystum under controlled conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The brown algal genus Sargassum (Phaeophyceae) serves as one of the largest sources of natural seaweed worldwide and can be found abundantly along the benthic reefs and low intertidal zones of tropical and subtropical regions (Phillips and Fredericq 2000; Komatsu et al. 2007). Previous reports estimated that the genus Sargassum is currently comprised of about 300 species (Guiry and Guiry 2017), and some of these represent economically and ecologically important seaweeds (Trono 1999).
Sargassum polycystum C. Agardh, one of the most common species of the genus in tropical areas, usually grows between intertidal and subtidal zones in the western Pacific and eastern Indian oceans (Chiang et al. 1992). Sargassum polycystum, an edible seaweed, is rich in dietary fiber, vitamins, minerals, and polysaccharides; consequently, this seaweed serves as a common food source in Asian countries (e.g., Hong et al. 2007; Nagappan et al. 2017). Recently, various bioactive compounds have been identified in S. polycystum, including some with antioxidative, antibacterial, and antitumor properties; collectively, these properties render this seaweed very useful for a range of biomedical applications (e.g., Anggadiredja et al. 1997; Thangaraju et al. 2012). Moreover, because of the relatively high yield and quality of extracted alginate from this taxon relative to other Sargassum species, S. polycystum is also harvested as one of the main sources of alginate products (e.g., Calumpong et al. 1999; Saraswathi et al. 2003).
In China, Sargassum provides valuable food fodder for a variety of aquatic animals because it is a good source of minerals, carbohydrates, several essential amino-acids and is also rich in beta-carotene and vitamins (Yu et al. 2014). However, populations of natural Sargassum along the coast of tropical China are now being harvested more frequently to meet the growing demand for Sargassum as both fodder and an industrial material, which has resulted in a loss of natural habitat for Sargassum over time (Ang 2006). Consequently, establishing aquacultural methods for cultivating Sargassum species is very important in order to prevent the over-exploitation of this highly economically important species.
Environmental factors such as temperature, salinity, rainfall, irradiance, and pH are all known to affect the seasonal growth of Sargassum species (e.g., Steen 2004; Ang 2006; Yeong and Wong 2013). A previous study demonstrated that the seasonal growth pattern of S. polycystum strongly affects the yield and constituents of alginate, and thus its economic value (Saraswathi et al. 2003). However, little is known about the effects of these environmental factors on the growth of S. polycystum under aquaculture. Understanding the potential effects of these factors is vital to establishing a robust method for the large-scale cultivation of this alga.
Being a typical intertidal alga, in natural environments, Sargassum often suffers from unfavorable environmental conditions such as extremes of temperature, salinity, and irradiance. To understand the physiological status of plants under various environmental conditions, the relative growth rate (RGR) serves as a classical analytical tool for characterizing plant growth (Hurt 1982; Hoffmann and Poorter 2002). Additionally, because photosystem II (PSII) plays an important role in the photosynthetic response to environmental perturbation (Baker 1991), and because PSII production declines more quickly than other physiological activities when plants encounter stressful conditions, chlorophyll fluorescence measurements have been widely used to assess photosynthetic features and stresses. Several chlorophyll fluorescence parameters are frequently used as indicators of plant physiological status, including non-photochemical quenching (NPQ) and the maximal photochemical quantum yield of PSII (F v /F m) (Maxwell and Johnson 2000). When plants are exposed to stressful conditions, reductions of F v/F m are frequently observed and reflect the potential quantum efficiency of PSII (Baker 2008). In addition, increased NPQ values become evident and imply the excessive energy dissipated from the PSII reaction center is being released as heat; this represents a classical feature of the photoprotective response of plants (Müller et al. 2001; Baker 2008).
Samples in the present study were subjected to various laboratory growth conditions to examine the effects of temperature, salinity, and irradiance on the growth rate, photosynthetic pigment content, and photosynthetic activities of S. polycystum germlings isolated from Haitou, Danzhou city, Hainan Province, China. The aim of this study was to characterize the physiological responses of S. polycystum to various environmental conditions and to assess the tolerance for S. polycystum to those conditions and to assess the optimal cultivation conditions for this species. Collectively, resultant data will provide a useful foundation for the large-scale commercial cultivation of this alga.
Material and methods
Sampling and pre-culture of S. polycystum
Specimens of Sargassum polycystum were collected from a subtidal reef in the coastal area of Haitou, Danzhou city, Hainan Province (19°34.61′N, 108°59.17′E) in April 2015. The specimens were immediately transported back to the laboratory in natural seawater. Then, 9–10-cm long branches of S. polycystum were selected and gently cleaned with a brush to remove epiphytic algae and small animals; specimens were then rinsed several times with sterilized seawater. Next, the algae specimens were pre-cultured in plastic pots, each containing 2 L of sterilized natural seawater (32 practical salinity units [PSU]), and supplied with continuous air bubbling at 20 °C. After 7 days cultivation, samples that showed similar growth behavior were selected for subsequent experimentation. Light was provided by white fluorescent tubes (40 μmol photons·m−2·s−1) with a 12:12 h light and dark (L/D) cycle.
Temperature, salinity, irradiance, and light quality
After 7 days of pre-culture, S. polycystum samples were transferred to growth chambers to test the effects of different temperatures, salinities, and levels of irradiance upon growth. For all treatments, at least three replicates were carried out for each condition. Seawater was exchanged with fresh seawater once every 3 days.
Temperature
The average water temperature in the sampling area ranged from 18.1 °C in January to 30.4 °C in July, the minimum temperature in winter could fall below 15 °C, while the maximum temperature during the summer could reach up to 33 °C. Thus, the effects of temperature were examined under seven different temperature conditions (10, 15, 18, 20, 23, 25, and 30 °C ± 0.5 °C) with a 12:12 h, light/dark cycle, irradiated at the same 40 μmol photons·m−2·s−1 for all temperatures, and cultured in separate plastic pots containing sterilized natural seawater (32 PSU).
Salinity
Ultra high-purity water and sodium chloride was used to adjust the seawater to a range of salinities (16, 20, 24, 28, 32, 36, and 40 PSU). Salinities were determined using a refractometer (DSA28, Hangzhou Hui’er Instrument Equipment Co., Ltd., China). Salinity experiments were carried out under similar conditions as the temperature experiments described above, with temperature held constant at 23 ± 0.5 °C.
Irradiance
Six different irradiance levels were studied (10, 20, 40, 80, 120, and 200 μmol photons·m−2·s−1). Irradiance was measured with a Light Meter (GLZ-C, Top Instrument Co., Ltd., China). Other culture conditions were similar to those described above, with temperature and salinity held constant at 23 ± 0.5 °C, and 32 PSU, respectively.
Growth rate measurements
The fresh weight (W 1) of each S. polycystum thallus was measured at the beginning of treatment, and the changed fresh weight (W 2) for each sample was re-measured after t days of culture under each treatment condition.
The relative growth rate (RGR) was then calculated as follows:
The growth rate of each sample was measured weekly, and at least three replicates of each condition were used for weight measurement.
Determination of photosynthetic pigment contents
Approximately 0.05 g of S. polycystum fresh leaves were ground in 2.0 mL of 80% (v/v) acetone using a mortar and transferred to a 10-mL centrifuge tube. Each sample was then left to stand for 24 h in darkness, after which 80% (v/v) acetone was added to a total volume of 10 mL. To determine the concentration of chlorophyll, the absorbance of each sample was measured at wavelengths of 480, 510, 630, and 664 nm. The concentrations of chlorophylls a and c as well as carotenoid were then calculated according to Eqs. (1)–(3):
where V = total volume (10 mL) and W = sample weight.
At least five replicates of each condition were used to determine the content of photosynthetic pigments.
Chlorophyll fluorescence parameters
Sargassum polycystum samples which were exposed to light treatments were pre-treated in the dark for 10 min. Chlorophyll fluorescence parameters, were measured by pulse amplitude modulation (Min-PAM; Walz, Germany) at room temperature. First, the initial fluorescence level (F 0) was measured under 0.3 μmol photons·m−2·s−1; then a saturating light pulse (4000 μmol photons·m−2·s−1) for 0.8 s was used to induce maximum fluorescence (F m). For measurements of NPQ, an actinic light of 120 μmol photons·m−2·s−1 was used to illuminate sample leaves, and illuminated by 0.8 s saturating light pulse every 20 s, until the F m′ (maximum fluorescence in the light) values were stable. F v/F m and NPQ were then calculated according to eqs. (4) and (5) (Cosgrove and Borowitzka 2011):
Statistical analysis
SPSS software version 22 was used for all statistical testing. All data were analyzed by one-way analysis of variance (ANOVA), and Tukey’s honest significant difference tests were applied for multiple comparisons. P values <0.05 were considered statistically significant.
Results
The effects of temperature, salinity, and irradiance upon the growth rate
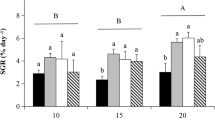
Temperature had a significant effect upon the growth rate of S. polycystum over the two weeks of cultivation (one-way ANOVA, F = 51.12, P < 0.05). RGR initially increased as water temperature rose from 10.0 to 23.0 °C and peaked at 5.51 ± 0.125 and 3.72 ± 0.071% day−1 at 23.0 °C, over the first and second weeks, respectively (Fig. 1a). However, RGR began to decrease as temperature continued to rise, and growth was arrested when temperature reached 30.0 °C by the end of the second week as the algal leaves bleached and became rotten. In addition, it appeared that prolonged low temperatures (10.0 °C) had a more harmful effect upon S. polycystum, because growth was arrested from the first week.
Relative growth rates of S. polycystum subjected to different treatments during a period of 2 weeks: (a) water temperature, (b) salinity, and (c) irradiance. Error bars show standard deviation. Bars marked with different letters are significantly different according to Tukey’s multiple comparison test (P < 0.05)
Salinity appeared to exert only a relatively weak effect upon the RGR of S. polycystum, because our samples were able to survive at salinities from 16 to 40 PSU, and significant differences in RGR were not observed among different salinity conditions until 2 weeks of cultivation (one-way ANOVA, F = 10.39, P < 0.05; Fig. 1b). The optimum salinities for growth of S. polycystum were 32–36 PSU, at which the mean growth rates (3.12 ± 0.179 and 2.98 ± 0.084% day−1 for 32 and 36 PSU, respectively) were more than twice as high as mean growth rates at 16 PSU (1.38 ± 0.469% day−1).
Irradiance had a significant effect on the growth of S. polycystum over the 2 weeks of cultivation (one-way ANOVA, F = 6.85, P < 0.05). Sargassum polycystum grew much faster at 20–80 μmol photons·m−2·s−1 with a maximum RGR of 3.69 ± 0.523% day−1 at 20 μmol photons·m−2·s−1 over the first week, and 3.25 ± 0.493% day−1 at 40 μmol photons·m−2·s−1 over the second week. However, RGR began to decrease as the level of irradiance rose from 80 to 200 μmol photons·m−2·s−1, and the minimum RGR of 1.32 ± 0.064% day−1 occurred at 200 μmol photons·m−2·s−1 (Fig. 1c). These results suggest that the optimum irradiance for the growth of S. polycystum was 20–80 μmol photons·m−2·s−1.
Effect of temperature, salinity, irradiance, and light quality upon photosynthetic pigment content
A direct relationship between temperature and the accumulation of photosynthetic pigment was not observed in S. polycystum, because no significant differences were detected in terms of the effects of temperature upon chlorophyll a (F = 2.52, P > 0.05), chlorophyll c (F = 6.59, P > 0.05), or carotenoid content (F = 5.47, P > 0.05). Furthermore, the content of each photosynthetic pigment did not change with increasing temperature (Fig. 2a).
The content of chlorophyll a (F = 6.48, P < 0.05) first increased and then decreased as salinity increased, peaking at 32 PSU (0.19 ± 0.026 mg/g; Fig. 2b). However, no significant effects were observed in terms of content of chlorophyll c (F = 0.90, P > 0.05) and carotenoids (F = 2.66, P > 0.05).
The contents of chlorophyll a and carotenoids exhibited similar response patterns in relation to changes in irradiance, which both decreased gradually with increasing irradiance and were significantly lower at higher levels of irradiance (120 and 200 μmol photons·m−2·s−1) compared with other irradiance levels (chlorophyll a: F = 28.13, P < 0.05; carotenoid: F = 12.36, P < 0.05). No significant difference was observed in terms of chlorophyll c content (F = 2.15, P > 0.05; Fig. 2c).
Effects of temperature, salinity, irradiance, and light quality upon chlorophyll fluorescence parameters
Because chlorophyll fluorescence parameters have been widely used to assess photosynthetic features and can be used as indicators of the physiological status of algae, we investigated the maximal quantum yield of F v/F m and NPQ in S. polycystum leaves after 0 and 1 h exposure to different treatments, respectively.
Compared with the 0 h treatment (control), F v/F m and NPQ decreased significantly at 10 °C after treatment for 1 h (P < 0.05). No significant differences were observed in either F v/F m or NPQ among the other temperature treatment groups (Fig. 3a and b). However, no significant difference (P > 0.05) was observed in F v/F m and NPQ values among each of the salinity treatments (Fig. 3c and d), although slight non-significant decreases and increases of F v/F m and NPQ were observed at a salinity of 16 PSU, respectively. In terms of irradiance treatment, the values of F v/F m at 120 and 200 μmol photons·m−2·s−1 significantly decreased by 10.1 and 24.2%, respectively (P < 0.05), compared to their controls (Fig. 3e and f). Under actinic light illumination (120 μmol photons·m−2·s−1), NPQ values significantly decreased (P < 0.05) as irradiance increased from 80 μmol photons·m−2·s−1.
Maximal photochemical yield (F v /F m ) and non-photochemical quenching (NPQ) of S. polycystum after exposure to different treatments for 0 and 1 h: (a) and (b) temperature, (c) and (d) salinity, (e) and (f) irradiance and light quality, respectively. Data are shown as means ± standard deviation (n = 3). Different letters indicate statistical significance (P < 0.05)
Discussion
Sargassum polycystum, a common species in tropical areas, has nutritional properties and capacity that allow rapid growth and make this an economically important seaweed for large-scale cultivation. However, until now, little was known about the physiological responses of S. polycystum to various environmental conditions. Understanding these mechanisms is essential to the development of aquaculture techniques. In order to explore suitable culture conditions, we investigated the effects of various environmental factors, including temperature, salinity, and irradiance upon RGR, photosynthetic pigment content, and chlorophyll fluorescence characteristics of S. polycystum.
Both length and weight are effective indicators for evaluating plant growth. In the study of growth characteristics in Sargassum species, Choi et al. (2008) reported that the RGRs of S. horneri for weight were greater than those for length, because the blades of S. horneri grew mainly in width and thickness. In this present study, we found that the length of some S. polycystum individuals did not increase as fast as weight. This was mainly due to the fact that several lateral branches grew more rapidly and also because the size and number of leaves increased significantly. Similar growth behavior was observed in the 3-month artificial culture experiment of S. polycystum conducted in the sea around Danzhou, which showed the RGRs for weight were also greater than those for length throughout the entire culture period (Zou et al. 2016). Thus, it is speculated that weight might be more sensitive than length in terms of reflecting the growth of several Sargassum species; consequently, we ultimately used weight to calculate the RGRs of S. polycystum in the present study.
Physical environmental factors, such as temperature, salinity, and light play important roles in the growth, reproduction, and distribution of seaweed (Lüning 1984; Lobban and Harrison 1994). Several studies have reported that these factors significantly affect the seasonal growth of Sargassum species (De Wreede 1976; Norton 1977, Díaz-Villa et al. 2005; Yeong and Wong 2013). Of these environmental factors, temperature is strongly correlated with biomass for tropical Sargassum species in both Hawaii (Glenn et al. 1990) and South Africa (Gillespie and Critchley 1999). Similarly, in the present study, we found that the RGR of S. polycystum was significantly affected by temperature, which first increased and then decreased as temperature rose, peaking at 23 °C. Despite the fact that intertidal macroalgae possess a relatively broad temperature tolerance and can adapt to changeable environmental conditions (Einav et al. 1995; Martone et al. 2010), extreme temperatures that are beyond the survival threshold can have a devastating effect upon plants and can disrupt cellular homeostasis and uncouple major physiological processes (Suzuki and Mittler 2006). Here, we found that the growth rate of S. polycystum decreased at temperatures above 25 °C, and the thallus suffered bleaching and decayed when the temperature was lower than 10 °C or higher than 30 °C. This suggests that these conditions might extend beyond the range of temperature tolerance for S. polycystum growth. The observation of temperature on the growth of S. polycystum in the present study was in agreement with their various seasonal growth patterns along the coast of Hainan Island, where S. polycystum start to grow rapidly from December to February (winter; monthly water temperature was 22.3 °C) in the southernmost region, but attained faster growth about 2 months later in the northern regions (spring; monthly water temperature rose to 22 °C until late March). Furthermore, populations of S. polycystum in the southern and northern regions both died back in the summer, mainly due to the high water temperature (above 30 °C) which was beyond their tolerable range.
However, the temperature for optimum growth, and tolerance limits, is not constant for S. polycystum populations across the world. Hwang et al. (2004) reported a similar optimum temperature (25 °C) for S. polycystum populations in Southern Taiwan and found that although the abundance of S. polycystum growth was negatively related to water temperature, the upper temperature limit could be increased from 25 to 30 °C in S. polycystum by nutrient enrichment. Algal blooms have occurred in the small South Pacific atoll nation of Tuvalu since 2011, which were dominated by the non-native species S. polycystum (N’Yeurt and Iese 2015), thus providing good evidence for the high adaptability of this species to environment changes (e.g., global warming and nutrient pollution of the seawater). Although the reasons for this sudden algal bloom remain unclear, high nutrient levels found in the bloom area might be related to the increased tolerance limit to the warmer water temperature conditions (30.3–31.7 °C).
On the other hand, extreme temperature can have large impacts on plant growth and development. It has been reported that extreme temperature stress may affect electron transport efficiency within the mitochondria and chloroplasts, resulting in energy production deficiencies followed by bleaching and rotting of the alga (Kobayashi et al. 2014). Although photosynthetic pigment content was not directly affected by temperature in the present study, F v/F m and NPQ decreased significantly at low temperatures. PSII is very sensitive to temperature stress, and under unfavorable temperature stress, the activity of PSII decreased rapidly (Berry and Bjorkman 1980). Our results showed no changes in F v/F m and NPQ at higher temperatures, but temperatures as low as 10 °C induced a significant reduction in these parameters. This indicates that S. polycystum is relatively sensitive to low-temperature stress and that the photo-reaction-system might be affected under such conditions. Low-temperature stress has been previously shown to have a significant negative effect on growth of higher plants and algae. Low temperature inhibits the repair of PSII in higher plants and algae by suppressing the synthesis and processing of several proteins (e.g., D1 protein) which are needed for the assembly of the active PSII complex (Kanervo et al. 1997; Allakhverdiev and Murata 2004; Murata et al. 2007). Thus, we speculate that damage incurred by the photosynthetic system may be a significant reason for the detrimental effects of low temperature on S. polycystum growth; further studies, however, will be required to reveal the specific mechanism(s) involved.
Salinity is another important factor that can influence the growth and distribution of marine algae, and marine algae growing in different regions exhibit different ranges of salinity tolerance (Kirst 1989; Karsten 2012). Being an intertidal macroalga, S. polycystum was expected to have a relative high salinity tolerance that ranged from 16 to 40 PSU (or even boarder) in the present study. Optimal growth salinities occurred in the range of 24–36 PSU, close to the mean salinity (33 PSU) of surface seawater that this species inhabits. Similar levels of salinity tolerance have been reported for other members of the genus Sargassum. For example, S. fulvellum can survive in 15–35 PSU (Dawes and Tomasko 1988), S. muticum germlings were able to survive in salinities as low as 5 PSU (Steen 2004), and S. thunbergii germlings exhibited a strong tolerance to fluctuating salinity (Chu et al. 2012). Collectively, these results indicate that most species within the genus Sargassum exhibit broad salinity tolerance.
In addition, the content of chlorophyll a initially increased but then decreased as salinity increased in our experiments, peaking at 28–36 PSU. This suggests that optimal salinity conditions can enhance the activity of enzymes involved in the synthesis of chlorophyll a. Short-term high- or low-salinity shock has been reported to significantly affect photosynthetic rates of S. thunbergii germlings after exposure to adverse salinity conditions for up to 6 h (Liang et al. 2013). However, in our study, we found no significant changes in F v/F m and NPQ values over all salinity conditions investigated. This implies that the salinity treatments in this study may be moderate stressors for S. polycystum, or that the short-term salinity shock (1 h treatment) may not be long enough to affect the PSII activity of S. polycystum. However, the slight decrease of F v/F m, and increase of NPQ, at low salinity (16 PSU) indicates that the negative effects of low salinity stress caused by photosynthetic activity might begin to emerge over time.
Light is an indispensable factor for the growth of algae and plants. Light had a notable effect on algal growth in this study. The growth rate and photosynthetic pigment content of S. polycystum declined as irradiance increased to 120 μmol photons·m−2·s−1. Furthermore, F v/F m and NPQ were also significantly affected, indicating that lower light levels are more amenable to the growth of S. polycystum, which coincides with the benthic habitat of this species. Like higher plants, algae also develop several photo-acclimation strategies to adapt to variable light conditions. However, little is known about how Sargassum species respond to changing conditions of irradiance until now. A recent study of the response of S. thunbergii germlings to different light intensities found that chronic photo-inhibition occurred under high light conditions and that germlings acclimated to oversaturating conditions probably by accumulating closed PSII reaction centers (Li et al. 2014). In the present study, we found that the chlorophyll a content of S. polycystum under relatively high light conditions (above 120 μmol photons·m−2·s−1) was significantly lower than that under lower light conditions (10–80 μmol photons·m−2·s−1). The highest chlorophyll a content was detected at the lowest irradiance condition tested (10 μmol photons·m−2·s−1). This suggests that Sargassum deploys a light adaptive strategy in that it changes its chlorophyll content to avoid photo-inhibition during times of excessive irradiance under high light conditions. It also suggests this species increases the amount of photosynthetic pigments in the thallus to improve the utilization ratio of light energy under low light environments. Similar changes in chlorophyll a content have been reported in other alga (Geider et al. 1997), suggesting that this might represent a general light acclimation mechanism which adjusts chlorophyll content under different light conditions. However, the nature of the mechanisms associated with pigment content, photosynthetic activity, and optimal growth remains uncertain and further studies are now required to clarify these issues.
In conclusion, this study investigated the effect of 20 levels of temperature, salinity, and irradiance on the growth of S. polycystum. To reduce these effects on different growth stages of S. polycystum samples, we used specimens which were collected at the same sampling time, and were thus of similar growth stages. However, limited by experimental conditions, we only conducted a short-term experiment under an indoor controlled environment. Our results showed that S. polycystum possessed a relatively broad tolerance to various environmental conditions, and survived under a wide range of temperature, salinity, and irradiance conditions. Optimal conditions were identified which maximized growth and thus, improved yields. The results of the short-term indoor experiment were also supported by our artificial cultivation experiment from March to June 2015 in the sea around Danzhou, China (Zou et al. 2016). This artificial cultivation experiment showed that S. polycystum grow fast from late March to late May, when the monthly mean sea surface temperature ranged from 21.1–23.3 °C, and the salinity of seawater ranged from 27 to 34 PSU in the experimental sea area; this was similar to the conditions of the present study under which we observed the maximum RGR of S. polycystum 23 °C and 32 PSU. Furthermore, we found that S. polycystum under artificial culture grew significantly better at a water depth under 80 cm (P < 0.05), where the irradiance was much lower than that at the sea surface. These results further indicated the low levels of light adaptation in this species.
The present study has provided essential information regarding the physiological characteristics of S. polycystum under various environmental conditions. The results are likely to be highly valuable in the development and management of aquacultural conditions for this economically important species of alga. However, only three environment parameters were tested in this laboratory experiment. Further studies should be carried out on other environmental parameters and the effects on seasonal changes, such as nutrition, pH, and day-length changes should also be taken into account, which can also exert an influence over the growth and yield of S. polycystum.
References
Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage–repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1657:23–32
Ang PO (2006) Phenology of Sargassum spp. in Tung Ping Chau Marine Park, Hong Kong SAR, China. J Appl Phycol 18:629–636
Anggadiredja J, Andyani R, Hayati M (1997) Antioxidant activity of Sargassum polycystum (Phaeophyta) and Laurencia obtusa (Rhodophyta) from Seribu Islands. J Appl Phycol 9:477–479
Baker NR (1991) A possible role for photosystem II in environmental perturbations of photosynthesis. Physiol Plant 81:563–570
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Calumpong HP, Maypa AP, Magbanua M (1999) Population and alginate yield and quality assessment of four Sargassum species in Negros Island, central Philippines. Hydrobiologia 398:211–215
Chiang Y-M, Yoshida T, Ajisaka T, Trono JG, Tseng CK, Lu B (1992) Distribution and variation in Sargassum polycystum C.A. Agardh (Fucales, Phaeophyta). In: Abbott IA (ed) Taxonomy of economic seaweeds with reference to some Pacific and Western Atlantic species, vol 3. California Sea Grant College, La Jolla, pp 35–42
Choi HG, Lee KH, Yoo HI, Kang PJ, Kim YS, Nam KW (2008) Physiological differences in the growth of Sargassum horneri between the germling and adult stages. J Appl Phycol 20:729–735
Chu SH, Zhang QS, Tang YZ, Zhang SB, Lu ZC, Yu YQ (2012) High tolerance to fluctuating salinity allows Sargassum thunbergii germlings to survive and grow in artificial habitat of full immersion in intertidal zone. J Exp Mar Biol Ecol 412:66–71
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prásil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 1–17
Dawes CJ, Tomasko DA (1988) Physiological responses of perennial bases of Sargassum filipendula from three sites on the west coast of Florida. Bull Mar Sci 42:166–173
De Wreede RE (1976) The phenology of three species of Sargassum (Sargassaceae, Phaeophyta) in Hawaii. Phycologia 15:175–183
Díaz-Villa T, Sansón M, Afonso-Carrillo J (2005) Seasonal variations in growth and reproduction of Sargassum orotavicum (Fucales, Phaeophyceae) from the Canary Islands. Bot Mar 48:18–29
Einav R, Breckle S, Beer S (1995) Ecophysiological adaptation strategies of some intertidal marine macroalgae of the Israeli Mediterranean coast. Mar Ecol Prog Ser 125:219–228
Geider RJ, Macintyre HL, Kana TM (1997) Dynamic model of phytoplankton growth and acclimation: responses of the balanced growth rate and the chlorophyll a:carbon ratio to light, nutrient-limitation and temperature. Mar Ecol Prog Ser 148:187–200
Gillespie RD, Critchley AT (1999) Phenology of Sargassum spp. (Sargassaceae, Phaeophyta) from Reunion Rocks, KwaZulu-Natal, South Africa. Hydrobiologia 398/399:201–210
Glenn EP, Smith CM, Doty MS (1990) Influence of antecedent water temperatures on standing crop of a Sargassum spp.-dominated reef flat in Hawaii. Mar Biol 105:323–328
Guiry MD, Guiry GM (2017) AlgaeBase. World-wide electronic publication. http://www.algaebase.org. Accessed Searched on 28 Feb 2017
Hoffmann WA, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot 90:37–42
Hong DD, Hien M-H, Son PN (2007) Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J Appl Phycol 19:817–826
Hurt R (1982) Plant growth curves. The functional approach to plant growth analysis. Cambridge University Press, Cambridge
Hwang R-L, Tsai C-C, Lee T-M (2004) Assessment of temperature and nutrient limitation on seasonal dynamics among species of Sargassum from a coral reef in southern Taiwan. J Phycol 40:463–473
Kanervo E, Tasaka Y, Murata N, Aro EM (1997) Membrane lipid unsaturation modulates processing of the photosystem II reaction-center protein D1 at low temperatures. Plant Physiol 114:841–849
Karsten U (2012) Seaweed acclimation to salinity and desiccation stress. In: Wiencke C, Bischof K (eds) Seaweed biology: ecological studies (analysis and synthesis). Springer, Berlin, pp 87–107
Kirst GO (1989) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol Plant Mol Biol 40:21–53
Kobayashi Y et al (2014) Algae sense exact temperatures: small heat shock proteins are expressed at the survival threshold temperature in Cyanidioschyzon merolae and Chlamydomonas reinhardtii. Genome Biol Evol 6:2731–2740
Komatsu T et al (2007) Distribution of drifting seaweeds in eastern East China Sea. J Mar Syst 67:245–252
Li X, Zhang Q, He J, Yu Y, Liu H (2014) Photoacclimation characteristics of Sargassum thunbergii germlings under different light intensities. J Appl Phycol 26:2151–2158
Liang Z, Sun X, Wang F, Wang W, Liu F (2013) Impact of environmental factors on the photosynthesis and respiration of young seedlings of Sargassum thunbergii (Sargassaceae, Phaeophyta). Am J Plant Sci 04:27–33
Lobban CS, Harrison PJ (1994) Seaweed ecology and physiology. Cambridge University Press, Cambridge
Lüning K (1984) Temperature tolerance and biogeography of seaweeds: the marine algal flora of Helgoland (North Sea) as an example. Helgol Mar Res 38:305–317
Martone PT, Alyono M, Stites S (2010) Bleaching of an intertidal coralline alga: untangling the effects of light, temperature, and desiccation. Mar Ecol Prog Ser 416:57–67
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence- a practical guide. J Exp Bot 51:659–668
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421
N’Yeurt DRA, Iese V (2015) The proliferating brown alga Sargassum polycystum in Tuvalu, South Pacific: assessment of the bloom and applications to local agriculture and sustainable energy. J Appl Phycol 27:2037–2045
Nagappan H, Pee PP, Kee SHY, Ow JT, Yan SW, Chew LY, Kong KW (2017) Malaysian brown seaweeds Sargassum siliquosum and Sargassum polycystum: low density lipoprotein (LDL) oxidation, angiotensin converting enzyme (ACE), α-amylase, and α-glucosidase inhibition activities. Food Res Int. https://doi.org/10.1016/j.foodres.2017.01.023
Norton TA (1977) Ecological experiments with Sargassum muticum. J Mar Biol Assoc UK 57:33–43
Phillips N, Fredericq S (2000) Biogeographic and phylogenetic investigations of the pan-tropical genus Sargassum (Fucales, Phaeophyceae) with respect to Gulf of Mexico species. Gulf of Mexico Science 2000:77–87
Saraswathi SJ, Babu B, Rengasamy R (2003) Seasonal studies on the alginate and its biochemical composition I: Sargassum polycystum (Fucales), Phaeophyceae. Phycol Res 51:240–243
Steen H (2004) Effects of reduced salinity on reproduction and germling development in Sargassum muticum (Phaeophyceae, Fucales). Eur J Phycol 39:293–299
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51
Thangaraju N, Venkatalakshmi RP, Chinnasamy A, Kannaiyan P (2012) Synthesis of silver nanoparticles and the antibacterial and anticancer activities of the crude extract of Sargassum polycystum C. Agardh. Nano Biomedicine Eng 4:89–94
Trono G Jr (1999) Diversity of the seaweed flora of the Philippines and its utilization. Hydrobiologia 398/399:1–6
Yeong BM-L, Wong C-L (2013) Seasonal growth rate of Sargassum species at Teluk Kemang, Port Dickson, Malaysia. J Appl Phycol 25:805–814
Yu Z, Zhu X, Jiang Y, Luo P, Hu C (2014) Bioremediation and fodder potentials of two Sargassum spp. in coastal waters of Shenzhen, South China. Mar Poll Bull 85:797–802
Zou X, Lin Y, Zhu J, Huang H, Bao S (2016) Study on the artificial cultivation technology of Sargassum polycystum. Fishery Modernization 43:29–33 (in Chinese with English abstract)
Funding information
This work was supported by the Ocean Public Welfare Scientific Research Project (No. 2014050402), Natural Science Foundation of Hainan Province, China (No. 20163119, 317261), Grants from National Project of Marine economy innovation development area demonstration (12PYY001SF08-ZGRKY-1), and Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 1630052016011, No. 1630012017009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, XX., Xing, SS., Su, X. et al. The effects of temperature, salinity and irradiance upon the growth of Sargassum polycystum C. Agardh (Phaeophyceae). J Appl Phycol 30, 1207–1215 (2018). https://doi.org/10.1007/s10811-017-1282-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1282-4