Abstract
Three marine algal biomasses, namely Gelidium latifolium (Grev.) Bornet et Thuret, Ulva lactuca Linnaeus and Colpomenia sinuosa (Mertens et Roth) Derbes et Solier, were used in their raw dried forms to biosorb Al3+, Fe3+, and Zn2+ from aqueous solutions. The optimum biosorption conditions were initial element concentration, 1000 mg L−1; temperature, 40 °C; contact time, 1 h; pH, 4, 3, and 6 for Al3+, Fe3+, and Zn2+, respectively. The highest biosorption efficiency reached 63.78, 65.95, and 111.57 mg g−1 for Al3+, Fe3+, and Zn2+, respectively, using the brown algal biomass C. sinuosa. Examination of algal biomass surface using SEM showed several morphological changes in the cell wall surface due to biosorption such as rupturing, wrinkling, some cavity appearance, protuberance, and roughness as well as each algal type had a unique surface structure in its raw form. FTIR was used to characterize algal biomasses, and the contributing groups were variable according to heave metals and algal type as well. The thermodynamic studies revealed that the biosorption was nonspontaneous, endothermic, and chemical in nature. Freundlich isotherm model fitted slightly better than the Langmuir model in case of Zn2+ biosorption; meanwhile, Langmuir model fitted better in case of trivalent Al3+ and Fe3+. The algal biomasses were efficiently regenerated and reused for four cycles via 0.01 M Na2EDTA. Algal biomasses were applied under the optimum concluded conditions to treat 21 actual polluted industrial effluents from Borg El-Arab region, Egypt. The removal efficiency reached 80.81, 38.25, 91.79, 59.96, 95.33, 98.54, 27.39, 88.42, 36.59, and 96.98% for Al, Co, Cr, Cu, Fe, Mn, Mo, Ni, V, and Zn, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial wastewater is one of the important pollution sources for the aquatic environment. During the last century, a huge amount of industrial wastewater was discharged into rivers, lakes, and coastal areas. This has resulted in serious pollution problems in the aquatic environment and caused negative effects to the eco-system and human life (Aboulsoud 2008). Heavy metal pollution has become a global issue of concern due to their higher toxicities, nature of non-biodegradability, high capabilities in bioaccumulation in human body and food chain, and carcinogenicities to humans (He and Chen 2014).
In general, heavy metals are introduced into water streams as a result of mining operations, refining ores, sludge disposal, fly ash from incinerators, processing of radioactive materials, metal plating, or the manufacture of electrical equipment, paints, alloys, batteries, pesticides, or preservatives (Ahalya et al. 2003). On the other hand, heavy metals are sometimes naturally found in water resources. Growing attention is being given to the potential health hazard presented by heavy metals to the environment. Many heavy metals have chronic ill-health effects on human particularly children, such as lung tumors, allergic dermatitis, chronic bronchitis, irritation of nose, mouth and eyes, headache, stomachache, dizziness, and diarrhea (Sud et al. 2008). Increasing environmental awareness and stringent government regulations have made the treatment of industrial wastewater mandatory. Further, if valuable, effective recovery of such heavy metals is indeed the need of the hour (Shukla and Shukla 2013).
Biosorption is a term that describes the removal of pollutants by the passive binding to biological biomass from an aqueous solution (Davis et al. 2003). Biosorption process has several possible mechanisms taking place essentially in the cell wall such as physical and chemical adsorption, electrostatic interaction, ion exchange, complexation, chelation, and microprecipitation (Aksu 2005).
The objectives of this paper were firstly, determining the optimum conditions to biosorb Al3+, Fe3+, and Zn2+ from aqueous solutions via batch biosorption using three different Egyptian algal biomasses; secondly, application of the optimum conditions in the treatment of actual polluted industrial effluents collected from Borg El-Arab region.
Materials and methods
Marine algal biomasses were collected from Abou-Keer and Almontazah shores, Alexandria (Egyptian Mediterranean Sea) during April, 2013. The algal taxa were identified and classified according to Aleem (1993) and Zinova (1967). In the field, the collected algae were immediately washed with the surrounding water to remove extraneous matter, sand particles, epiphytes, and water squeezed out. In the laboratory, the algal biomasses were washed several times with distilled water prior to air drying.
Biosorption experiments (batch procedures)
Single and multi-metal solutions were prepared by dissolving metallic salts in demineralized water. The used salts ZnSO4·7H2O, FeCl3, and Al2(SO4)3· (NH4)2SO4·24H2O were analytical grade (AR) reagents purchased from Aldrich Chemical Company, Germany.
All batch experiments were carried out in triplicate. Unless otherwise stated, 0.025 g of algal biomass was put in contact with 25 mL of metal aqueous solution at room temperature for 1 h. The solution was then filtered, and the remained metal concentration was analyzed using Inductively Coupled Argon Plasma, iCAP 6500 Duo (Thermo Scientific, England). Multi-element certified standard solution of 1000 mg L−1 (Merck, Germany) was used as stock solution for instrument standardization. pH of the heavy metal solutions was adjusted using pH meter by addition of dilute solutions of HCl and NaOH. The maximum pH was 4, 3, and 6 for Al3+, Fe3+, and Zn2+, respectively, as metal precipitation as hydroxides occurred at higher pH.
For the reusability and regeneration experiment, the metal-loaded algal biomass was separated by filtration, air-dried, and put in contact with a known Na2EDTA volume. The algal biomass was separated, thoroughly washed with demineralized water, and dried prior to reuse in a new metal removal cycle.
Calculations and data evaluation
-
(a)
The amount of biosorbed element to algal biomass was expressed according to Vitor and Corso (2008) and Kumar et al. (2005):
where q 1 is the amount of sorbed element onto the unit amount of the biomass (mg g−1), C i is the initial concentration of element in aqueous solution (mg L−1), Cf is the final (remaining) concentration of element in aqueous solution (mg L−1), V is the volume of element aqueous solution (L), M is the biomass weight (g), and q 2 is the amount of removed element from the aqueous solution (%).
-
(b)
In biosorption isotherm modeling, the linearized Langmuir and Freundlich adsorption isotherms were applied according to Salima et al. (2013) and Farah and El-Gendy (2013) where
C e is the element concentration in aqueous solution at equilibrium (mg L−1), q e is the experimental amount of adsorbed element at equilibrium (mg g−1), Q e is the calculated amount of adsorbed element at equilibrium (mg g−1), and K L is the Langmuir constant indicating the adsorption affinity of the binding sites (L mg−1). By plotting C e /q e versus C e , Q e and K L can be determined from the slope and intercept of the obtained straight line, respectively.
Whereas the linearized logarithmic Freundlich equation assumes as follows:
where K F is the Freundlich constant indicating adsorption capacity, n is the Freundlich constant indicating adsorption intensity. By plotting Log q e versus Log C e , n and K F can be determined from the slope and intercept of the obtained straight line, respectively.
-
(c)
The thermodynamic parameters such as changes in standard free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) are determined by using the following equations according to Farah and El-Gendy (2013) and Saibaba and King (2013), where
K c is the equilibrium constant, C a is the adsorbed element “initial concentration-final concentration” (mg L−1), C e is the remained “final concentration” (mg L−1), ΔH is the change in enthalpy “heat content” (J mol−1), R is the gas constant (8.314 J mol−1 K−1), T is the temperature (K), ΔS is the change in entropy “randomness” (J mol-1 K-1), and ΔG is the change in Gibbs’ free energy of element removal (J mol−1). The ∆H and ∆S values can be obtained from the slope and intercept, respectively, of the Van’t Hoff plots of ln K c versus 1/T. Meanwhile, ∆G values are calculated based on ∆H and ∆S values.
-
(d)
The amount of desorbed element from algal biomass (de-loaded) using disodium ethylene diamine tetra acetate (Na2EDTA) was expressed according to Bulgariu and Bulgariu (2014) and Kanwal et al. (2013), where Desorption % = (qdesorbed/qsorbed) × 100
-
(e)
The amount of resorbed element to algal biomass (re-loaded) was expressed according to Aboulsoud (2008), where Resorption % = (qresorbed/qsorbed) × 100
Examination of algal surface by scanning electron microscopy
The dried algal samples (raw and elements loaded biomasses) were coated by gold sputter coater (SPI-Module). The coated samples were examined by Scanning Electron Microscope (JSM-5500 LV, JEOL, Japan). The accelerating voltage used was 18 kV.
Determination of functional groups onto the algal biomass by Fourier transform infrared
Fine powdered dried algal samples (raw and element loaded biomasses) were pressed into KBr pellets prior to analysis by FTIR Spectrophotometer (8201 DC, Shimadzu, Japan) (wavenumber range from 400 to 4000 cm−1).
Application of algal biomasses in the treatment of real polluted industrial effluents in Borg El-Arab region
Twenty-one wastewater samples were collected from Borg El-Arab city during March to December 2010. The brown algal biomass Colpomenia sinuosa was applied under the concluded optimum conditions from the batch experiments (S/L ratio 1:1000 (w/v), contact time: 1 h, temperature: 40 °C). No pH adjustment was done in order to avoid the precipitation of some metals. Heavy metals (Al, Co, Cr, Mo, and V) and trace elements (Fe, Mn, Cu, Ni, and Zn) were determined by inductively coupled argon plasma (iCAP 6500 Duo, Thermo Scientific, England). The removal percentage was calculated using the following equation: Removal (%) = (Ci-Cf/Ci) × 100, where C i is the initial element concentration before treatment and Cf is the final element concentration after treatment.
Statistical analysis
All determinations were made in triplicate for all assays. Data were subjected to an analysis of variance (ANOVA) with statistical significance at P < 0.05 being tested using the Duncan’s test (Waller and Duncan 1969). In the tables means having the same letters in the same column are not significant at P (significance probability value) = 0.05 level.
Results and discussion
Effect of contact time on element biosorption
Data in Table 1 shows that the efficiency of biosorption process increased with increasing of contact time in each case. The biosorption process is very fast during the initial stage, and afterwards, the rate of biosorption process becomes slower near to equilibrium, which is practically obtained after 1 h. The biosorption efficiency remained more or less constant thereafter up to 24 h. Bakatula et al. (2014) stated that biosorption occurred in two steps: the first step corresponds to the dissociation of the complexes formed between metals in solution and water hydronium ions followed by the interaction of metal with algae functional groups.
Effect of pH on element biosorption efficiency
It can be observed from Table 1 that the biosorption efficiency of the three investigated metals increases with increasing pH value. The optimum pH for biosorption of Al3+, Fe3+, and Zn2+ are 4, 3, and 6, respectively, in case of the three used algal biomasses.
Both the ionic forms of the metal ions in solution and the electrical charges of the algal cell wall components depend on the solution pH (Aboulsoud 2008). The low degree of biosorption at low pH values can be explained by the fact that at low pH, the H+ ion concentration is high, and therefore, protons can compete with the metal cations for surface sites (Sarı and Tuzen 2009).
Effect of initial metal concentration on biosorption of metals
As can be seen in Table 2, the metal biosorption efficiency increases with increasing the initial metal concentrations to give the highest removal values at 1000 mg L−1 where equilibrium is attained (the optimum concentration).
This attitude may be interpreted by the increase of interaction probability between heavy metals and functional groups from biosorbent surface with increasing of initial concentration of studied metal ions (Bulgariu and Bulgariu 2014). The binding sites at the cell wall of the alga seemed to become saturated with the metal ions at high concentration, which led to a corresponding steadiness in the efficiency of the metal uptake (Elrefaii et al. 2012).
Effect of temperature on metal biosorption
The biosorption of metals is investigated as a function of temperature ranging from 298 to 333 K (25–60 °C). Working above this range of temperature is avoided at high temperatures, the structure of the biomass might be changed and the active sites destroyed (Aboulsoud 2008).
As shown in Table 2, the metal biosorption is slightly increased with rise in temperature from 298 to 333 K showing a slight endothermic nature. In general, the formation of coordination complexes between transition metal cations and carboxylate ligands is endothermic (Vilar et al. 2005). Although there is no significant difference between biosorption efficiency at 40° and 60 °C, 40 °C was chosen as the optimum temperature for metal biosorption as it is as similar as possible to the Egyptian climatic conditions.
Thermodynamic studies
Figure 1 shows the Van’t Hoff plots of ln K c versus 1/T, and Table 3 shows the thermodynamic parameters that are calculated from the slopes and intercepts of the straight lines.
The positive values of (∆G) indicated the nonspontaneous nature of adsorption for the three tested metals using all algal biomasses (Aksu 2002). The positive values of (∆H) show the endothermic nature of the biosorption, which is an indication of the existence of a strong interaction between algal biomasses and the three metals under study. The positive enthalpy of adsorption obtained indicates chemical adsorption. This suggests that the chemical bonds between the algal surface and the metal molecules are strong enough and the metal molecules cannot be easily desorbed by physical means such as simple shaking or heating (Farah and El-Gendy 2013). The negative values of (∆S) show the decreased randomness at the solid/solution interface during the biosorption of the metals on algal biomass, reflecting that the metal molecules were orderly adsorbed on the surface of the algal biomasses (Saibaba and King 2013).
Summarizing these results, the biosorption mechanism of the three studied metals is nonspontaneous, endothermic, chemical, and orderly adsorption on the surface of the algal surface.
Biosorption isotherm modeling
As can be observed from Table 3 and Figs. 2 and 3, the calculated (Qe) values match with the experimental ones (qe (exp)) that are determined in the level of high concentrations. These results reflect the applicability of biosorption in the treatment of water samples that contain low and high concentrations of metals. On the other hand, K L values indicate the adsorption affinity of the binding sites where the good sorption is indicated by low values of Langmuir parameter (Kumar et al. 2005). This assumption is confirmed as the higher (KL) calculated values are found to be inversely proportional to the actual high biosorption capacities (qe (exp)) and vice versa.
The values of Freundlich constant (n) that are greater than unity indicate the favorability of the algal biomass to biosorb metals from water. Also, the higher adsorption capacity (KF) indicates the strong electrostatic attraction force (Kumar et al. 2005); therefore, the higher (KF) calculated values are found to be proportional to the actual high biosorption capacities (qe (exp)) and vice versa.
The best-fit equilibrium model was determined based on the linear regression correlation coefficient (R 2). It can be reported that Freundlich isotherm model fitted slightly better than the Langmuir model in case of divalent Zn biosorption; meanwhile, Langmuir model fitted better in case of trivalent Al and Fe. This finding indicates that probably the sorption of Zn2+ was a multilayer coverage (heterogeneous sorption); meanwhile, the sorption of Al3+ and Fe3+ was monolayer coverage (homogeneous sorption). This finding agrees with Sarı and Tuzen (2009) and Bakatula et al. (2014) where they reported the best fitting of Langmuir model in case of Al3+ and Fe3+ biosorption using the brown alga Padina pavonica and the green alga Oedogonium sp., respectively. On the other hand, Liu et al. (2009) reported the best fitting of Freundlich model in case of Zn biosorption using the brown alga Saccharina (Laminaria) japonica.
The three algal biomass affinity to heavy metal biosorption follows the same sequence of Zn2+ > Fe3+ > Al3+. This affinity order may be attributed to the more or less decrease of ionic radii in the same sequence: 0.75, 0.55, and 0.53 Å, respectively (Wells 1975). As a general rule, there is a preferential binding of heavier ions to active sites which can be due to stereo-chemical effects; larger ions might better fit a binding site with two distinct active groups (Figueira et al. 2000). Another explanation may be conducted on the light of the equilibrium isotherm results in the current study that reveals the multilayer coverage of Zn2+ versus the monolayer coverage of Al3+ and Fe3+ that leads to higher Zn2+ biosorption.
Reusability and regeneration of algal biomasses
High numbers of cycles of biosorption-desorption-resorption that the biosorbent can achieve are desirable to make the process more economical. The reuse of the biomass and desorbent is an important feature for its possible utilization in continuous systems in industrial processes (Deng et al. 2006).
Figure 4 shows the desorption efficiency using 0.01 M Na2EDTA for four reuse cycles of sorption-desorption. After one reuse cycle, the desorption % in case of G. latifolium reaches 91.33, 91.94, and 93.89% for Al3+, Fe3+, and Zn2+, respectively. The desorption % in case of U. lactuca reaches 91.17, 92.02, and 91.66% for Al3+, Fe3+, and Zn2+, respectively. The desorption % in case of C. sinuosa reaches 90.65, 90.58, and 90.64% for Al3+, Fe3+, and Zn2+, respectively. Similar results were obtained by Deng et al. (2006) where 0.01 M Na2EDTA recovered 94.7% of Cu2+ and 82.5% of Pb2+ bound to the green biomass Cladophora fascicularis but within 18 h.
The desorption efficiency decreases with increasing cycle number, as after four reuse cycles reach 62.15, 68.37, and 68.2% for Al3+, Fe3+, and Zn2+, respectively, in case of G. latifolium, while the desorption % in case of U. lactuca reaches 65.34, 70.01, and 67.1% for Al3+, Fe3+, and Zn2+, respectively. The desorption % in case of C. sinuosa reaches 65.29, 65.82, and 66.53% for Al3+, Fe3+, and Zn2+, respectively.
Figure 5 shows the resorption efficiency of Al3+, Fe3+, and Zn2+ for four reuse cycles of desorption-resorption. After one reuse cycle, the highest resorption % reaches 77.4, 76.88, and 77.17% in case of C. sinuosa for Al3+, Fe3+, and Zn2+, respectively. Then, the middle resorption % reaches 76.09, 75.7, and 76.59% in case of U. lactuca for Al3+, Fe3+, and Zn2+, respectively. The lowest resorption % reaches 73.96, 75.04, and 75.07% in case of G. latifolium for Al3+, Fe3+, and Zn2+, respectively.
The resorption efficiency decreases with increasing cycle number, as after four reuse cycles, the highest resorption % reaches 51.28, 51.63, and 53.28% for Al3+, Fe3+, and Zn2+, respectively, using C. sinuosa. Then, the middle resorption % reaches 49.09, 49.14, and 51.98% in case of U. lactuca for Al3+, Fe3+, and Zn2+, respectively. The lowest resorption % reaches 45.16, 48.16, and 48.98% in case of G. latifolium for Al3+, Fe3+, and Zn2+, respectively. Similar results were obtained by Aboulsoud (2008) who stated that resorption % after five reuse cycles reached 45.28% using Na2EDTA.
As can be seen, both of desorption and resorption efficiencies decrease with increasing the reusability cycle number. A possible cause of reduction in the biosorption capacity of algal biomass can be attributed to the adverse effect of the eluent on the binding sites of the algal cell wall components (Tüzün et al. 2005). Also, accumulation of remaining metal molecules inside the algal biomass in each cycle acts to decrease the further resorption efficiency in the next cycle. The algal biomasses can be reused up to four cycles with a reasonable efficiency in all cases.
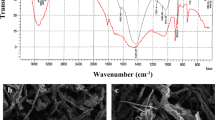
Examination of algal biomass surface using scanning electron microscope
The SEM micrographs of the biosorbent material before and after metal biosorption are illustrated in Fig. 6. By comparing with the surface of the raw biomasses between algal biomasses, it is clear that each algal type shows a unique surface structure. The surface of the red alga G. latifolium appears smooth and non-porous, the green alga U. lactuca poses a papillary surface structure supplying a large exposed surface area for biosorption; meanwhile, the brown algal biomass C. sinuosa surface is stratified in shape and extensively papillary providing a larger exposed surface area for biosorption.
It is obviously seen that the morphological changes in the cell wall surface due to metal biosorption. The red algal biomass G. latifolium is ruptured due to loading with Al3+; meanwhile, little cavities appeared due to Fe2+ loading and the surface appeared wrinkled in case of Zn2+ loading. The green algal biomass U. lactuca shows a surface protuberance in case of Al3+ loading; also, Fe2+-loaded biomasses have a plump shape surface; meanwhile, Zn2+ loading shows a more protuberance accompanied with wrinkling of surface. The brown algal biomass C. sinuosa surface becomes rough due to Al3+ loading while preserving of the stratified structure; oppositely, the stratified algal surface changes and shows large cavities due to the Fe2+ loading and becomes ruptured due to Zn2+ loading.
The morphology change according to Tamilselvan et al. (2013) might be due to the binding of metals in the surface of the biomass. Moreover, this change is probably caused due to strong cross-linking due to the chemisorption (concluded from thermodynamic studies) between the metals’ molecules and the active groups in the cell wall matrix.
Similar results were obtained by Arief et al. (2008), Natarajan et al. (2011), and Shukla and Shukla (2013).
Fourier transform infrared analysis of algal biomasses
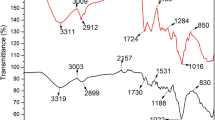
Table 4 shows the FTIR spectra of raw algal biomasses and loaded ones by metals under investigation. The FTIR spectra are in the range of 400–4000 cm−1. It exhibits absorption bands indicating the presence of C–N–S, alcoholic C–O, S=O, carboxylic C–O, carboxylic C=O, NH, and OH groups. The frequencies of functional groups can be firmly discussed as follows:
-
C–N–S group:
The spectra of C–N–S display transmittance band at 474, 470, and 532 cm−1 in case of the red algal biomass G. latifolium, the green algal biomass U. lactuca, and the brown algal biomass C. sinuosa, respectively. After metal biosorption, these bands are shifted to wavenumbers ranging from 459 to 597 cm−1 indicating the participation of C–N–S group in Al3+, Fe3+, and Zn2+ biosorption process especially in case of the brown algal biomass where the band shift is acute and more obvious. Bulgariu and Bulgariu (2014) in their study on U. lactuca stated the thiocyanate (C–N–S) shearing due to polypeptide structure of algae cells at 574–464 cm−1.
-
C–O of alcoholic group:
The spectra of C–O of alcoholic group display transmittance band at 1114 cm−1 for the three algal biomasses. Only in G. latifolium, these bands are shifted after metal biosorption to lower wavenumbers is ranged from 1072 to 1076 cm−1 indicating the participation of C–O of alcoholic group in Al3+, Fe3+, and Zn2+ biosorption process. Meanwhile, no band shift is observed in case of green and brown algal biomasses in case of all metals under study revealing that C–O of alcoholic group is not participated in such cases. Bulgariu and Bulgariu (2014) in their study on U. lactuca also reported C–OH band at 1031 cm−1.
-
S=O of sulfonate group:
The spectra of S=O of sulfonate group display transmittance band at 1284, 1280, and 1280 cm−1 in G. latifolium, U. lactuca, and C. sinuosa, respectively. Only in case of Fe3+ biosorption by the three algal biomasses, these bands are shifted to lower wavenumbers ranging from 1261 to 1203 cm−1 indicating the participation of S=O of sulfonate group in Fe3+ biosorption process. The band shift is acute and more obvious in C. sinuosa. No shifting is observed after Al3+and Zn2+ biosorption reflecting that this group is not contributed in their biosorption by the three algal biomasses. It is worth to mention that Figueira et al. (1999) reported the contribution of sulfonate group in removal of Fe3+ by the brown algal biomass Sargassum fluitans. Fakhry (2013) observed absorbance band at 1200 cm−1 corresponding to the sulfonate (–OSO3) group in the alga Padina pavonica.
-
Carboxylic C–O:
The spectra of C–O of carboxylic group display transmittance band at 1411, 1404, and 1411 cm−1 in G. latifolium, U. lactuca, and C. sinuosa, respectively. After Al3+, Fe3+, and Zn2+ biosorption, these bands are shifted to lower wavenumber of 1384 cm−1 for all algal biomasses indicating the participation of C–O of carboxylic group in Al3+, Fe3+, and Zn2+ biosorption process. Aboulsoud (2008) observed absorbance band at 1411 cm−1 corresponding to the C–O of carboxylic group in biomass of Sargassum hornschuchii.
-
Carboxylic C=O:
The spectra of C=O of carboxylic group display transmittance band at 1635, 1631, and 1631 cm−1 in G. latifolium, U. lactuca, and C. sinuosa, respectively. After Al3+, Fe3+, and Zn2+ biosorption by the three algal biomasses, these bands are shifted to higher wavenumbers that are ranged from 1627 to 1639 cm−1 indicating the participation of C=O of carboxylic group in Al3+, Fe3+, and Zn2+ biosorption process. This finding agrees with the thermodynamic studies in the present study where the biosorption process was proven to be endothermic in nature, as Vilar et al. (2005) stated that the formation of coordination complexes between transition metal cations and carboxylate ligands is endothermic. Fakhry (2013) observed absorbance band at 1670 cm−1 corresponding to the C=O of carboxylic group in the alga Padina pavonica.
-
NH group:
The spectra of NH group display transmittance band at 3417, 3414, and 3444 cm−1 in G. latifolium, U. lactuca, and C. sinuosa, respectively. After Al3+and Zn2+ biosorption by the three algal biomasses, these bands are shifted to wavenumber of 3421 cm−1 indicating the participation of NH group in Al3+ and Zn2+ biosorption process. Meanwhile, no shift is observed in case of Fe3+ biosorption reflecting that this group is not contributed in its biosorption by the three algal biomasses. Fakhry (2013) observed absorbance band at 3400 cm−1 corresponding to the NH group in P. pavonica.
-
OH group:
The spectra of OH group display transmittance band at 3645, 3749, and 3749 cm−1 in G. latifolium, U. lactuca, and C. sinuosa, respectively. After Al3+, Fe3+, and Zn2+ biosorption, these bands are shifted to wavenumbers that are ranged from 3946 to 3973 cm−1 in case of G. latifolium and C. sinuosa indicating the participation of OH group in Al3+, Fe3+, and Zn2+ biosorption process. No band shift is observed in case of Al3+, Fe3+, and Zn2+ biosorption by U. lactuca reflecting that this group does not contribute metal biosorption by this algal type. Jones (2000) reported the absorbance band of OH group at 3600–3700 cm−1.
Application of biosorption optimum conditions in the treatment of real polluted industrial wastewater samples from Borg El-Arab region
The batch experiment results show that the brown algal biomass is an effective and economical biosorbent material for the removal and recovery of heavy metal ions. The highest biosorption efficiency reached 63.78, 65.95, and 111.57 mg g−1 for Al3+, Fe3+, and Zn2+, respectively, using C. sinuosa under the optimum biosorption conditions (initial element concentration, 1000 mg L−1; temperature, 40 °C; contact time, 1 h; pH, 4, 3, and 6 for Al3+, Fe3+, and Zn2+, respectively); thus, it is applied in removal of heavy metals and trace elements from 21 industrial wastewater samples under the optimum concluded conditions. The quality of wastewater samples was assessed according to the Egyptian Law No. 4 of 1994 promulgating the environment Law and its amendments in Law No. 9 of 2009. Only the violating concentrations are focused in the following discussion.
In the textile dyeing sector, the violating concentrations reached 14.11, 26.84, and 1.35 mg L−1 for Zn, Fe, and Mn, respectively, regarding to the guidelines of 5, 1.5, and 1.00 mg L−1, respectively. These concentrations were lowered to reach 1.87, 1.25, and 0.438 mg L−1 by removal percentage of 86.68, 95.33, and 67.6%, respectively. These violating concentrations of Fe and Mn may result from the “metallization” process during the textile dyeing using azodyes (Straley and Fisher 1959). On the other hand, most used dyes contain Zn or other metal atoms in their composition; thus, Zn concentration in dyeing wastewater (acid dyes on wool) reaches 3.4 mg L−1 as mentioned by Bisschops and Spanjers (2003); therefore, the high result of Zn concentration probably originated from the dye residues in wastewater.
In the food production sector, the violating concentrations reached 39.03, 84.11, and 83.58 mg L−1 for Al, Fe, and Mn, respectively (biscuits production factory). This is regarding to the Egyptian guidelines of 3, 1.5, and 1.00 mg L−1 for Al, Fe, and Mn, respectively. These concentrations were lowered to reach 12.67, 9.38, and 4.391 mg L−1 by removal percentage of 67.54, 88.84, and 94.75%, respectively. It is noticed that food industrial wastewater is the most polluted effluent among the factories under study. High Al concentration is mostly due to the residue of baking powder (containing the additive sodium aluminum phosphate) that was reported to contain 2.3 g Al (100 g)−1 (Orme and Ohanian 1990). On the other hand, Fe is extremely in high concentration which is probably due to the “fortification” process of some food staff with iron as it is regularly added to food in many countries to prevent iron deficiency anemia. For example, Fe concentration in different biscuits reaches 15.96 mg (100 g)−1 as reported by Doner and Ege (2004). Meanwhile, Mn is one of the essential minerals and a main component of plenty of food staff. For example, Mn concentration reaches 2.79 mg (100 g)−1 in tea biscuits (Vitali et al. 2008).
In the oil production sector, only Fe concentration reached 2.436 mg L−1 and was violating the guidelines of 1.5 mg L−1. This concentration was lowered to be 0.671 mg L−1 (72.45 removal %). For many years, Fe is used in the form of FeCl3 as coagulant in demulsifying oily wastes (Abo-El Ela and Nawar 1980).
In the paper and carton production sector, the violating concentrations reached 3.135, 2.818, and 0.6241 mg L−1 for Al, Fe, and Ni, respectively. This is in regard to the guidelines of 3, 1.5, and 0.10 mg L−1 for Al, Fe, and Ni, respectively. Their concentrations were lowered to reach 0.964, 0.873, and 0.072 mg L−1 by removal percentage of 69.25, 68.8, and 88.42%, respectively. As manufacturing of paper is actually a proprietary activity which is strictly protected by each manufacturer, it is difficult to say where certain elements come from, taking into account that the used additives are not of high chemical purity, so it is possible that some elements are introduced as impurities. It is worth to mention that Fe and Ni are main components in paper composition as their concentrations reach 753.4 and 55 mg kg−1, respectively (Rozic et al. 2005).
In the spinning and textile production sector, only Mn concentration was violating the guidelines reaching 3.833 mg L−1 regarding to 1.00 mg L−1 as guideline concentration. Its concentration was lowered to reach 0.056 mg L−1 by 98.54 removal %. This concentration of Mn may result from the metallization process during textile dyeing (Straley and Fisher 1959).
In the soap production sector, four elements, Al, Fe, Mn, and Zn, were exceeding the guidelines as their concentrations reached 9.09, 31.93, 3.76, and 23.67 mg L−1, respectively, with regard to the guidelines of 3, 1.5, 1.00, and 5 mg L−1, respectively. Their concentrations were lowered to reach 3.21, 2.35, 0.87, and 3.21 mg L−1 by removal percentage of 64.6, 92.63, 76.84, and 86.42%, respectively. Al and Fe may result from alum and FeCl3 that are used in demulsifying oily wastes (Abo-El Ela and Nawar 1980), whereas Mn was reported to be a constituent of soap composition and was found in soap effluent by 0.351 mg L−1 (Faremi and Oloyede 2010). Also, Zn is involved in the composition of soap in the form of zinc pyrithione and zinc carbonate as antimicrobial agent and discoloration inhibitor, respectively (Smith et al. 2012).
In the metals, steel, and concrete production sector, the violating concentrations reached 21.37, 4.388, and 5.01 mg L−1 for Mn, Al, and Fe, respectively. This is regarding to the guidelines of 1, 3, and 1.5 mg L−1, respectively. Their concentrations were lowered to reach 0.45, 0.93, and 0.87 mg L−1 by removal percentage of 97.86, 78.67, and 82.5%, respectively. The cooling water resulted during steel manufacturing was reported to be polluted with different metals such as Mn (Strugariu and Heput 2012).
In the wood production sector, Fe and Zn concentrations in sample reached 7.743 and 26.22 mg L−1 comparing with the Egyptian guidelines of 1.5 and 5 mg L−1, respectively. Their concentrations were lowered to reach 1.471 and 2.65 mg L−1 by removal percentage of 81.00 and 89.89%, respectively. Fe may release in wastewater as a result of cellular degradation of wood, where the brown rot fungi produce extracellular H2O2 reacts with Fe from wood itself leading to production of Fenton’s reagent “Fe++ + H2O2” (Xu and Goodell 2001). On the other hand, many Zn compounds are used as wood preservatives (Goettsche and Reuther 1993).
In the car filter production sector, Cr, Fe, and Zn concentrations were violating the guidelines of 1.00, 1.5, and 5 mg L−1 where they reached 10.87, 5.606, and 34.96 mg L−1, respectively. These concentrations were lowered to reach 0.89, 1.33, and 4.78 mg L−1 by removal percentage of 91.79, 76.24, and 86.32%, respectively. These three contaminants are among the main components in this industrial sector (Kulekci 2008; Abdullah 2010 and WQA 2013).
In the cosmetics production sector, only Fe concentration is violating the guidelines of 1.5 mg L−1 where it reached 3.372 mg L−1. This concentration was lowered to be 0.82 mg L−1 (75.65 removal %). The source of Fe may be the iron oxide that used in skin cosmetics as dispersible agent (Yoshio and Tadashi 1995).
In the pharmaceutical production sector, only Zn concentration is greater than the guidelines of 5 mg L−1 where it reached 8.144 mg L−1. This concentration was lowered to be 1.38 mg L−1 (83.06 removal %). Zn is a component of multivitamin drugs and reported to reach 2.9 mg L−1 in wastewater of pharmaceutical industry (Tisler and Koncan 1999).
Borg El-Arab city includes 9 residential areas and 4 industrial zones. All the domestic sewage as well as the industrial wastewater is primary treated at an oxidation pond treatment station (Hussein et al. 2005). Concentrations of Al, Fe, and Zn in the collective industrial wastewater reached 11.29, 8.076, and 17.29 mg L−1 comparing with the Egyptian guidelines of 3, 1.5, and 5 mg L−1, respectively. These concentrations were lowered to reach 2.167, 1.14, and 4.37 mg L−1 by removal percentage of 80.81, 85.85, and 74.72%, respectively. On the other hand, concentrations of Al and Fe in the oxidation pond reached 6.156 and 7.894 mg L−1, respectively. Their concentrations were lowered to reach 1.23 and 1.34 mg L−1 by removal percentage of 80 and 83%, respectively.
It was achieved that all the violating concentrations of heavy metals are lowered to be less than the guidelines except of Al and Fe in soap production factory that are lowered from 9.091 to 3.218 mg L−1 (by 64.6 removal %) and from 31.93 to 2.354 mg L−1 (by 92.63 removal %), respectively; and Al, Fe and Mn in food production factory that are lowered from 39.03 to 12.67 mg L−1 (by 67.54 removal %), from 84.11 to 9.387 mg L−1 (by 88.84 removal %) and from 83.58 to 4.391 mg L−1 (by 94.75 removal %), respectively. However, the violating concentrations are lowered to a great extent, but the incomplete removal of heavy metals in these two samples may be attributed to the very high concentrations of the violating metals that are accompanied with low pH values (3.3 and 3.8 for sample Nos. 18 and 20, respectively), where H+ competes with heavy metal ions for the algal biomass active sites.
In general, the removal efficiency for the determined elements reached 80.81, 38.25, 91.79, 59.96, 95.33, 98.54, 27.39, 88.42, 36.59, and 96.98% for Al, Co, Cr, Cu, Fe, Mn, Mo, Ni, V, and Zn, respectively, through all studied samples.
Conclusions
The studied algal biomasses are natural, low-cost, reusable, and eco-friendly tool showing promising results in the field of water treatment and removal of heavy metals. Biosorption of metals by the algal biomass followed by the recovery achieves four aims: firstly, improving quality of water resources and elimination of the environmental pollution, secondly recovery of the removed metals, thirdly let the algal biomass application is not for one use but is used for many times till four cycles of sorption-desorption with a reasonable capacity, and fourthly secure the final getting rid of the algal biomass after exhaustion, as the further decomposition of died algal biomass will not lead to release of the metals again into the environment.
References
Abdullah SB (2010) Heavy metals removal from industries wastewater by using seaweed through biosorption process. Ph.D. Thesis, Faculty of Civil Engineering & Earth Resources, University Malaysia Pahang, Malaysia
Abo-El Ela SI, Nawar SS (1980) Treatment of wastewater from an oil and soap factory via dissolved air flotation. Environ Inter 4:47–52
Aboulsoud YIEM (2008) Removal of certain heavy metals by biomaterials derived from some Egyptian algae. M.Sc. Thesis, Faculty of Science, Ain Shams University, Egypt
Ahalya N, Ramachandra TV, Kanamadi RD (2003) Biosorption of heavy metals. Res J Chem Environ 7:71–79
Aksu Z (2002) Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto Chlorella vulgaris. Process Biochem 38:89–99
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026
Aleem AA (1993) Marine algae of Alexandria, Egypt. 138 pp., 55 plates
Arief VO, Trilestari K, Sunarso J, Indraswati N, Ismadji S (2008) Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: characterization, biosorption parameters and mechanism studies. Clean 36:937–962
Bakatula EN, Cukrowska EM, Weiersbye IM, Mihaly-Cozmuta L, Peter A, Tutu H (2014) Biosorption of trace elements from aqueous systems in gold mining sites by the filamentous green algae (Oedogonium sp.) J Geochem Explor 144:492–503
Bisschops I, Spanjers H (2003) Literature review on textile wastewater characterization. Environ Technol 24:1399–1411
Bulgariu L, Bulgariu D (2014) Enhancing biosorption characteristics of marine green algae (Ulva lactuca) for heavy metals removal by alkaline treatment. J Bioprocess Biotech 4:146
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Deng L, Su Y, Su H, Wang X, Zhu X (2006) Biosorption of copper (II) and lead (II) from aqueous solutions by nonliving green algae Cladophora fascicularis: equilibrium, kinetics and environmental effects. Adsorption 12:267–277
Doner G, Ege A (2004) Evaluation of digestion procedures for the determination of iron and zinc in biscuits by flame atomic absorption spectrometry. Analyt Chim Acta 520:217–222
Elrefaii AH, Sallam LA, Hamdy AA, Ahmed EF (2012) Optimization of some heavy metals biosorption by representative Egyptian marine algae. J Phycol 48:471–474
Fakhry EM (2013) Padina pavonica for the removal of dye from polluted water. Am J Plant Sci 4:1983–1989
Farah JY, El-Gendy NS (2013) Performance, kinetics and equilibrium in biosorption of anionic dye acid red 14 by the waste biomass of Saccharomyces cerevisiae as a low-cost biosorbent. Turkish J Eng Env Sci 37:146–161
Faremi AY, Oloyede OB (2010) Biochemical assessment of the effects of soap and detergent instrumental effluents on some enzymes in the stomach of albino rats. Res J Environ Toxicol 4:127–133
Figueira MM, Volesky B, Mathieu HJ (1999) Instrumental analysis study of iron species biosorption by Sargassum biomass. Environ Sci Technol 33:1840–1846
Figueira MM, Volesky B, Ciminelli VST, Roddick FA (2000) Biosorption of metals in brown seaweed biomass. Water Res 34:196–204
Goettsche R, Reuther W (1993) Wood preservative based on polymeric nitrogen compounds and metal-fixing acids. US Patent 5:186,947
He J, Chen PJ (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour Technol 160:67–78
Hussein RA, El-Sebaie OD, El-Sharkawy FM, Mahmoud AH, Ramadan MH (2005) Assessment of the waste stabilization pond performance, new Borg el-Arab city. JEPHAss 80:1–25
Jones RW (2000) Infrared technology, vol 14, ECT 4th edn. Ames Laboratory, United States Department Of Energy (USDOE), Washington, pp 379–416
Kanwal F, Rehman R, Mushtaq MW, Batool A, Naseem S (2013) Use of Opuntia dillenii seeds for sorptive removal of acidic textile dyes from water in benign way. Asian J Chem 25:7710–7714
Kulekci MK (2008) Magnesium and its alloys applications in automotive industry. Int J Adv Manuf Technol 39:851–865
Kumar KV, Sivanesan S, Ramamurthi V (2005) Adsorption of malachite green onto Pithophora sp., a fresh water algae: equilibrium and kinetic modeling. Process Biochem 40:2865–2872
Liu Y, Cao Q, Luo F, Chen J (2009) Biosorption of Cd2+, Cu2+, Ni2+ and Zn2+ ions from aqueous solutions by pretreated biomass of brown algae. J Hazard Mater 163:931–938
Natarajan ST, Jayaraj R, Thanaraj PJ, Prasath PMD (2011) The removal of heavy metal chromium (VI) from aqueous solution by using marine algae Gracilaria edulis. J Chem Pharm Res 3:595–604
Orme J, Ohanian E (1990) Assessing the health risks of aluminum. Environ Geochem Health 12:55–58
Rozic M, Macefat MR, Orescanin V (2005) Elemental analysis of ashes of office papers by EDXRF spectrometry. Nucl Instru Meth Physics Res B 229:117–122
Saibaba NKV, King P (2013) Equilibrium and thermodynamic studies for dye removal using biosorption. IMPACT: IJRET 1(3):17–24
Salima A, Benaouda B, Noureddine B, Duclaux L (2013) Application of Ulva lactuca and Systoseira stricta algae-based activated carbons to hazardous cationic dyes removal from industrial effluents. Water Res 47:3375–3388
Sarı A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on aluminum biosorption from aqueous solution by brown algae (Padina pavonica) biomass. J Hazard Mater 171:973–979
Shukla PM, Shukla SR (2013) Biosorption of cu(II), Pb(II), Ni(II), and Fe(II) on alkali treated coir fibers. Sep Sci Technol 48:421–428
Smith E, Wang J, Wang X, Jiang C (2012) Bar soap comprising pyrithione sources. US Patent Appl 2012/0220516A1
Straley JM, Fisher JG (1959) Metallizable Azo dyes prepared from a benzothiazole derivative and β-naphthol. US Patent 2:875,190
Strugariu ML, Heput T (2012) Monitoring results on industrial wastewater pollutants in steel industry. Acta Technica Corviniensis- Bulletin of Engineering, Faculty of Engineering, Hunedoara, Romania, October–December 33–36
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions - a review. Bioresour Technol 99:6017–6027
Tamilselvan N, Hemachandran J, Thirumalai T, Sharma VC, Kannabiran K, David E (2013) Biosorption of heavy metals from aqueous solution by Gracilaria corticata varcartecala and Grateloupia lithophila. JCLM 1:102–107
Tisler T, Koncan JZ (1999) Toxicity evaluation of wastewater from the pharmaceutical industry to aquatic organisms. Water Sci Tech 39:71–76
Tüzün İ, Bayramoğlu G, Yalçın E, Başaran G, Çelik G, Arıca MY (2005) Equilibrium and kinetic studies on biosorption of hg(II), cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. J Environ Manag 77:85–92
Vilar VJP, Botelho CMS, Boaventura RAR (2005) Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Process Biochem 40:3267–3275
Vitali D, Dragojevic IV, Sebecic B (2008) Bioaccessibility of Ca, mg, Mn and cu from whole grain tea-biscuits: Impact of proteins, phytic acid and polyphenols. Food Chem 110:62–68
Vitor V, Corso CR (2008) Decolorization of textile dye by Candida albicans isolated from industrial effluents. J Ind Microbiol Biotechnol 35:1353–1357
Waller RA, Duncan DB (1969) A Bayes rule for the symmetric multiple comparisons problem. J Amer Stat Assoc 64:1484–1503
Wells AF (1975) Structural inorganic chemistry, 4th edn. Oxford Univ. Press, Oxford 259p
WQA (Water Quality Association) (2013) National primary drinking water standards and treatment methods. [Online], Retrieved March 1, 2013, from http://www.wqa.org/consumer/alltables.cfm?SubTitleID=1&MainTitleID=1
Xu G, Goodell B (2001) Mechanisms of wood degradation by brown-rot fungi: chelator-mediated cellulose degradation and binding of iron by cellulose. J Biotechnol 87:43–57
Yoshio S, Tadashi F (1995) Skin cosmetics. European Patent. EP0612516
Zinova AD (1967) Key of green, brown and red algae of southern seas of USSR. Prin. Nauka Acad. Nauk USSR, 310pp
Acknowledgements
Special gratitude is expressed to sewage Company of Alexandria and New Borg El-Arab city Authority for sincere help and cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaaban, AS.M., Badawy, R.K., Mansour, H.A. et al. Competitive algal biosorption of Al3+, Fe3+, and Zn2+ and treatment application of some industrial effluents from Borg El-Arab region, Egypt. J Appl Phycol 29, 3221–3234 (2017). https://doi.org/10.1007/s10811-017-1185-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1185-4