Abstract
This work mainly aimed to compare the growth and tolerance of fungal–algal cultures in various dilutions of anaerobically digested swine wastewater (ASW) and to determine their nutrient removal efficiency and role in biogas upgrading. Three species of microalgae, namely, Chlorella vulgaris, Scenedesmus obliquus, and Pseudokirchneriella subcapitata, were selected for ASW purification and biogas upgrading in photobioreactors with four different concentrations of 1300, 1000, 700, and 300 mg L−1 COD (chemical oxygen demand). Co-cultivation of the fungus Ganoderma lucidum and C. vulgaris in 300 mg L−1 COD was the most efficient for reduction of COD, total nitrogen, and total phosphorus by 79.74 ± 4.87%, 74.28 ± 6.13% and 85.37 ± 6.84%, respectively. With respect to biogas upgrading, co-cultivation of G. lucidum and P. subcapitata in 700 mg L−1 COD showed the maximum CO2 removal efficiency of 84.77 ± 3.04%. These results show the feasibility of simultaneously removing nutrients in ASW and CO2 in biogas using fungi-microalgae pellets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Swine wastewaters are considered as serious agricultural contaminants. Anaerobic digestion is a promising technology not only to reduce the pollution of swine wastewaters but also to produce clean energy biogas. However, large volumes of wastewaters with numerous nutrients including organic carbon, nitrogen, and phosphorus are generated during anaerobic digestion. Anaerobically digested swine wastewater (ASW) will lead to environment pollution if directly discharged into the river without further treatment (Anam et al. 2012). Furthermore, CO2 ranks second only to methane in biogas and needs to be removed before the biogas can be used for vehicle fuels. Although many methods have been applied for ASW treatment, such as oxidation ponds, artificial wetlands, soil infiltration, sequencing batch reactors, and so on (Zhao et al. 2014), there are some disadvantages of these ASW treatment methods, such as chemical oxygen demand (COD) removal efficiency, large land requirements, and significant sludge generation. The common biogas upgrading technologies mainly include water washing, cryogenic separation, physical and chemical absorption, membrane separation, and biological upgrading methods. However, most of these result in methane loss and high energy consumption. Compared to other wastewater treatment methods and biogas upgrading technologies, microalgae systems have distinct advantages, including high-efficiency, low-energy consumption and no chemical addition (García et al. 2017). Furthermore, nutrients in ASW can be used for microalgal growth and CO2 in biogas is important for microalgal photosynthesis. Some researchers have demonstrated a good capability of COD, total nitrogen (TN), total phosphorus (TP) removal, and algal accumulations for biodiesel production by microalgal photosynthesis (Prachanurak et al. 2014; Zhou et al. 2014; Prandini et al. 2016). However, the high cost of microalgae harvesting is one of the major bottlenecks for industrialization of algae-based technologies because of the small size (5–50 μm), negative surface charge on the algae, and the low concentration of algae biomass (Packer 2009; García et al. 2017). Microalgal co-cultivation strategy can play a key role on biological purification of biogas slurry and biogas upgrading because of the large pellets for easy harvest (Zhou et al. 2012; Muradov et al. 2015; Sun et al. 2016).
Microalgae grow rapidly together with fungi. The photosynthetically produced O2 can be assimilated by the fungi for their growth, and fungally generated CO2 can be used by the algae. Studies also have been carried out to study the potential of bacterial and algal partnership to treat wastewater and upgrade biogas (Wrede et al. 2014; Lebrero et al. 2016; Yan et al. 2016; Sun et al. 2016). Wrede et al. (2014) showed the additive and synergistic effects of co-cultivation of fungal and microalgal cells on microalgal biomass production, lipid production, and wastewater treatment. Lebrero et al. (2016) investigated biogas upgrading in a photobioreactor (PBR) through co-cultivation of green algae (Chlorella sp.) and aerobic sludge. They showed that more than 62% of CO2 was removed from biogas at pH 8.1. Yan et al. (2016) studied the effects of different light wavelengths, light intensities, and photoperiods on biogas upgrading and biogas slurry purification. The removal of COD, TN, and TP in raw biogas slurry were in the range of 30.7–70%, 30–77% and 31–79%, respectively. The CO2 removal efficiency reached 57%, which distinctly improved the biogas grades (Yan et al. 2016). Sun et al. (2016) investigated the influences of different CO2 concentrations on nutrient removal and biogas upgrading using three different strains co-cultured with activated sludge (Sun et al. 2016). The co-cultivation of Scenedesmus obliquus and activated sludge with CO2 concentration of 45% performed well in nutrient removal and biogas upgrading. The COD removal was close to 75%, which was higher than the results of Yan and Zheng (2014). The nutrient removal efficiency and biogas upgrading depend on microalgal strains, operational conditions, and culture modes. Further research on the co-cultivation of microalgae and fungi for improving ASW purification and biogas upgrading is necessary, especially the comparison of co-cultivation of fungi with different algae strains.
Most previous studies aimed to improve the microalgae growth and the nutrient removal (Christenson and Sims 2011; Yan and Zheng 2014;). Furthermore, there is a lack of studies comparing the performance with actual biogas of different algae species associated with fungi when treating ASW under different concentrations of nutrients in ASW. Co-cultivation of microalgae with fungi is necessary to investigate biogas upgrading and ASW purification simultaneously. In this study, three algal strains were selected to co-culture with fungi in a photobioreactor. Furthermore, ASW should be diluted to accommodate the growth of algae-fungal cells to avoid inhibition by high COD. Therefore, the main objectives of this work were (1) to select the optimal microalgae for ASW purification and biogas upgrading and (2) to determine the optimal dilution ASW for the co-cultivation of fungal and microalgal cells and the nutrient removal and biogas upgrading in optimal conditions.
Materials and methods
Microalgal strains and Ganoderma lucidum
Three green microalgae stored in our laboratory on BG11 medium (Rippka et al. 1979), Chlorella vulgaris FACHB-25, Scenedesmus obliquus FACHB-13, and Pseudokirchneriella subcapitata FACHB-271, were selected on account of their high growth rates (in the range of 0.33–0.45 day−1) and nutrient removal ability (more than 60% removal of COD) in ASW (Zhao et al. 2015).
Ganoderma lucidum 5.765 was obtained from the China General Microbiological Culture Collection Center. An inoculum was prepared by inoculating 100 mL of a synthetic medium (glucose, 10 g L−1; NH4NO3, 2.0 g L−1; K2HPO4, 1.0 g L−1; NaH2PO4·H2O, 0.4 g L−1; MgSO4·7H2O, 0.5 g L−1; and yeast extract, 2.0 g L−1; pH 6.5) with 25 mycelial discs (Zárate-Chaves et al. 2013).
Raw biogas and ASW
The raw biogas and ASW were collected from an anaerobic digestion reactor in a livestock wastewater treatment plant of a pig farm in Jiaxing, Zhejiang, China, and fed to the PBR. The H2S in biogas was removed to less than 50 ppm (v/v) by absorption on Fe2(SO4)3 solution (Chung et al. 2006). The desulfurized raw biogas consists of CH4 (57.32 ± 4.65%, v/v), CO2 (39.25 ± 3.14%, v/v), O2 (0.75 ± 0.06%, v/v), and H2O (2.67 ± 0.13%, v/v).
ASW samples were centrifuged to remove large particles before being filtered through Whatman grade no. 41 filter paper (pore size 20–25 μm). The ASW was autoclaved at 121 °C and cooled to room temperature and then stored at 4 °C. Table 1 presents the characteristics of the raw wastewater. The concentration of other inorganic nitrogen forms such as concentration of NO3 −-N was below detection limits. The PBR was fed with ASW diluted with distilled water to avoid potential inhibition by high COD concentration on growth of the G. lucidum–algal pellets. The nutrient concentration of the diluted ASW corresponded to 10%, 25%, 35% and 45% of raw wastewater.
Pelletization and fungal-assisted flocculation
Ganoderma lucidum was incubated at 25 ± 1 °C on a rotary shaker at 160 rpm for 7 days. The obtained biomass was washed with sterile distilled water and homogenized with 100 mL of sterile distilled water in a laboratory blender. Subsequently, the culture was gradually acclimated in ASW with nutrient concentration range from 10 to 45%, until G. lucidum biomass production was not affected. Afterward, the obtained strains were used for the co-cultivation with microalgal cells.
All the three microalgae were incubated separately in a PBR (400 L) at 25 ± 1 °C, a light intensity of 150 ± 5 μmol photons m−2 s−1 produced by cool white fluorescent lights, and under a light/dark cycle of 12 h:12 h for 7 days before pelletization. Periodic agitations were performed three times a day. The algal cultures were precipitated, washed, and resuspended before being mixed with fungal pellets to achieve a final concentration of 158.37 ± 14.26 mg L−1. Each algae culture suspension (100 mL) was mixed with 5 mL of G. lucidum pellet suspension and shaken at 160 rpm for 168 h under constant light (200 μmol photons m−2 s−1) at 25 °C for pelletization. All of the experiments were replicated at least three times. The daily biomass concentrations were measured by direct drying for G. lucidum/C. vulgaris (Gl/Cv), G. lucidum/S. obliquus (Gl/So), and G. lucidum/P. subcapitata (Gl/Ps) pellets during operational periods in 7 days.
Experimental setup of simultaneously purifying ASW and upgrading biogas
The PBR consisted of two interconnected 16.8-L (individual) glass-made cylinder blocks (diameter = 0.2 m, height = 0.6 m) filled with 14 L raw biogas and 2.8 L ASW and illuminated under 200 μmol photons m−2 s−1 by six fluorescent lamps arranged in a circular configuration (20 W, 110 V) for left-cylinder block (Fig. 1). The reactors were hermetically sealed by rubber stoppers after the G. lucidum–algal pellets were harvested by filtration and injected into the PBR. ASW was continuously added from the right-cylinder block to the left of the PBR and the raw biogas was fed via a PBR headspace under ambient temperature for 10 days.
Analytical procedures
The biogas was collected with syringe for component analysis. The media were sampled daily from PBR for determination of pH, COD, TN, TP, and fungal–algal growth rate starting from inoculation of the G. lucidum–algae pellets. The pH was measured using a pH meter (Orion 250 Aplus ORP Field Kit, USA). The COD, TN, and TP contents in ASW were determined using standard methods (APHA 1995). The concentrations of CH4, CO2, O2, H2S, and H2O (v/v) in biogas were analyzed by a gas analyzer (GA94, Onuee Co., Ltd., China). The dry weight of microalgae was measured by direct drying. Firstly, 20 mL of culture suspension was filtered using a glass microfiber filter (GF/C, 1.2 μm pore size; Whatman, USA). Second, microalgae cells together with the filter were dried at 100 °C for 12 h and then cooled to room temperature in a desiccator and then the dry weight was determined from the weight difference before and after filtration (Zhao et al. 2015).
The biomass productivity (P, g L−1 day−1) of the fungal–algal pellets was calculated by Eq. (1):
where DW i is the biomass concentration (g L−1) at time t i and DW 0 is the initial biomass concentration (g L−1) at t 0 (days).
Statistical analysis
Statistical analyses were performed using SPSS (SPSS, 2003) by one-way analysis of variance. A 95% confidence level (p ≤ 0.05) was applied for all analyses. Duncan’s multiple range test at 5% level was used to assess the significant differences among different treatments of the same fungal–algal pellets.
Results
Fungal–algal pellet growth in the ASW
Biomass productivity was periodically determined during co-cultivation of fungal–algal pellets on the different dilutions of ASW, and the results are shown in Table 2. As can be seen from Table 2, the dilution ratio of ASW affected the maximum algae–fungi pellet biomass and mean daily productivity significantly (p < 0.05). The maximum algae–fungi pellet biomass was 4.77 g L−1 and the mean daily productivity was 0.45 g L−1 day−1 with co-culture of fungi and C. vulgaris. Ten percent was considered as the best dilution ratio of ASW for all the fungal–algal pellets to obtain the highest maximum biomass and mean daily productivity. However, in view of Gl/Cv, there was no significant difference in maximum biomass between 10% and 35% ASW as well as 45% and 25% ASW. However, for other two fungal–algal pellets (Gl/So and Gl/Ps), there was no significant difference on maximum biomass and mean daily productivity in 25%, 35%, and 45% ASW, but the maximum biomass and mean daily productivity in 10% dilution biomass was significantly higher than the other three dilutions. In addition, Gl/Cv pellets grew faster at 10% and 35% ASW than at other ASW, which corresponded to Wrede’s research (Wrede et al. 2014), which demonstrated high biomass production of Aspergillus fumigatus/Tetraselmis chuii pellets in 10% ASW. As a result, 10% ASW treatments were selected for microalgal growth.
Nutrient removal
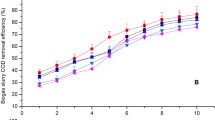
COD removal amount and removal rates of ASW using three kinds of fungal–algal pellets in the different dilution ratios of ASW during the 10-day batch co-cultivations are presented in Fig. 2. Both COD removal amount and COD removal rates of these three kinds of fungal–algal pellets in different dilution ratios of ASW all ranked Gl/Cv > Gl/So > Gl/Ps pellets. The higher the dilution ratio of ASW, the lower the COD removal amount and the removal rate obtained. Besides, there was no significant difference observed in 45% and 25% of ASW for mean COD removal (p > 0.05) (Table 2). The mean COD removal efficiencies (REs) of Gl/Cv in 10% ASW were significantly higher than those of the ASW dilutions (p < 0.05). With an eye to the final COD removal amount and removal rate, 45% is the appropriate dilution ratio for purification of ASW using the mentioned three kinds of fungal–algal pellets according to Fig. 2. The highest COD-RE (%) Gl/Cv pellets were 13.4% and 10.6% higher than Gl/So and Gl/Ps pellets from 10% ASW, respectively.
Nitrogen removal RE was investigated as a function of operating time to evaluate nitrogen RE with different dilution ratios of ASW (Fig. 3). The dilution ratio with maximum mean TN removals for all fungal–algal pellets was 10% (Table 2). As can be seen from Fig. 3, the maximum final TN removal can be obtained with 10% ASW for Gl/Cv and Gl/So pellets and 25% ASW for Gl/Ps, respectively. Gl/Cv in 10% ASW can remove the TN more than other dilutions. At 10% ASW, 85% of TN was removed by Gl/Cv pellets after 120 h of incubation (Fig. 3a). There was no significant differences between 35% and 10% ASW in TN-RE using co-cultured microalgae (p > 0.0.5) as well as the difference between 45% and 25%. However, the TN-RE of 35% and 10% ASW using co-cultured microalgae were significantly higher than that of 45% and 10% (p < 0.05). Therefore, 10% ASW was considered as the appropriate ASW for nitrogen removal.
The effects of dilution ratio on phosphorus removal by the three fungal–algal pellets are shown in Fig. 4. The maximum final TP removal amounts and removal rates can be obtained with 10% ASW using Gl/Cv. Under the dilution ratios of 45%, 35%, and 10%, the TP removal rate of three fungal–algal pellets ranked Gl/Cv > Gl/Ps > Gl/So. In view of the highest TP removal rate, 45% ASW was considered as the optimal ASW using Gl/Cv. According to Table 2, Gl/So had lower mean TN-RE than Gl/Cv and Gl/Ps in different ASW. However, no significant differences in mean TN-RE were found between 25% and 35% ASW for Gl/Ps and Gl/So (p > 0.05). The highest TP removal rate of Gl/Cv was 113.3 mg L−1 day−1 with a dilution ratio of 45%, which was 3.7% and 2.5% higher than Gl/So and Gl/Ps. Totally, the 10% ASW was regarded as the optimum using Gl/Cv.
Biogas upgrading
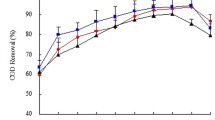
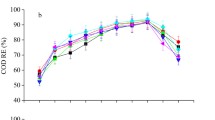
CO2 removal (%), CH4, CO2, O2, and H2O amount (v/v) were investigated as a function of operating time to evaluate differences in biogas upgrade with varying dilution ratios for the three fungal–algal strains (Fig. 5 and Table 3). As seen from Table 3, the dilution ratio of ASW has a significant effect on CO2 removal (p < 0.05).The highest CO2 removal efficiency could be found in these three fungal–algal pellets under the 25% ASW dilution. Thus, the methane contents in biogas were enhanced. The highest methane content for biogas upgraded by Gl/Ps under dilution ratio of 25% was 92.4% with the CO2 removal of 84.77% and the lowest O2 content in three fungal–algal pellets. As can be seen from Table 3, for Gl/Cv, there were no significant differences between ASW dilution ratio of 25% and 35% in the CH4 content in biogas (p > 0.05). However, the CO2 content in upgraded biogas under ASW dilution ratio of 25% was much less than that of 35%. Similar patterns could be observed for Gl/Ps and Gl/So pellets. Therefore, Gl/Ps revealed the best for upgrading biogas with the 25% ASW. The final methane content reached 92.41 ± 3.14%, which was close to the standard required of fuels in this research (Ryckebosch et al. 2011).
Discussion
Biomass productivity
Biomass productivity is a key parameter which is used to assess the nutrient removal potential of fungal–algal pellets in ASW and CO2 in biogas. According to Table 2, the maximum biomass and mean daily productivity of C. vulgaris was shown in 10% ASW. This corresponds to the findings of Muradov et al. (2015), who achieved 4.4±1.1 g L-1 biomass for co-cultivation of the marine microalga Tetraselmis suecica and Aspergillus fumigatus. It was also indicated that NH4 +-N and PO4 3−-P were almost completely removed after 48-h incubation at 10% ASW. In addition, nutrient uptake by A. fumigatus/Thraustochytrid and A. fumigatus/T. chuii pellets led to 2.1- and 1.6-fold increase in their biomass production after 48 h of treatment, respectively (Wrede et al. 2014). Therefore, a low ASW concentration can promote the accumulation of biomass and provide adequate concentration of nutrients for fungal–algal growth. The mean daily productivities of all these three fungal–algal pellets did not increase as the dilution ratio of ASW increased. This implies that the nutrient concentration in ASW were sensitive for growth of different fungal–algal pellets in a certain nutrient concentration range. For Gl/Ps and Gl/So pellets, the maximum biomass productions in 10% ASW were significantly higher than others (p < 0.05). However, for Gl/Cv pellets, the maximum biomass production in 10% and 35% ASW were higher than 25% and 45% ASW, respectively. This finding may be explained by the higher tolerance on higher nutrient in ASW for Gl/Cv pellets.
The maximum biomass of the co-cultivation of G. lucidum with C. vulgaris was much higher than S. obliquus (Table 2), which corroborates with efficient flocculation of the C. vulgaris with a number of cultured filamentous fungal strains, including Aspergillus sp., Aspergillus niger, and Aspergillus oryzae (Zhou et al. 2014; Zhang and Hu 2012; Xie et al. 2013, 2014). Biomass production generally is affected by operational factors, including microalgal species, nutrient, sunlight, pH, CO2 availability, and desired final products (Gultom and Hu 2013; Borowitzka 1999). On the basis of the results in this study of the high biomass production of different G. lucidum–algal pellets, C. vulgaris was selected as the optimal strain for co-cultivation with G. lucidum in 10% ASW (Xu et al. 2015; Zhao et al. 2015).
Nutrient removal from ASW
Fungal–algal pelletization has been proven as a promising technology for microalgae harvesting, which can be used to remove nutrients from ASW (Zhou et al. 2012). Besides, fungi–algae pellets have been used as efficient bioremediation agents for wastewater treatment (Muradov et al. 2015; Wrede et al. 2014; Xia et al. 2014). After wastewater anaerobic digestion, further treatment is required to remove nutrients before discharge (Zhou et al. 2012). According to Table 2 and Fig. 2, 10% ASW was suitable for COD removal by fungi–algae. The maximum RE of the Gl/Cv pellets reached 79.7%, which is much higher than those reported for G. lucidum/Aspergillus sp. pellets (Zhou et al. 2012). COD-RE from 10% ASW was significantly higher (p < 0.05, Table 2) than other dilutions. The highest specific growth rate of the microalgae with the COD concentration of 400 mg L−1 (about 12.5% ASW) was reported. Microalgae grew better under the relatively low COD concentration of ASW (Xu et al. 2015). Therefore, the cultivation of Gl/Cv pellets in 10% ASW was appropriate to increase the COD removal amount and removal rates for ASW purification, which was in accordance with the analysis of fungal–algal growth above.
As can be seen from Fig. 3, high TN removals from Gl/Cv were superior to the results from A. fumigatus (Muradov et al. 2015; Sun et al. 2016). Some papers report that TN can be efficiently removed by Aspergillus sp./C. vulgaris pellets (Zhou et al. 2012, 2014). In this study, the highest mean TN removal rate for 10% of ASW using Gl/Cv was 74.28% which was 66% higher than found by Zhou et al. (2014). The reduction of TN could be largely attributed to the physical absorption by fungi–algae symbionts because of their unique structure (Li et al. 2011). The results also showed that co-cultivation of C. vulgaris and S. obliquus with G. lucidum can be used for TN reduction in ASW.
As can be seen from Fig. 4, the higher the dilution ratio was, the lower TP removal rate was obtained for all the algae–fungi pellets. When the fungus–alga pellets were grown in 10% ASW, the sphere structure of fungus–alga pellets was relatively stable and could not easily break into small pieces. In terms of TP-RE in ASW, the results in this study were almost 1.5 times higher than our previous results (Xu et al. 2015; Zhao et al. 2015). The same algae were mono-cultured in the same ASW for wastewater purification. It could be that co-cultivation of algae with fungi was superior to mono-culture on the nutrient removal. In the algae–fungi symbiotic system, it can be complementary and mutually beneficial to each other on the metabolic function (Su et al. 2011). The CO2 produced by fungal decomposition can also be used as a carbon source for microalgae growth for rapid growth. Microalgae use solar energy to fix the CO2 in the biogas through photosynthesis to produce their own sugars and nutrients and release O2. The O2 will increase the dissolved oxygen in the biogas slurry and promote the metabolic activity of the fungus (Gultom et al. 2014; Gultom and Hu 2013). In addition, co-cultivation of microalgae and fungi can increase the secretion of extracellular enzymes or secretion of certain specific enzymes or growth factors to promote the growth of each other (Zhang and Hu 2012). Therefore, the fungus-assisted immobilized algal cells with phosphorus removal can be effectively used for ASW treatments by using dominant microalgal strains (Wrede et al. 2014).
Biogas upgrading
The results shown in Table 3 and Fig. 5 indicate that the fungal–algal pellets cultured in the PBR demonstrated a high CO2 removal capacity (71–85%). The Gl/Ps pellets achieved the highest CO2 removal in 25% ASW, while the Gl/Cv pellets grew well at 10% ASW. It was worth noting that the pH value of ASW was weakly alkaline after 144 h of culture. The results also showed that the reduced nutrient concentration gradient of ASW from 45% to 25% at a high pH promoted CO2 removal up to 85% (Prandini et al. 2016). Thus, a better biogas upgrading performance in the PBR was expected under relatively high pH (Kao et al. 2012). Stable pH is extremely important for obtaining high microalgae biomass (Jeong et al. 2013). In this study, the pH increased less than 5% compared to the initial value of pH. It has been reported that the high biomass had a positive effect on biogas purification by microalgae (Unnithan et al. 2014). Moreover, Wang et al. (2016) demonstrated that the activity of fungal–algal pellets was not inhibited by a CH4 amount (v/v) of up to 80% (Wang et al. 2016). The estimated maximum CH4 amount (92.4%, v/v) was higher than the previously reported values from mono-cultured C. vulgaris (86.5%, v/v) supplemented with artificial biogas (65% CH4 and 35% CO2) (Sun et al. 2016). Furthermore, the amounts (v/v) of O2 and H2O during the operational period were nearly unchanged (Table 3). The presence of O2 and H2O in upgraded biogas was not expected to exert negative effects on the growth of fungi–algae pellets given that raw biogas was always saturated with water (Zhao et al. 2013). Although the CO2 removal in the PBR was restricted by the mass transport of this biogas pollutant to the aqueous phase, REs of CO2 reaching 60–65% were still recorded in 10% ASW.
Conclusions
The co-cultivation of microalgae with G. lucidum was better than mono-cultured microalgae to remove nutrients in ASW and remove CO2 in biogas. The Gl/Cv grew better than the other two other fungal–algal pellets in the same diluted ASW. Gl/Cv was selected for removing the nutrient in 10% ASW. Co-culturing of Gl/Ps performed the best on CO2 removing in biogas in 25% ASW. The biogas upgraded by Gl/Cv and Gl/Ps was close to the standard of fuels. The findings in this work confirmed the potential of the symbiosis between microalgae and fungi as a technological platform for the simultaneous removal of CO2 from raw biogas and nutrient from ASW.
Reference
Anam K, Habibi MS, Harwati TU, Susilaningsih D (2012) Photofermentative hydrogen production using Rhodobium marinum from bagasse and soy sauce wastewater. Int J Hydrogen Energ 37:15436–15442
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Christenson L, Sims R (2011) Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol Adv 29:686–702
Chung Y, Ho K, Tseng C (2006) Treatment of high H2S concentrations by chemical absorption and biological oxidation process. Environ Eng Sci 23:942–953
García D, Alcántara C, Blanco S, Pérez R, Bolado S, Muñoz R (2017) Enhanced carbon, nitrogen and phosphorus removal from domestic wastewater in a novel anoxic-aerobic photobioreactor coupled with biogas upgrading. Chem Eng J 313:424–434
Gultom S, Hu B (2013) Review of microalgae harvesting via co-pelletization with filamentous fungus. Energies 6:5921–5939
Gultom S, Zamalloa C, Hu B (2014) Microalgae harvest through fungal pelletization-co-culture of Chlorella vulgaris and Aspergillus niger. Energies 7:4417–4429
Jeong H, Lee J, Cha M (2013) Energy efficient growth control of microalgae using photobiological methods. Renew Energ 54:161–165
Kao C-Y, Chiu S-Y, Huang T-T, Dai L, Hsu L-K, Lin C-S (2012) Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Appl Energ 93:176–183
Lebrero R, Toledo-Cervantes A, Munoz R, del Nery V, Foresti E (2016) Biogas upgrading from vinasse digesters: a comparison between an anoxic biotrickling filter and an algal-bacterial photobioreactor. J Chem Technol Biot 91:2488–2495
Li Y, Zhou W, Hu B, Min M, Chen P, Ruan RR (2011) Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: strains screening and significance evaluation of environmental factors. Bioresour Technol 102:10861–10867
Muradov N, Taha M, Miranda AF, Wrede D, Kadali K, Gujar A, Stevenson T, Ball AS, Mouradov A (2015) Fungal-assisted algal flocculation: application in wastewater treatment and biofuel production. Biotechnol Biofuels 8:24
Packer M (2009) Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 37:3428–3437
Prachanurak P, Chiemchaisri C, Chiemchaisri W, Yamamotob K (2014) Biomass production from fermented starch wastewater in photo-bioreactor with internal overflow recirculation. Bioresour Technol 165:129–136
Prandini JM, da Silva MLB, Mezzari MP, Pirolli M, Michelon W, Soares HM (2016) Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour Technol 202:67–75
Rippka R, Deruelles J, Waterbury J, Herdman M, Stanier R (1979) Generic assignments, strain histories and properties of pure culture of cyanobacteria. J Gen Microbiol 111:1–61.
Ryckebosch E, Drouillon M, Vervaeren H (2011) Techniques for transformation of biogas to biomethane. Biomass Bioenergy 35:1633–1645
Su Y, Mennerich A, Urban B (2011) Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res 45(11):3351–3358
Sun S, Ge Z, Zhao Y, Hu C, Zhang H, Ping L (2016) Performance of CO2 concentrations on nutrient removal and biogas upgrading by integrating microalgal strains cultivation with activated sludge. Energy 97:229–237
Unnithan VV, Unc A, Smith GB (2014) Mini-review: a priori considerations for bacteria–algae interactions in algal biofuel systems receiving municipal wastewaters. Algal Res 4:35–40
Wang Z, Zhao Y, Ge Z, Zhang H, Sun S (2016) Selection of microalgae for simultaneous biogas upgrading and biogas slurry nutrient reduction under various photoperiods. J Chem Technol Biotechnol 91:1982–1989
Wrede D, Taha M, Miranda AF, Kadali K, Stevenson T, Ball AS, Mouradov A (2014) Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS One 9(11):e113497
Xia C, Wei W, Hu B (2014) Statistical analysis and modeling of pelletized cultivation of Mucor circinelloides for microbial lipid accumulation. Appl Biochem Biotech 172:3502–3512
Xie S, Sun S, Dai SY, Yuan JS (2013) Efficient coagulation of microalgae in cultures with filamentous fungi. Algal Res 2:28–33
Xu J, Zhao Y, Zhao G, Zhang H (2015) Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl Microbiol Biot 99:6493–6501
Yan C, Zheng Z (2014) Performance of mixed LED light wavelengths on biogas upgrade and biogas fluid removal by microalga Chlorella sp. Appl Energ 113:1008–1014
Yan C, Zhu L, Wang Y (2016) Photosynthetic CO2 uptake by microalgae for biogas upgrading and simultaneously biogas slurry decontamination by using of microalgae photobioreactor under various light wavelengths, light intensities, and photoperiods. Appl Energ 178:9–18
Zárate-Chaves C, Romero-Rodríguez M, Niño-Arias F, Robles-Camargo J, Linares-Linares M, Rodríguez-Bocanegra M, Gutiérrez-Rojas I (2013) Optimizing a culture medium for biomass and phenolic compounds production using Ganoderma lucidum. Braz J Microbiol 44:215–223
Zhang J, Hu B (2012) A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour Technol 114:529–535
Zhao Y, Wang J, Zhang H, Yan C, Zhang Y (2013) Effects of various LED light wavelengths and intensities on microalgae-based simultaneous biogas upgrading and digestate nutrient reduction process. Bioresour Technol 136:461–468
Zhao B, Li J, Leu S-Y (2014) An innovative wood-chip-framework soil infiltrator for treating anaerobic digested swine wastewater and analysis of the microbial community. Bioresour Technol 173:384–391
Zhao Y, Sun S, Hu C, Zhang H, Xu J, Ping L (2015) Performance of three microalgal strains in biogas slurry purification and biogas upgrade in response to various mixed light-emitting diode light wavelengths. Bioresour Technol 187:338–345
Zhou W, Cheng Y, Li Y, Wan Y, Liu Y, Lin X, Ruan R (2012) Novel fungal pelletization-assisted technology for algae harvesting and wastewater treatment. Appl Biochem Biotech 167:214–228
Zhou Q, Zhang P, Zhang G (2014) Biomass and carotenoid production in photosynthetic bacteria wastewater treatment: effects of light intensity. Bioresour Technol 171:330–335
Acknowledgments
We thank members of the Analysis and Testing Centre, Jiaxing University, for their mass biomass survey work. Editors at NPG Language Editing provided editing assistance to the authors during the preparation of this manuscript. This study was sponsored by the National Science Foundation of China (Grant Nos. 31670511 and 31370520), the Zhejiang Provincial Natural Science Foundation (Grant No. LY16C030003), and the Public Science and Technology Projects of Zhejiang Province (Analytical Test No. 2014C37049).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Guo, G., Cao, W., Sun, S. et al. Nutrient removal and biogas upgrading by integrating fungal–microalgal cultivation with anaerobically digested swine wastewater treatment. J Appl Phycol 29, 2857–2866 (2017). https://doi.org/10.1007/s10811-017-1207-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1207-2