Abstract
This study was conducted to evaluate dietary inclusion effects of Arthrospira (Spirulina) platensis (SP) meal on the growth, survival, biochemical composition, and particularly reproductive performance during eight successive spawning events in the caridean red cherry shrimp, Neocaridina davidi, for 11 months. Juveniles were fed with six diets containing 0 (control), 1, 3, 5, 8, and 10% of SP levels. Most growth indicators in terms of final weight, specific growth rate, and average daily growth rate were significantly higher in the shrimp fed with SP10. The highest survival of females (75–81%) was recorded in shrimp fed SP8–10, being different from the other groups (25–73%). No changes were observed in lipid and moisture contents, and protein content showed an increasing trend with dietary Spirulina levels up to 10%. Absolute fecundity (eggs per female) was significantly affected in order of the highest to lowest viz. 45, 39, 30, 28, 27, and 24 with different incorporations. The relative fecundity (eggs per female’s body weight) of shrimp fed SP8–10 showed differences with the other groups. Except for the control, all Spirulina-fed females of SP1–5 showed hatching percentages of 78–84% compared with those of 91–94% for SP8–10. Relative fecundity in females fed SP8–10 showed differences from the fourth to the sixth spawning events compared with the other groups; this trend decreased non-significantly until the eighth spawning. The results obtained suggest that dietary inclusion of S. platensis meal at a level of 8–10% can improve the growth, survival and, in particular, reproductive performance of red cherry shrimp in commercial aquaria during maturity only up to the sixth spawning event.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of numerous aquaculture species has increased considerably since 2000, with crustaceans representing 10% of the total production in 2012 (FAO 2014). The values of international trade of ornamental production have been estimated to worth around US$717 million in 2009, increasing at an average growth rate of approximately 14%/year (FAO 2014).
In recent years, ornamental shrimp have drawn attention due to color variations, relatively a high price, easy maintenance, high adaptability to water quality parameters, high appetite to feed on algal diets, detritus, and micro-organisms on the substrate, and also on other remains in aquaria. The atyid red cherry shrimp (RCS) (Neocaridina davidi, Bouvier, 1904), formerly known as Neocaridina heteropoda, belongs to the sub-order Caridina (family Atyidae), has undoubtedly been the most popular freshwater shrimp in the hobby aquarium industry since 2003 as well as a model shrimp in ecotoxicological and reproduction investigations (Heerbrandt and Lin 2006; Tropea and Lopez Greco 2015). The genus Neocaridina inhabits various types of inland water bodies (lakes, reserviors, ponds, and rivers) being distributed throughout Russia, China, Japan, Korea, Taiwan, and Vietnam (Cai 1996; His and Yixiong 2007). Full-grown adults of cherry shrimp in aquaria with pH and temperature levels of 6.5–8 and 14–29 °C, respectively, can reach maturity in 2–2.5 months, and approximate total lengths of 1.5–3.5 cm (up to 4 cm) have been reported for ages of 1–2 years (Heerbrandt and Lin 2006). The common practice of commercial RCS shrimp breeding under captive conditions is that the domesticated broodstocks are propagated for seed production. After the first spawning, it typically takes 30 to 45 days for the females to re-develop their ovaries in preparation for a second spawn. However, with substantial increases in the larval supply for RCS shrimp sales in the local markets of Iran, there are increasing interests in re-maturing the broodstocks to produce several consecutive spawning for seed production.
Future improvements concerning maturation diet formulation may allow the broodstock to achieve a high reproductive performance for longer periods. In captivity, reproductive brooders are totally dependent on the nutrients provided by maturation diets to fulfill the high nutritional requirements related to reproduction, in which any dietary deficiencies or imbalances are reflected in lower reproductive performances. On the other hand, the size of females quantitatively affects larval production; however, shrimp size and age are closely related and senescence occurring in larger (and older) shrimp may negatively influence reproductive indices and offspring quality (Rothlisberg 1998; Coman and Crocos 2003; Tropea et al. 2012).
Spirulina platensis (currently recognized name Arthrospira platensis), a multicellular filamentous cyanobacterium (Cyanobacteria; family Phormidiaceae), has received a great attention for the use in aquaculture feeds (Spolaore et al. 2006; Wells et al. 2017). Spirulina biomass contains high quantities of proteins (up to 55–70% of dry weight), lipids (6–11%), and good amounts of essential fatty acids (e.g., gamma-linolenic acid), antioxidant pigments (carotenoids), vitamins (especially vitamin B12 and pro-vitamin A; β-carotene), minerals (especially iron), and compounds that promote the attractability and palatability of the diets (Belay et al. 1996; Habib et al. 2008). A number of feeds (additives or supplements) have been utilized in the shrimp farming industry, but Spirulina is one of the best microalgal additives demonstrated to be benefitial to growers offseting the initial cost and providing a significant cost/performance resulting from dietary inclusion levels ranging from 5 to 20% (Habib et al. 2008; Wells et al. 2017). Several studies have been conducted using microalgae and/or dried Spirulina as a complementary dietary ingredient for the white shrimp Litopenaeus schmitti (Jaime-Ceballos et al. 2005), Pacific white shrimp Litopenaeus vannamei (Hanel et al. 2007; Macias-Sancho et al. 2014), and several fish species including tilapia, carp, and sturgeon (Olvera-Novoa et al. 1998; Nandeesha et al. 2001; Lu and Takeuchi 2004; Palmegiano et al. 2005). Dietary incorporation of many microalgal species for the growers and particularly broodstocks of shrimp is still at early research stages (Ju et al. 2009; Tank et al. 2010); only few studies have examined shrimp with respect to feed additives (Cuzon et al. 1981; Nakagawa and Gomez-Diaz 1995; Jaime-Ceballos et al. 2005; Hemaiswarya et al. 2010), feed attractants (Silva-Neto et al. 2012), and/or partial or total replacements of feed protein sources (Tank et al. 2010; Macias-Sancho et al. 2014; Abdul Basri et al. 2015).
During the last three decades, most researches have focused on the improvement of the quality and quantity of different nutrients through broodstock diets on the stimulation of sexual maturation, increased fecundity, egg quality, and larval survival as well as developments in aquatic vertebrates and invertebrates (Harrison 1990; Wouters et al. 2001; Calado et al. 2009; Wu et al. 2010). To date, most investigations conducted on ornamental RCS shrimps have addressed the physiology of digestive traits (Wang et al. 2010), the effects of temperature on biochemical composition, growth, and reproduction (Tropea et al. 2015), female growth and offspring quality over successive spawning events (Tropea and Lopez Greco 2015), morphology of the initial post-hatching larval stages (Pantaleao et al. 2017), and low-cost biofilm-based methods with zero water exchange for sustainable culture (Viau et al. 2016). Accordingly, studies aiming at the improvements of the growth and particularly reproductive performance of RCS shrimp are scarce. Furthermore, there are no reports or data on the influences of dietary Spirulina meal on the capabilities of re-matured RCS shrimp broodstock to allow reliable evaluation of reproductive performance and offspring production within several successive spawnings in captivity. Thus, the main objective of the present study was to evaluate the effects of different dietary incorporation levels of S. platensis meal on the growth, survival, body composition, and particularly, female’s reproductive indices in the red cherry shrimp by the end of the eight consecutive spawnings for 11 months.
Materials and methods
Experimental diets
Spirulina platensis (SP) meal was obtained from SINA Microalgae Company (Qeshm Island, Hormozgan Province, Iran). The proximate compositions of dry matter, crude protein, and lipid in S. platensis meal used were, respectively, 92, 58, and 8.2%.
Six iso-nitrogenous (46.4% crude protein), iso-lipidic (9.4% crude lipid), and iso-energetic (17.2 kJ g−1) practical diets were formulated in the laboratory. A diet without algal meal inclusion was served as a control group, and five experimental diets containing S. platensis meal were formulated to contain 1, 3, 5, 8, and 10% of the meal (designated as SP1, SP3, SP5, SP8, and SP10, respectively). Dietary S. platensis meal was supplemented at different percentages, and the chemical compositions of diets were re-adjusted mainly with fishmeal, soybean meal, and other ingredients. The dry ingredients were first sifted through sieve (4 mm mesh size) and mixed using a Hobart feed mixer (Hobart Grinder, Model MG 4366) for approximately 30 min to ensure that the mixture was well homogenized. Then, water was added (20–30%, V/W) to give a pelletable mixture. Diets were prepared as pellets with appropriate diameters of 3 mm and dried in a drying oven (Fan Azma Gostar, Model BM 55) at 55 °C for 24 h. The diets were pelletted every 3 weeks, kept in separate, tightly packed plastic bags, and finally stored at −20 °C until use (Table 1).
Experimental RCS shrimp, culture conditions, feeding trials, and sex determination
The experiments were conducted in the experimental facilities of the Hamoon Ornamental Fish and Crustacean Culture Center (Marlik Town, Tehran, Iran) for a period of 11 months during 2015–2016. RCS juveniles (n = 400, mean initial weight of 0.45 ± 0.02 mg, 35 days old) were obtained from a local ornamental hatchery center (Hamoon Ornamental Center, Iran). Before the start of the experiments, juveniles were adapted to the experimental conditions in six 80 L aquaria for 10 days and fed (ad libitum) once per day with a commercial diet (CP 9910) containing 30% crude protein and 4% crude lipid. The aquaria were aerated continuously in a closed system. Then, the juveniles were randomly distributed in 18 rectangular glass aquaria (50 cm × 40 cm × 40 cm; 80 L), each stocked with 22 juveniles. Mild aeration was provided to each aquarium through an air stone connected to a central air compressor in a closed system. Juveniles were reared under 12 h light:12 h dark photoperiod throughout the feeding trials and hand-fed three times a day (09:00, 13:00, and 17:00 hours) with the experimental diets in six inclusion levels of S. platensis meal based on 3% of wet body weight. During the experimental period, water temperature, pH, dissolved oxygen, ammonia, and hardness were monitored and ranged from 26 to 28 °C, 7 to 7.2, 5.8 to 6.1 mg L−1, <0.2 mg L−1, and 85 to 90 mg L−1, respectively. The experimental aquaria were cleaned once every 3 days by siphoning out fecal pellets and other residual feeds, and water was replaced completely once every 10 days.
Before the first stage of breeding, morphological differences of the male and female brooders were determined on the basis of size, coloration, and saddle situation (Cai 1996; Pantaleao et al. 2015). Females are larger than the males, have a much darker red coloration, and the lower section of the abdomen has a curved shape. The males are smaller, have very little red coloration, and there is a straight line with no curved shape at the lower section of the abdomen. Females have a “saddle,” which is a common name for the eggs still in the ovaries, located behind the head on the top of the bottom.
Afterwards, the brooders were first distinguished on the basis of the morphological characteristics and then divided into six treatments (three replicates per treatment) in 18 experimental aquaria (each with a volume of 30 L; 25 cm × 12 cm × 10 cm) with ten females per aquarium reared for the subsequent experiments up to 10 months. All females at different treatments were fed daily three times at 09:00, 13:00, and 17:00 hours at a ration of 3% total wet biomass. Prior to daily feeding, feces and uneaten feed were removed by siphoning. Due to the high sensitivity of the females in keeping eggs, the stocks were exposed to minimum stressful conditions during all stages of feeding, manipulation, and breeding. During the experiments, survival of the females was recorded every day in the morning. Over the experimental period, all aquaria were gently aerated and factors such as water quality and environmental conditions for culture were maintained as in the previous phase. The water quality parameters were within the suitable ranges for N. davidi broodstocks farming.
At the end of each experimental stage, shrimps were fasted for 24 h prior to sampling. Every 30 days, brooders (nine shrimp from each treatment) were randomly removed from the respective aquaria and after removing excess water with paper tissues, mean wet body weight was measured individually by a precision balance (to the nearest 0.001 g), and total length was determined with a digital caliper (to the nearest 0.01 mm). Accordingly, the mean final weight of females in each spawning event fed with the diets containing different inclusion levels of S. platensis meal were measured during eight consecutive spawnings over the experimental period.
Evaluation of growth performance
The growth indices of brooder females were calculated according to data obtained in the last spawning event as follows:
where W f and W i are mean final and initial weights (mg), L f and L i are mean final and initial lengths (mm), N f and N i are final and initial numbers, and T is the number of days for feeding trials (Hardy and Barrows 2002).
Reproductive performance, hatching percentage, and spawning intervals
The specificaitons of first reproduction time in shrimp were calculated at different treatments according to a level of 50% population maturation in each replicate (aquarium). In order to mate, three males (already fed control diet) were randomly selected and introduced to each experimental aquarium containing females for 3 days. Immediately after egg laying, the brooders were taken out of the aquarium and the attached eggs were carfully separated among the swimming legs by holders.
To determine absolute fecundity, total cumulative numbers of eggs from healthy females were taken from each aquarium and mean data calculated for the total egg production per female. Relative fecundity was calculated according to the total egg production per female per mean wet body weight of each female. The whole eggs obtained from each treatment were hatched in vertical incubators and the hatchability percentage (%) were determined in vitro at 28 ± 1.0 °C based on the total number of hatched larvae (juveniles)/total number of fertilized eggs × 100. Actual fecundity was calculated by the total number of hatched juveniles within the incubation period. Moderate aeration provided oxygenation and ensured that the eggs would not settle in incubators. The whole water was replaced daily in each incubator and when hatching started, newly hatched larvae were recorded every 12 h when all newly hatched larvae were collected from the incubators. The above procedures continued until all eggs hatched out or were finally confirmed to be dead.
After reproduction in each step, all the brooders were immediately placed in the relevant aquaria and nutritional trials continued in the different treatments until the next mating event. The frequency and feeding schedules of females and water quality conditions in each aquarium were completely identical in all treatments. The time required between two successive spawnings (spawning intervals) was determined according to the shrimp’s life cycle by introducing males to each aquarium followed by mating and the next egg laying step. To evaluate the effects of feeding with S. platensis meal, all brooders reproduced in eight consecutive steps for 10 months at different treatments. All the trials were conducted during 11 months.
Shrimp’s body biochemical composition
To determine the moisture, protein, lipid, and ash contents of whole body biochemical analysis of shrimp, samples were randomly obtained from each treatment and prepared according to AOAC (2002) and then stored at −20 °C. Before the sampling, the shrimp were starved for 24 h. Moisture was determined by oven drying at 105 °C for 24 h. Crude protein (N × 6.25) was measured by the Kjeldahl method after acid digestion using an auto-Kjeldahl System (1030-Auto-analyzer, Tecator, Sweden). Crude lipid was determined by the ether-extraction method using Soxtec System HT (Soxtec System HT6, Tecator, Sweden). Ash content was estimated using a muffle furnace at 550 °C for 24 h.
Statistical analyses
Data were first subjected to arcsine transformation when they did not follow a normal distribution. Mean values (means ± SD) for growth, survival, and reproductive performances were analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests to verify significant differences among the treatments at a level of P < 0.05. Changes among successive spawning events were assessed by an ANOVA design with repeated measures test. All the statistical analyses were carried out using SPSS software version 22.
Results
Growth performance
The growth indicators of RCS shrimp fed with different inclusion levels of Spirulina meal showed significant differences (P < 0.05) between the treatments during the feeding trials (11 months). Higher levels (5–10% SP) of algal meal inclusion in RCS shrimp diets resulted in significant enhancements in most growth parameters during the experimental period. Final weight was significantly (P < 0.05) higher in shrimps fed 10% SP compared with other treatments showing a weight gain of 1.6 times the control group. The highest final length (26.3–27.8 mm) was recorded in shrimps fed 5–10% dietary SP in comparision with the other treatments (25.3–25.7 mm) (P < 0.05). Length increments of females showed increasing trends by the inclusion of 1–10% of SP meal, though no differences (P > 0.05) were obtained in final length between SP inclusion levels of 5 and 10%. RCS shrimps fed with 3, 1, and control groups of Spirulina (%) in the diets had significantly (P < 0.05) greater FCRs (3–3.1) than those fed diets with 10, 8, and 5% of SP (FCR; 2.66–2.79, respectively). SGR values were highest (P < 0.05) in shrimps fed 10% SP whereas there were no significant differences (P > 0.05) in shrimp fed with 3–8% SP (Table 2).
Reproductive indices and survival rate of females
The reproductive parameters of RCS shrimp up to first spawning showed that the brooders with final weights of 64–66 mg reached the first sexual maturity within 11.7–15.7 days after initial stocking at different treatments. The time required for the maturation of shrimp fed different incorporation levels showed significant differences (P < 0.05) between treatments. On the other hand, Spirulina-fed groups with higher inclusion levels (5–10%) displayed accelerated sexual maturity. The time interval (days) between two successive egg productions (inter-spawning intervals) showed a decreasing trend with dietary Spirulina levels up to 10% (Table 3).
Mean absolute fecundity (eggs per female) varied (P < 0.05) among treatments resulting from the incorporation levels being highest in the order of SP10 (45), SP8 (39), and SP5 (30) followed by SP3 (28), SP1 (27), and the control (24). Significant (P < 0.05) declines in relative fecundities (eggs per gram female’s wet body weight) were recorded in the control up to SP5 (35–39) group compared with those observed in the other treatments (SP8 and SP10, 49–52 eggs/g female wet body weight, respectively). Except the control group, all Spirulina-fed shrimps in levels of 1–5% had comparable (P < 0.05) hatching percentages (78–84%) compared with those in SP8 (91%) and SP10 (94%). The resultant larval length in brooders fed 10% Spirulina was significantly (P < 0.05) larger compared with the other groups, and no significant differences (P > 0.05) were detected between the control and the other treatments received dietary Spirulina levels of 1–8%. The survival rate of female shrimps tended to elevate with increasing dietary Spirulina levels up to 10% with the higher rates (75–81%) in shrimps fed diets containing 8–10% Spirulina, which were significantly different (P < 0.05) from those found in the other algal-fed groups (40–73%). Shrimps fed with the control diet showed the lowest survival value (25.7%) after 11 months (Table 3).
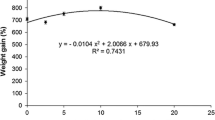
The mean final weight of RCS broodstocks showed differences (P < 0.05) in each spawning event with different S. platensis inclusion levels. Apart from the control, all Spirulina-fed groups revealed no significant differences (P > 0.05) in final body weight up to the 5th stage of spawning. However, the broodstocks fed 8–10% Spirulina were significantly different from other treatments in 6–7th spawning event being more noticeable in the 8th spawning step in shrimp fed SP10 (P < 0.05) compared with other treatments. The average weight of the females from the first to the eighth spawning events varied between 66 and 70, 66 and 79, 66 and 87, 65 and 91, 65 and 98, and 66 and 113 mg in the control, 1, 3, 5, 8, and 10% SP, respectively. Consequently, lower weight gain was found in the control group compared with the other treatments fed Spirulina (Fig. 1).
Mean final weight of red cherry shrimp (Neocaridina davidi) females (n = 3, mean ± SD) fed with diets containing different inclusion levels of Spirulina platensis meal in each spawning event (eight consecutive spawning) during experimental periods. Values with the same superscript are not significantly different (P > 0.05)
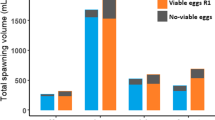
The absolute and relative fecundities of algal-fed females showed differences (P < 0.05) in all stages of the spawning events at different S. platensis inclusion levels. The spawners fed with 10% Spirulina had noticably higher absolute fecundity in all steps of spawning. However, the shrimp fed 8 and 10% Spirulina showed no significant differences with each other (P > 0.05); both groups showed significant differences (P < 0.05) with the other groups in the seventh and eighth steps of spawning (Fig. 2).
Mean egg production per female (absolute fecundity) of red cherry shrimp (Neocaridina davidi) broodstocks (n = 3, mean ± SD) in each spawning event (eight successive step) fed with diets containing different levels of Spirulina platensis meal. Values with the same superscript are not significantly different (P > 0.05)
The relative fecundity in the brooders fed with 8–10% algal diet showed relatively significant differences (P < 0.05) compared with the other groups from the fourth to the sixth events of the spawning. However, the relative fecundity in broodstock fed 5–10% Spirulina were not different from each other (P > 0.05), all three groups revealed differences (P < 0.05) with the other groups during the 7th–8th spawning steps (Figs. 3 and 4).
Mean egg production per wet body weight of female (relative fecundity) of red cherry shrimp (Neocaridina davidi) (n = 3, mean ± SD) in each of the spawning events (eight successive step) fed with diets containing different incorporation levels of Spirulina platensis meal. Values with the same superscript are not significantly different (P > 0.05)
Body proximate composition
Lipid (0.45–0.83%) and moisture (70.74–73.87%) contents of whole shrimp body indicated no changes among all treatments, and protein content showed an increasing trend with dietary Spirulina levels up to 10%. Except the control group, the protein contents (20.8–25.34%) were not affected by the different dietary Spirulina levels. The lowest ash content (1.40–1.62%) was recorded in SP10 treatment (Table 4).
Discussion
Unlike most previous studies, this is the first investigation that evaluated the influence of dietary incorporation of S. platensis meal on the reproductive performance in red cherry shrimp (N. davidi), as a model of ornamental shrimps, over multiple spawnings. According to the results, the algal diet containing 10% Spirulina yielded better performances in terms of growth indices, final weight, and length of shrimp with statistical differences with other treatments. Most of the growth characteristics displayed increasing trends by the algal meal inclusions from 1 to 10% during feeding trials so that all the diets containing Spirulina (even 1% SP) led to relatively better weight gains compared with the control. These results suggest that S. platensis meal contains high levels of protein that might have a promising impact on growth indices of RCS shrimp (WG, LG, and SGR) compared with the group fed an algal-free diet. Our findings in N. davidi are in agreement with the results of Cuzon et al. (1981), who incorporated 8% of lipid free fraction of Spirulina meal and observed promoted growth and survival in Penaeus japonicus. In another study, Nakagawa and Gomez-Diaz (1995) found significant improvements in growth, survival, pigmentation, and protein utilization of giant freshwater shrimp (Macrobrachium rosenbergii) fed 5–10% inclusion of whole Spirulina meal diets and attributed the enhancements to protein assimilation promotion. Also, enhanced growth shrimp (L. vannamei) was reported by the inclusion of 9% whole microalgae Thalassiosira weissflogii or Nannochloropsis meals (Ju et al. 2009). In a recent study, Abdul Basri et al. (2015) reoported that a meal derived from ″green water cultures” proved to be a good source of carotenoids and was considered as an acceptable alternative protein source for the shrimp, L. vannamei, with a dietary inclusion level of about 10% with no significant negative effects on survival, FCR, and SGR.
The beneficial effects of micro- and macroalgae inclusions (in a range of 0.05–20%) in the diets of fish and shrimp species have previously been declared in terms of enhanced growth, nutritional performance, health, and coloration (Mustafa and Nakagawa 1995; Habib et al. 2008; Güroy et al. 2012; Silva-Neto et al. 2012; Teimouri et al. 2013; Zhu et al. 2015). A tendency toward better growth performance was detected at 5, 8, and 10% Spirulina inclusion levels in the current study, which suggests that the microalgal incorporation might have improved food absorption efficiency by increasing bacterial colonization in the digestive tract as well as through the decomposition of indigestible feed components to extractable nutrients in intestinal flora (James et al. 2006) or as a result of promoted diet attractiveness (Silva-Neto et al. 2012). Previous researchers proposed that bioactive compounds (such as growth hormones, nucleotides, vitamins, and minerals, free amino acids and fatty acids, pigments, and compounds inducing gene expression) in the diets containing Spirulina might constitute effective agents to enhance the attractability of the feed (Distel et al. 1992; Fafournoux et al. 2000; Brown 2002; Clarke et al. 2002; Jaime-Ceballos et al. 2005; Spolaore et al. 2006). For example, Jaime-Ceballos et al. (2005) determined that S. platensis included at 5% in the diet of L. schimitti improved the attractability of the feed owing to bioactive compounds such as nucleotides, amino acids and/or pigments. The researchers found that Spirulina has approximately 8.7% of glutamic acid, 6.6% of aspartic acid, and 5% of leucine; hence, the higher growth performance in shrimps fed diets containing Spirulina might be attributed due to the high contents of glutamic acid as a promoter of feeding effector and/or a chemoattraction agent for penaeid shrimp. Ju et al. (2012) reported that a low inclusion (3% of defatted microalgal meal (DDM), Haematococcus pluvialis) significantly enhanced the growth of Pacific white shrimp resulting from some bioactive compounds in DDM or from some health benefits of DMM. Although the fatty acid and amino acid profiles of diets were not analyzed in this study, the outcomes of growth performance demonstrated significant effects of the diet containing the highest Spirulina level on the final body weights during all spawning events.
In the present study, the average survival rate of RCS broodstocks was highly influenced by the inclusion levels of S. platensis, showing positive supplementary effects of the diets formulated with microalgal biomass on considerable health enhancement. The survival rates of RCS shrimps in all groups fed with diets containing Spirulina were remarkably higher (1.5–3.2 times) than the control throughout the experimental rearing period. Significant improvement in immunological factors was found in Pacific white shrimp when fishmeal was replaced with S. platensis meal, indicating that microalgae may stimulate and/or enhance the immune system of shrimp (Macias-Sancho et al. 2014; Wells et al. 2017). Higher FCR values were observed in shrimps fed with either the control or lower inclusion diets; however, diets containing 5–10% SP showed more satisfactory results in terms of FCR. Our findings are in agreement with Silva-Neto et al. (2012), who conducted a study with S. platensis as an attractant and observed better FCRs in the shrimp studied. They concluded that this microalga had the ability to stimulate food intake even at low inclusion levels. Such observations have been reported in fish, Zhu et al. (2015), for instance, examined white spotted snapper Lutjanus stellatus juveniles with 5% inclusion level of Ulva lactuca, which revealed better performances in terms of WG, SGR, and FCR than those in other (10–20%) inclusions.
The protein contents of shrimps fed S. platensis in the current experiment increased parallel to increasing dietary inclusion levels. The palatability of Spirulina meal in the diets may improve feed consumption, which in turn directly increases the body carcass composition (Habib et al. 2008; Radhakrishnan et al. 2016). However, the replacement of fish meal with H. pluvialis meal in the diet of Pacific white shrimp did not affect the whole body proximate composition (Ju et al. 2012). In the present study, the lipid content decreased in shrimps fed on diets containing increasing SP levels. This result is in accordance with that of Radhakrishnan et al. (2016) in M. rosenbergii fed on diets having higher levels of fishmeal replacement (75 and 100%) by A. platensis meal.
Reproductive performance indices
To date, studies aiming at supplementing shrimp diets with Spirulina meal have been restricted to growth, survival, body pigmentation, and flesh quality. Few researches have also investigated the influence of dietary algal carotenoids on the reproductive performance of shrimp (Wyban et al. 1997; Regunathan and Wesley 2006). Positive effects of Spirulina supplementation on reproductive indices have been only reported for four fish species including swordtail, cichlid, three-spot gourami (ornamental fish), and tilapia (commercial fish) (Lu and Takeuchi 2004; James et al. 2006; Güroy et al. 2012; Khanzadeh et al. 2016; Karadal et al. 2016). Since the RCS juveniles were not fed with the experimental diets during the first 45 days of the culture period, the first maturity cannot be definitively attributed to the impacts of diets containing Spirulina. However, shrimps fed diets containing Spirulina levels of 5–10% experienced a significantly early maturity.
Among the treatments, SP10 noticeably increased total egg production and absolute fecundity of females as 1.8 times the control. Additionally, the females fed the algal diets (8 and 10% SP) exhibited higher values of relative fecundities and hatching percentages in comparison with the other groups. These results clearly signify the positive effects of higher inclusion levels of algal diets on the reproductive performance of RCS females. Similarly, Millamena and Quinitio (2000) reported that provision of a formulated diet in combination with natural food resulted in improved consistency in the reproductive performance of mud crab (Scylla serrata). Similar results were presented with significantly higher fecundity in the female swordtail, Xiphophorus helleri, fed 8% of Spirulina meal compared with other groups (5, 3, 1, and 0% algal meal) (James et al. 2006). In a recent study on blue gourami (Trichopodus trichopterus), Khanzadeh et al. (2016) have found highly increased total egg production and relative fecundity in females fed diets containing 8 to 9.5% of S. platensis meal. In addition, Regunathan and Wesley (2006) reported that a dietary Spirulina inclusion of 3% in a maturation diet supplied the required carotenoids to shrimps undergoing maturation, which enhanced the number of eggs per spawn, nauplii per spawn, and nauplii viability. The mean fecundity of female N. davidi recieved algal diets of 5–10% SP in the current study revealed somewhat higher estimates compared with a range of 20–55 eggs per female over consecutive spawns reported by Tropea and Lopez Greco (2015). Meanwhile, absolute fecundity over successive spawnings in our study was highest in the last spawns compared with the first one. This result contradicts findings reported by Tropea and Lopez Greco (2015), who obtained the highest fecundity in the first spawn, which remained relatively constant until the fifth spawn in N. davidi. Arcos et al. (2003) found no differences in the number of eggs per spawn between the first and successive broods of L. vannamei under controlled conditions.
The fatty acid composition and total carotenoid contents of diets and N. davidi eggs were not analyzed in this study over consecutive spawns, but existing evidence indicates that dietary fatty acid profile reflects eggs fatty acids. S. platensis has been found to be the best algal source of n-6 fatty acid (41.2% of total fatty acids), especially of linoleic and γ-linolenic acids (Kim et al. 2012) both of which are precursors of arachidonic acid, a major component in the formation of prostaglandins as mediators of steroidogenesis, oocyte maturation, and ovulation (Patino and Sullivan 2002). Also, S. platensis contains natural pigments such as phycocyanin chlorophylls, and carotenoids, vitamins A and E, and phenolic components, which together exert important physiological actions in crustaceans (Goiris et al. 2012; Wade et al. 2015). For example, carotenoids have previously been suggested to have the capacity to trigger shrimp vitellogenesis being directly related to the transcription of hormone genes involved in ovary maturation (Dall et al. 1995; Linan-Cabello and Paniagua-Michel 2004). Several researchers (Wyban et al. 1997; Regunathan and Wesley 2006) have highlighted that there is a need to investigate the most appropriate sources of dietary carotenoids that should be incorporated in broodstock shrimp diets in order to prevent declined reproductive performances of broodstock kept in captivity after several spawning events. These issues and also the influences of maturation algal diets on the aging needs and biochemical composition of eggs over successive spawnings should be investigated in the red cherry shrimp in the future. However, the main reasons accounting to increased reproduction performance in shrimp broodstocks fed on diets containing Spirulina are still unclear.
A positive relationship was reported between both absolute and relative fecundities and female weight, independent of spawning order, in penaeid and caridean shrimps (Peixoto et al. 2004; Lara and Wehrtmann 2009). Comparisons of relative fecundities within eight successive spawning events in our RCS shrimps showed that 8–10% Spirulina-fed brooders had significantly higher fecundities (>50 eggs g-1 body weight) up to the 5–6th spawning stages in comparison with those (<50 eggs g-1 body weight) fed the other incorporation levels up to the 8th spawning step. Interestingly, it was observed that the relative fecundity of females fed with 8–10% Spirulina showed an increase only up to sixth spawning event followed by a decreasing trend (with no significant differences) until the eighth spawning event. Despite statistical differences in the body weight of females fed with higher incorporation levels (8–10% Spirulina) with other nutritional groups at 7th and 8th spawns, the relative fecundity showed a decreasing trend with increasing weight (and/or age) (Fig. 4). Rothlisberg (1998) highlighted the possibility of senescence occurring in larger (older) penaeid shrimps negatively affecting their reproductive performance; Calado et al. (2005), on the other hand, did not find any evidence of senescence in embryo production by Lysmata seticaudata or even biochemical composition of developing embryos. In contrast, Tropea and Lopez Greco (2015) have reported that total lipid and energy contents of N. davidi eggs decreased in the 4th and 5th spawns as a consequence of multiple spawning, and that females could not accumulate and/or mobilize the same levels of lipids in the re-maturing ovary over consecutive spawnings. With respect to the effect of multiple spawning on egg biochemical composition in L. vannamei, Palacios and Racotta (2003) and Arcos et al. (2003), respectively, presented evidence of a similar transfer of reserves to the eggs or even an increased transfer over successive spawns. In view of these results, our finding of a decreasing trend in relative fecundity up to the sixth event could have probably caused by decreased lipid deposits in the eggs resulting from multiple spawning. This corresponds to the findings of Tropea and Lopez Greco (2015), who detected decreased lipid concentrations and energy contents in eggs of the fourth and fifth spawns, which may indicate a decrease in the maternal supply over multiple spawning.
Considering the fact that the feed consumption level is very low in broodstocks of this shrimp species, higher inclusion levels of Spirulina (8–10%) in this study is financially justifiable. Based on the obtained results, it is recommended that an addition of 8–10% Spirulina meal is a viable strategy to increase survival rate and reproductive indices in red cherry shrimp during maturity. However, a higher relative fecunditiy was only observed up to the sixth spawning event, which gradually decreased afterwards up to the eighth event, indicating that it negatively affects the reproductive performance of this species.
References
Abdul Basri N, Muhamad Shaleh SR, Matanjun P, Noor MN, Shapawi R (2015) The potential of microalgae meal as an ingredient in the diets of early juvenile Pacific white shrimp, Litopenaeus vannamei. J Appl Phycol 27:857–863
AOAC (Association of Official Analytical Chemists) (2002) Official methods of analysis of the Association of Official Analytical Chemists. Washington, DC.
Arcos FG, Ibarra AM, Palacios E, Vazquez-Boucard C, Racotta IS (2003) Feasible predictive criteria for reproductive performance of white shrimp Litopenaeus vannamei: egg quality and female physiological condition. Aquaculture 228:335–349
Belay A, Kato T, Ota Y (1996) Spirulina (Arthrospira): potential application as an animal feed supplement. J Appl Phycol 8:303–311
Brown MR (2002) Nutritional value and use of microalgae in aquaculture. CSIRO Mar Res 3:281–292. doi:10.1002/9780470995280.ch21
Cai Y (1996) A revision of the genus Neocaridina (Crustacea, Decapoda, Atyidae). Acta Zootaxon Sin 21:129–160
Calado R, Figueiredo J, Rosa R, Nunes ML, Narciso L (2005) Effects of temperature, density, and diet on development, survival, settlement synchronism, and fatty acid profile of the ornamental shrimp Lysmata seticaudata. Aquaculture 245:221–237
Calado R, Vitorino A, Reis A, Lopes da Silva T, Dinis MT (2009) Effect of different diets on larval production, quality and fatty acid profile of the marine ornamental shrimp Lysmata amboinensis (de Man, 1888), using wild larvae as a standard. Aquac Nutr 15:484–491
Clarke SD, Gasperikova D, Nelson C, Lapillonne A, Heird WC (2002) Fatty acid regulation of gene expression: a genomic explanation for the benefits of the Mediterranean diet. Ann N Y Acad Sci 967:283–298
Coman GJ, Crocos PJ (2003) Effect of age on the consecutive spawning of ablated Penaeus semisulcatus broodstock. Aquaculture 219:445–456
Cuzon G, Dos-Santos R, Hew M, Poullaouec G (1981) Use of Spirulina in shrimp (Penaeus japonicus) diet. Aquaculture 12:282–291
Dall W, Smith DM, Moore LE (1995) Carotenoids in the tiger prawn Penaeus esculentus during ovarian maturation. Mar Biol 123:435–441
Distel RJ, Robinson GS, Spiegelman BM (1992) Fatty acid regulation of gene expression. Transcriptional and post-transcriptional mechanisms. Biol Chem 267:5937–5941
Fafournoux DP, Bruhat A, Jousse C (2000) Amino acid regulation of gene expression. Biochemistry 351:1–12
Food and Agricultural Organization of United Nations (FAO) (2014) http://www.fao.org
Goiris K, Muylaert K, Fraeye I, Foubert I, Brabanter JD, De Cooman L (2012) Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J Appl Phycol 24:1477–1486
Güroy B, Sahin I, Mantoglu S, Kayali S (2012) Spirulina as a natural carotenoid source on growth, pigmentation and reproductive performance of yellow tail cichlid (Pseudotropheus acei). Aquacult Int 25:869–878
Habib MA, Parvin M, Huntington TC, Hasan MR (2008) A review on culture, production and use of Spirulina as food for humans and feeds for domestic animals and fish. FAO fisheries and aquaculture circular no. 1034, FAO, Rome, 33 p
Hanel R, Broekman D, de Graaf S, Schnack D (2007) Partial replacement of fishmeal by lyophylized powder of the microalgae Spirulina platensis in pacific white shrimp diets. Open Mar Biol J 1:1–5
Hardy RW, Barrows FT (2002) Diet formulation and manufacture. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, NY, pp 505–600
Harrison KE (1990) The role of nutrition in maturation, reproduction and embryonic development of decapod crustaceans: a review. J Shellfish Res 9:1–28
Heerbrandt TC, Lin J (2006) Larviculture of red front shrimp, Caridina gracilirostris (Atyidae, Decapoda). J World Aquacult Soc 37:186–190
Hemaiswarya S, Raja R, Ravi Kumar R, Ganesan V, Anbazhagan C (2010) Microalgae: a sustainable feed source for aquaculture. World J Microb Biotechnol 27:1737–1746
His T, Yixiong C (2007) Two new species of the land-locked freshwater shrimps genus, Neocaridina Kubo 1938 (Decapoda: Caridea: Atyidae) from Taiwan, with notes on speciation on the island. Zool Stud 46:680–694
Jaime-Ceballos B, Villareal H, Garcia T, Perez-Jar L, Alfonso E (2005) Effect of Spirulina platensis meal as feed additive on growth, survival and development in Litopenaeus schmitti shrimp larvae. Rev Invest Mar 26:235–241
James R, Sampath K, Thangarathinam R, Vasudevan I (2006) Effect of dietary Spirulina level on growth, fertility, coloration and leucocyte count in red swordtail, Xiphophorus helleri. Isr J Aquac 58:97–104
Ju ZY, Forster I, Dominy W (2009) Effects of supplementing two species of marine algae or their fractions to a formulated diet on growth, survival and composition of shrimp (Litopenaeus vannamei). Aquaculture 292:237–243
Ju ZY, Deng DF, Dominy W (2012) A defatted microalgae (Haematococcus pluvialis) meal as a protein ingredient to partially replace fishmeal in diets of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Aquaculture 354-355:50–55
Karadal O, Güroy D, Turkmen G (2016) Effects of feeding frequency and Spirulina on growth performance, skin coloration and seed production on kenyi cichlids (Maylandia lombardoi). Aquac Int. doi:10.1007/s10499-016-0017-x
Khanzadeh M, Esmaeili Fereidouni A, Seifi Berenjestanaki S (2016) Effects of partial replacement of fish meal with Spirulina platensis meal in practical diets on growth, survival, body composition, and reproductive performance of three-spot gourami (Trichopodus trichopterus) (Pallas, 1770). Aquac Int 24:69–84
Kim EK, Choi GG, Kim HS, Ahn CY, Oh HM (2012) Increasing γ-linolenic acid content in Spirulina platensis using fatty acid supplement and light-dark illumination. J Appl Phycol 24:743–750
Lara LR, Wehrtmann IS (2009) Reproductive biology of the freshwater shrimp Macrobrachium carcinus (L.) (Decapoda: Palaemonidae) from Costa Rica, central America. J Crustac Biol 29:343–349
Linan-Cabello MA, Paniagua-Michel J (2004) Induction factors derived from carotenoids and vitamin A during the ovarian maturation of Litopenaeus vannamei. Aquac Int 12:583–592
Lu J, Takeuchi T (2004) Spawning and egg quality of the tilapia Oreochromis niloticus fed solely on raw Spirulina throughout three generations. Aquaculture 234:624–640
Macias-Sancho J, Poersch LH, Bauer W, Romano LA, Wasielesky W, Tesser MB (2014) Fishmeal substitution with Arthrospira (Spirulina platensis) in a practical diet for Litopenaeus vannamei: effects on growth and immunological parameters. Aquaculture 426–427:120–125
Millamena OM, Quinitio E (2000) The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture 181:81–90
Mustafa MG, Nakagawa H (1995) A review: dietary benefits of alga as an additive in fish feed. Isr J Aquac 47:155–162
Nakagawa H, Gomez-Diaz G (1995) Usefulness of Spirulina sp. meal as feed additive for giant freshwater prawn, Macrobrachium rosenbergii. Suisanzoshoku 43:521–526
Nandeesha MC, Gangadhara B, Manissery JK, Venkataraman LV (2001) Growth performance of two Indian major carps, catla (Catla catla) and rohu (Labeo rohita) fed diets containing different levels of Spirulina platensis. Bioresour Technol 80:117–120
Olvera-Novoa M, Dominguez-Cen L, Olivera-Sastillo L, Martinez-Palacoice CA (1998) Effect of the use of the microalga Spirulina maxima as fish meal replacement in diets for tilapia, Oreochromis mossambicus (Peters) fry. Aquac Res 29:709–715
Palacios E, Racotta IS (2003) Effect of number of spawns on the resulting spawn quality of 1-year-old pond-reared Penaeus vannamei (Boone) broodstock. Aquac Res 34:427–435
Palmegiano GB, Agradi E, Forneris G, Gai F, Gasco L, Rigamonti E, Sicuro B, Zoccarato I (2005) Spirulina as a nutrient source in diets for growing sturgeon (Acipenser baeri). Aquac Res 36:188–195
Pantaleao JAF, Barros-Alves SP, Tropea C, Alves DFR, Negreiros-Fransozo ML, López-Greco LS (2015) Nutritional vulnerability in early stages of the freshwater ornamental “red cherry shrimp” Neocaridina davidi (Bouvier, 1904) (Caridea: Atyidae). J Crust Biol 35:676–681
Pantaleao JAF, Gregati RA, da Costa RC, Lopez-Greco LS, Negreiros-Fransozo ML (2017) Post-hatching development of the ornamental ‘red cherry shrimp’ Neocaridina davidi (Bouvier, 1904) (Crustacea, Caridea, Atyidae) under laboratorial conditions. Aquac Res 48:553–569
Patino R, Sullivan CV (2002) Ovarian follicle growth, maturation, and ovulation in teleost fish. Fish Physiol Biochem 26:57–70
Peixoto S, Cavalli RO, Wasielesky W, D’Incao F, Krummenauer D, Milach ÂM (2004) Effects of age and size on reproductive performance of captive Farfantepenaeus paulensis broodstock. Aquaculture 238:173–182
Radhakrishnan S, Belal IEH, Seenivasan C, Muralisankar T, Saravana Bhavan P (2016) Impact of fishmeal replacement with Arthrospira platensis on growth performance, body composition and digestive enzyme activities of the freshwater prawn, Macrobrachium rosenbergii. Aquac Rep 3:35–44
Regunathan C, Wesley SG (2006) Pigment deficiency correction in shrimp broodstock using Spirulina as a carotenoid source. Aquac Nutr 12:425–432
Rothlisberg PC (1998) Aspects of penaeid biology and ecology of relevance to aquaculture; a review. Aquaculture 164:49–65
Silva-Neto J, Nunes AJP, Sabry-Neto H, Vincius Carmo Sa M (2012) Spirulina meal has acted as a strong feeding attractant for Litopenaeus vannamei at a very low dietary inclusion level. Aquac Res 43:430–437
Spolaore P, Joannis-Cassan J, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Tank KV, James T, Nair CM, Bajaniya VC (2010) Evaluation of Arthrospira fusiformis as a protein source in the diet of Penaeus monodon Fabricius. J Appl Aquac 22:109–116
Teimouri M, Keramat Amirkolaie A, Yeganeh S (2013) The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquaculture (396-399):14-19
Tropea C, Lopez Greco LS (2015) Female growth and offspring quality over successive spawnings in a caridean shrimp Neocaridina davidi (Decapoda, Atyidae) with direct development. Biol Bull 229:243–254
Tropea C, Arias M, Calvo NS, Lopez Greco LS (2012) Influence of female size on offspring quality of the freshwater crayfish Cherax quadricarinatus (Parastacidae: Decapoda). J Crust Biol 32:883–890
Tropea C, Stumpf L, Lopez Greco LS (2015) Effect of temperature on biochemical composition, growth and reproduction of the ornamental red cherry shrimp Neocaridina heteropoda heteropoda (Decapoda, Caridea). PLoS One 10(3):e0119468
Viau VE, Marciano A, Iriel A, López Greco LS (2016) Assessment of a biofilm-based culture system within zero water exchange on water quality and on survival and growth of the freshwater shrimp Neocaridina heteropoda heteropoda. Aquac Res 47:2528–2542
Wade NM, Gabaudan J, Glencross BD (2015) A review of carotenoid utilisation and function in crustacean aquaculture. Rev Aquac. doi:10.1111/raq.12109
Wang HW, Cai DB, Zhao CL, Xiao GH, Wang ZH, Xu HM, Yang LK, Ma L, Ma JL (2010) Effects of dietary manganese supplementation on antioxidant enzyme activity in the shrimp (Neocaridina heteropoda). Isr J Aquac 62:78–84
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29:949–982
Wouters R, Lavens P, Nieto J, Sorgeloos P (2001) Penaeid shrimp broodstock nutrition: an updated review on research and development. Aquaculture 202:1–21
Wu X, Cheng Y, Zeng C, Wang C, Cui Z (2010) Reproductive performance and offspring quality of the first and the second brood of female swimming crab, Portunus trituberculatus. Aquaculture 303:94–100
Wyban W, Martinez G, Sweeney J (1997) Adding paprika to Penaeus vannamei maturation diet improves nauplii quality. World Aquac 28:59–62
Zhu D, Wen X, Xuan X, Li S, Li Y (2015) The green alga Ulva lactuca as a potential ingredient in diets for juvenile white spotted snapper Lutjanus stellatus Akazaki. J Appl Phycol 28:703–711
Acknowledgments
The authors are grateful to the Hamoon Ornamental Fish and Crustacean Culture Center (Marlik Town, Tehran, Iran). The research was funded by the Sari Agricultural Sciences and Natural Resources University (SANRU), Sari, Iran. Sincerest appreciations are credited to Dr. Mohammad Kazem Khalesi and Dr. Ali Jafarpour for editing the English revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Namaei Kohal, M., Esmaeili Fereidouni, A., Firouzbakhsh, F. et al. Effects of dietary incorporation of Arthrospira (Spirulina) platensis meal on growth, survival, body composition, and reproductive performance of red cherry shrimp Neocaridina davidi (Crustacea, Atyidae) over successive spawnings. J Appl Phycol 30, 431–443 (2018). https://doi.org/10.1007/s10811-017-1220-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1220-5