Abstract
Algae of the genus Nannochloropsis are attractive organisms for use in biotechnology due to their high lipid content. Genetic manipulation of marine Nannochloropsis species has already been reported; however, tools have not yet been developed to transform Nannochloropsis limnetica, the only known freshwater species of this genus. To establish N. limnetica as a model laboratory strain, we first tested the effects of 11 different antibiotics on growth of N. limnetica and the marine species N. oceanica and N. gaditana. These three microalgae responded very differently to antibiotic treatments, both in liquid cultures and on agar plates. In general, N. limnetica exhibited a much higher sensitivity to antibiotics than the marine strains, thus offering the potential for a large set of antibiotic resistance genes that may be applicable as artificial selection markers after transformation. We also developed a simple protocol using lysozyme to obtain high yields of viable N. limnetica protoplasts, as confirmed by flow cytometry and electron microscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Algae are promising organisms for use as cell factories for commercially valuable compounds such as fatty acids that can be esterified for biodiesel (Singh and Dhar 2011; Pereira et al. 2012) or used in other products (Lubián et al. 2000; Borowitzka 2013). The genus Nannochloropsis (Eustigmatophyceae) belongs to a group of algae that are attractive for the biotech industry as they accumulate fatty acids to high concentrations (Adarme-Vega et al. 2012; Ma et al. 2014). When cultured under normal growth conditions and in standard growth medium supplemented with nitrogen, the oil content in Nannochloropsis cells reaches about 30% of dry weight (Gouveia and Oliveira 2009; Rodolfi et al. 2009). This level can be further increased to 50–70% by cultivating under nitrogen limitation (Hu and Gao 2006; Rodolfi et al. 2009; Recht et al. 2012). The main drawback of nitrogen deprivation, however, is a slow growth rate, causing a significant decrease in biomass production (Hu and Gao 2006). Generally, it appears to be very difficult to achieve a profitable production system with selected algal species under optimized growth conditions (Aguirre et al. 2013; Mühlroth et al. 2013; Ho et al. 2014).
Current methods in molecular genetics potentially offer all tools needed to reprogram regulatory networks in Nannochloropsis cells in order to increase oil yield without a drastic reduction in biomass productivity. Indeed, genetic engineering has already been successfully employed to modify lipid production in several algal species (Dunahay et al. 1996; Radakovits et al. 2010), including Chlamydomonas reinhardtii (Li et al. 2010), Phaeodactylum tricornutum (Radakovits et al. 2011), Chlorella pyrenoidosa (Ramazanov and Ramazanov 2006), and Nannochloropsis oceanica (Kaye et al. 2015). The genomes of the marine species N. oceanica and N. gaditana have been recently sequenced, and nuclear transformation methods have been established by random incorporation or homologous recombination (Radakovits et al. 2012; Vieler et al. 2012; Kilian et al. 2011). However, nothing is known about the transformability of the only freshwater species of this genus, N. limnetica, and the genome of this alga has not yet been published. The production of polyunsaturated fatty acids by N. limnetica is very high in comparison to many other freshwater algae (Krienitz and Wirth 2006), and recent data indicate that when cultivated in marine medium, N. limnetica shows a similar fatty acid profile as marine Nannochloropsis species (Ma et al. 2014).
Establishing genetic tools for N. limnetica would increase the potential value of this alga in biotechnological applications, and it could serve as a molecular model for freshwater Eustigmatophyceae. As carried out previously for N. gaditana, N. oceanica, and N. oculata (Chen et al. 2008; Radakovits et al. 2012; Vieler et al. 2012), a strain-specific transformation protocol needs to be developed. Transformability is usually tested using a plasmid harboring a selection marker (typically an antibiotic resistance gene) under the control of an endogenous promoter. Knowledge about the sensitivity of a given strain to various antibiotics is therefore essential in order to develop a system for efficient screening and selection of transformants.
In this work, we have carried out an extensive analysis of the sensitivity of Nannochloropsis species, including N. limnetica, to a range of antibiotics. For this purpose, we developed a reliable and quantifiable method to determine antibiotic susceptibility of microalgae, both in liquid cultures and on agar plates. As the transformation of algae often requires production of permeabilized cells (protoplasts) (Jarvis and Brown 1991; Coll 2006; Leon and Fernandez 2007; Chen et al. 2008), a simple protocol for the production of N. limnetica protoplasts is also presented.
Material and methods
Strains and cultivation conditions
Axenic Nannochloropsis limnetica KRIENITZ 1998/3 (CCALA 864, Institute of Botany CAS, Czech Republic) was maintained in Bourrelli-modified medium at a photon flux density of 100 μmol photons m−2 s−1 and 150-rpm shaking at 20 °C (Krienitz and Wirth 2006). Agar plates were prepared using the same medium supplemented with 1.5% Bacto agar (Difco) and maintained at 100 μmol photons m−2 s−1 at 20 °C.
Nannochloropsis oceanica CCMP1779 and Nannocchloropsis gaditana CCMP526 (National Center for Marine Algae and Microbiota, USA) were grown photoautotrophically in F/2 medium without silica and 50% seawater, and agar plates were prepared with 1.5% Bacto agar (Difco) in the same medium. Liquid cultures were maintained at a photon flux density of 80 μmol photons m−2 s−1 and 170-rpm shaking at 23 °C. Agar plates were maintained at the same temperature and light intensity.
Sensitivity to antibiotics in liquid culture
Antibiotic tests in liquid culture were carried out in 24-well cell culture plates (Costar). Every well contained 1 mL of the appropriate growth medium (Bourrelli modified for N. limnetica and F/2 for N. oceanica and N. gaditana) supplemented with different concentrations of antibiotics, ampicillin (20, 10, or 100 μg mL−1), kanamycin (20, 10, or 100 μg mL−1), chloramphenicol (10, 20, 100, or 200 μg mL−1), streptomycin (1, 5, 10, or 40 μg mL−1), zeocin (1, 5, 10, or 40 μg mL−1), hygromycin (10, 20, 50, or 100 μg mL−1), paramomycin (1, 5, 10, 40, or 60 μg mL−1), cycloheximide (1, 5, 10, or 40 μg mL−1), gentamicin (20, 100, or 200 μg mL−1), geneticin (5, 10, 20, 100, or 200 μg mL−1), or nourseothricin (10, 50, or 100 μg mL−1).
Nannochloropsis strains were inoculated at a final concentration of 1–2 × 107 cells mL−1, and each antibiotic test was repeated at least three times. Cultures were maintained under the conditions described earlier, with gentle shaking for 6 days. Growth rates were monitored by multiple measurements of absorbance at 750 nm (A750) every 2 days in a plate reader (Infinite 200 Pro Multimode Reader, Tecan, Switzerland).
Sensitivity to antibiotics on agar plates
Antibiotic tests on agar plates were carried out in 6-well cell culture plates (Costar). Every well contained 7 mL of the appropriate agar medium (Bourrelli modified for N. limnetica and F/2 with 50% artificial sea water for N. oceanica and N. gaditana). Plates were supplemented with different amounts of antibiotics, ampicillin (50 or 100 μg mL−1), kanamycin (50 or 100 μg mL−1), chloramphenicol (10, 20, or 50 μg mL−1), streptomycin (10 or 50 μg mL−1), zeocin (1, 5, or 20 μg mL−1), hygromycin (25, 50, 100, or 200 μg mL−1), paramomycin (5, 20, 40, or 80 μg mL−1), cycloheximide (5 or 20 μg mL−1), or gentamycin (20 or 100 μg mL−1).
Nannochloropsis strains were inoculated at a final concentration of 2 × 108 cells mL−1, and 150 μL of each culture was spread onto a plate. Each antibiotic test was repeated three times. Agar plates were kept for 20 days under the conditions described in “Material and methods” section. The effects of antibiotics on cell proliferation were monitored by visual comparison.
Protoplast production and flow cytometry
In order to produce protoplast from N. limnetica, a 50-mL culture of the algae was grown as described above. Three days after inoculation (exponential phase), cells were harvested by centrifugation for 10 min at 8500×g and washed twice with fresh Bourrelli-modified medium. After washing, cells were resuspended in 1 mL of ½ Bourrelli-modified medium supplemented with 0.3 M sorbitol, 0.3 M mannitol, and one out of the following three different enzyme solutions: A 6% hemicellulase and 3% driselase; B 2% lysozyme; and C 1% lysozyme, 2% hemicellulase, and 1% driselase. In the second round of experiments, 0.5, 1, 2, or 4% lysozyme were tested. Cultures were incubated at 20 °C with the enzyme solution in multiwell plates for 4 h with gentle shaking (~100 rpm). To stop enzymatic degradation of the cell wall, cells were collected from plates, harvested by centrifugation (5 min at 400×g), washed twice with ½ Bourrelli-modified medium supplemented with 0.3 M sorbitol and 0.3 M mannitol, and resuspended in 1 mL of the same medium.
Flow cytometry
To monitor cell permeability and viability, 1 mL of protoplast suspension was incubated with 6.7 μL of propidium iodide (PI; Sigma, 1 mg mL−1) or 10 μL of fluorescein diacetate (FDA; Sigma, 1 mg mL−1) for 15 min, then loaded into a flow cytometer (BD Influx), and 5000 events were recorded. Samples were excited by a 488-nm laser, and fluorescence emission was collected on a detector with a 610/20-nm filter for PI or 530/40-nm filter for FDA.
Transmission electron microscopy
Nannochloropsis limnetica and N. oceanica cultures in logarithmic phase of growth (108 cells mL−1) were harvested by centrifugation (5 min at 5000×g) and resuspended in one volume of the growth medium mixed 1:1 with 5% (v/v) glutaraldehyde fixative in 0.2 M cacodylate buffer pH 7.2. After 15 min of rotary shaking at room temperature, cells were transferred to 0.1 M cacodylate buffer containing 2.5% (v/v) glutaraldehyde and fixed overnight at 4 °C. Pelleted cells were washed with cacodylate buffer and post-fixed with 1% (w/v) osmium tetroxide for 2 h. Followed by washing steps with the same buffer, cells were dehydrated through a graded series of acetone, embedded in low-viscosity Spurr resin (EMS), and polymerized at 60 °C for 48 h. Ultrathin sections of 60 nm were cut using an ultramicrotome (UCT, Leica). Sections were collected on Formvar-coated copper grids and stained with 1% (w/v) aqueous uranyl acetate for 10 min and with Sato’s lead citrate for 3 min (Sato 1968). Prepared sections were examined in a JEOL 1010 transmission electron microscope (JEOL) equipped with a Mega View III camera (SIS). Acquired pictures were analyzed by ImageJ software (Abràmoff et al. 2004).
Results
Sensitivity of Nannochloropsis species to antibiotics—liquid cultures
In order to select appropriate antibiotics for genetic manipulation of Nannochloropsis species, we developed a method to test the growth of N. limnetica, N. oceanica, and N. gaditana in liquid cultures. Eleven different antibiotics, at various concentrations, were assessed (see “Material and methods” section). Results showed that in liquid culture N. limnetica was very sensitive to most of the antibiotics tested, particularly streptomycin, zeocin, paromomycin, cycloheximide, and geneticin (G418), causing a 55–70% reduction in growth at a concentration of 5 μg mL−1 (Figs. 1 and S1). Nannochloropsis limnetica was slightly less sensitive to hygromycin and nourseothricin, exhibiting about 50% growth reduction at an antibiotic concentration of 10 μg mL−1 (Table 1 and Fig. S1), whereas gentamicin at a concentration of 20 μg mL-1 caused about 60% reduction in growth (Table 1 and Fig. S1). Kanamycin and chloramphenicol did not have a strong effect on N. limnetica growth at low concentrations, but higher concentrations (100 and 200 μg mL−1) resulted in 50% reduction in growth in 6 days (Fig. S1). Ampicillin was the only antibiotic tested that had no effect on growth of N. limnetica. Cultures exposed to concentrations of 200 μg mL-1 ampicillin grew indistinguishably from the control (Table 1 and Fig. S1).
Growth inhibitory effects of various antibiotics on three Nannochloropsis species. Cell densities of N. limnetica (a), N. oceanica (b), and N. gaditana (c) cultures were monitored by A750 for 6 days with ampicillin (min/max concentration 20/200 μg mL−1), kanamycin (20/200), chloramphenicol (10/200), streptomycin (1/40), zeocin (1/40), hygromycin (10/100), paromomycin (1/40), cycloheximide (1/20), gentamicin (20/200), geneticin (1/20 for N. limnetica and 5/200 for N. oceanica and N. gaditana), or nourseothricin (10/100). Histograms indicate the mean ± standard deviations of three replicates. Values for additional concentrations of all tested antibiotics and all three species are provided in the supplementary material S1, S2, and S3
In comparison with N. limnetica, N. oceanica cells exhibited extreme sensitivity to geneticin and cycloheximide, with a 70% reduction in growth at antibiotic concentrations of 20 and 1 μg mL−1, respectively (Fig. S2). Other antibiotics, including streptomycin, zeocin, and paromomycin, caused considerable growth inhibition at relatively low concentrations, 35, 53, and 54% reduction, respectively, at 40 μg mL−1 (Fig. S2). On the other hand, N. oceanica was less sensitive to chloramphenicol, hygromycin, and nourseothricin, showing roughly half of the growth rate at standard concentrations of these antibiotics (100 μg mL−1; Table 1 and Fig. S2). Ampicillin, kanamycin, and gentamicin had almost no effect after 6 days at standard concentrations (100 μg mL−1; Table 1 and Fig. S2).
The majority of antibiotics tested did not significantly affect growth of N. gaditana (Fig. 1, Table 1, and Fig. S3). Cultures supplemented with 200 μg mL−1 of ampicillin, kanamycin, or gentamicin or 40 μg mL−1 paramomycin grew at rates the same as without antibiotics (Table 1 and Fig. S3). Lower concentrations of streptomycin, hygromycin, and nourseothricin had no effect, and even high concentrations showed only mild reductions in growth (Fig. S3). Although this alga seems to be resistant to chloramphenicol and geneticin, growth was reduced when the culture was treated with a high (100 μg mL−1) concentration (Fig. S3). In contrast to other antibiotics, N. gaditana was highly sensitive to zeocin and cycloheximide, even at very low concentrations (Figs. 1 and S3). Zeocin and cycloheximide were thus the only antibiotics tested that, at low concentrations, were potent enough to inhibit growth of all Nannochloropsis species (Table 1).
Sensitivity of Nannochloropsis species to antibiotics—agar plates
Our tests in liquid medium allowed us to analyze the effects of antibiotics in a quantitative, repetitive, and reliable way. However, to provide comprehensive information about the concentrations of antibiotics appropriate for selection of transformants, we also assessed the effects of the same antibiotics on agar plates. All three strains were inoculated on agar plates (see “Material and methods” section), and cell viability was monitored over a period of 20 days. Based on cell density and color, the following four categories of antibiotic effectiveness were assigned: (−−) cells turned white with a dramatic loss of chlorophyll and cell death, (−) low cell density with obvious chlorosis, (+) medium cell density with green colonies, and (++) high cell density with colonies resembling control cells. Day 14 was chosen to determine antibiotic concentrations that resulted in unhealthy or dead cells (−− category; Table 2).
Ampicillin, kanamycin, chloramphenicol, and streptomycin, each at the highest concentrations tested, had no clear effect on growth of any of the three algal species (Table 2). However, responses of these species to the remaining antibiotics differed. Nannochloropsis limnetica was very sensitive to zeocin, paromomycin, cycloheximide, and hygromycin, where a minimal dose caused cell death (Table 2), but gentamicin did not reduce growth even at a high concentration. In contrast, N. oceanica was very sensitive to zeocin but had medium sensitivity to hygromycin and paromomicin. Nannochloropsis gaditana was only sensitive to zeocin. Cycloheximide and gentamicin were not tested on the marine species (Table 2).
Protoplast production
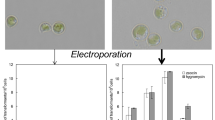
Production of protoplasts may be necessary, or at least helpful, in transformation (Gong et al. 2011; Qin et al. 2012; Schatz et al. 2013). Although the cell wall of N. limnetica is thinner than in N. oceanica (see supplementary material S4), this might still prevent transformation by electroporation. We therefore tested the capacity of the following three enzyme solutions to degrade the N. limnetica cell wall: A 6% hemicellulase and 3% driselase; B 2% lysozyme; and C 1% lysozyme, 2% hemicellulase, and 1% driselase. One of the effects that can be expected after enzymatic degradation of the cell wall is an increased permeability of the cell, as the cell membrane integrity could be affected as well. Permeability was measured by monitoring the penetration of PI, a DNA staining dye that is unable to cross an intact cell membrane/cell wall barrier. A non-treated sample without cell wall-degrading enzymes served as a negative control to establish the threshold below which the majority of cells do not show PI fluorescence. Figure 2 shows a representative experiment with the proportion of permeable cells after digestion with all three enzyme solutions. The histograms show the number of cells with different levels of PI fluorescence. In this particular experiment, the highest proportion of permeable cells (protoplasts) was obtained after incubation with 2% (w/v) lysozyme (Fig. 2c). This yielded the highest level of protoplasts in all three independent experiments, with an average of 66% permeable cells (Fig. 3). However, variability was high, probably due to several different factors including age and density of the culture, complete dissolution of the enzymes in the incubation medium, and variable flow cytometer measurements.
Permeabilization of N. limnetica cells after treatment with different enzyme cocktails. a Propidium iodide (PI) fluorescence of control cells; left panel represents the PI fluorescence according to cell size (FSC), and right panel represents the number of cells exhibiting the same PI fluorescence intensity. The proportion of permeabilized cells is shown above the bar. The red dashed line indicates a threshold point separating the intact, non-permeabilized cells (N) from the partially permeabilized protoplasts (P). b Alternation of PI fluorescence after digestion with a mixture of 6% hemicellulase and 3% driselase or with 2% lysozyme (c) or with a mixture of 1% lysozyme, 2% hemicellulase, and 1% driselase (d)
Proportion (%) of protoplasts obtained after digestion of the N. limnetica cell wall with three different enzymatic cocktails. The data were calculated from the PI fluorescence recorded in three independent experiments. Asterisks indicate the statistically significant differences, as tested using a paired Student’s t test (P = 0.02)
To optimize the lysozyme concentration for protoplast preparation, we incubated N. limnetica cells with 0.5, 1, 2, and 4% of the enzyme for 4 h and examined effects on the cell wall. Cell permeability was determined by PI staining and flow cytometry (Fig. 4a–d). We observed a similar level of permeabilization regardless of the lysozyme concentration used; an eightfold increase (from 0.5 to 4%) resulted in only a small increase (<5%) in permeabilized cells (Fig. 4a–d). Lysozyme-treated cells were also analyzed by electron microscopy, which revealed that 0.5% lysozyme eroded the cell wall along the entire surface, but a further increase in lysozyme concentration had no obvious effect (Fig. 5). Debris of the digested cell wall was also detected in the background of all samples except untreated controls (Fig. 5).
Permeabilization and viability of N. limnetica cells after treatment with different concentrations of lysozyme. a–d Proportion (%) of permeable cells (high PI fluorescence) obtained in control cells (a) or after digestion with 0.5% (b), 2% (c), or 4% (d) lysozyme. e–h Proportion (%) of viable cells (high FDA fluorescence) obtained in control cells (e) or after digestion with 0.5% (f), 2% (g), or 4% (h) lysozyme. Cells treated with 1% lysozyme showed very similar values to those treated with 2% lysozyme and are not shown
Transmission electron micrographs of permeabilized N. limnetica cells. a, b Control cells with no treatment. c–h Cells digested with 0.5% (c, d), 2% (e, f), or 4% (g, h) lysozyme. Zones with detached cell wall and debris of the digested cell wall are marked with black and white arrows, respectively. Cells treated with 1% lysozyme showed very similar values to those treated with 2% lysozyme and are not shown
To confirm that lysozyme-permeabilized cells were viable and could be used for transformation, we measured their ability to metabolize the “vital” dye, FDA (Brookes et al. 2000). Even after treatment with the highest concentration of lysozyme tested (4%), more than 90% of the cells were above the “alive threshold” established from the non-treated control (Fig. 4e–h). We also tested whether lysozyme-permeabilized cells were able to recover after treatment. Protoplasts were spread onto agar plates; no differences in growth were observed in comparison with non-treated cells (Fig. S5).
Discussion
It is notable that three algal species of the same genus, Nannochloropsis, exhibited quite different sensitivities to a wide spectrum of antibiotics (Tables 1 and 2). This suggests that detailed screening may be inevitable for any algal species with potential as a “new” laboratory strain. Our data are therefore important not only for the selection of transformants but also for maintenance of axenic cultures. Among the antibiotics tested, only ampicillin could be used at a standard concentration to eliminate bacterial contaminants from N. limnetica cultures. Very low concentrations of kanamycin and chloramphenicol, or a mixture, could be used as alternatives. Due to the high sensitivity of N. limnetica, all remaining antibiotics tested would be suitable as selection markers in transformation.
For N. oceanica, kanamycin, streptomycin, cycloheximide, gentamicin, geneticin, and nourseothricin were evaluated for the first time. Kanamycin and gentamicin had no effect (Tables 1 and 2) and could be applied to control contaminants in this strain. Streptomycin and nourseothricin showed a medium range effect, so they cannot be recommended as “contaminant cleaners” or “selection markers.” In contrast, cycloheximide and geneticin appear to be good candidates for the selection of transformants. Working concentrations of ampicillin, chloramphenicol, and zeocin were in the same range as reported (Vieler et al. 2012). However, our results show weaker effects of hygromycin and paromomycin on N. oceanica than previously reported (Vieler et al. 2012), suggesting that these antibiotics are not optimal for transformation testing.
The majority of antibiotics tested on N. gaditana did not cause strong growth inhibitory effects. Despite reports of genetic transformation of this algae using cassettes conferring resistance to zeocin (Radakovits et al. 2012), this is the first extensive study of antibiotic sensitivity in N. gaditana. Surprisingly, hygromycin and nourseothricin, widely used as selection markers for the transformation of eukaryotes (Radakovits et al. 2010; Anami et al. 2013; Mehrabi et al. 2015), had no effect even at high concentrations. Zeocin and cycloheximide seem to be the only suitable antibiotics for genetic transformation of N. gaditana. Other antibiotics showed only a medium-range effect, so cannot be recommended for this alga.
Although screening on agar plates followed the same trend as in liquid medium (Table 2), there were some differences in antibiotic concentrations required to completely inhibit growth. Whereas all Nannochloropsis species showed different sensitivities to kanamycin, chloramphenicol, and gentamycin in liquid culture, the same antibiotics caused no reduction in growth when used in agar plates. In addition, there were significant differences in concentrations used on plates compared to liquid cultures (compare Tables 1 and 2). These results suggest a need to perform screenings in both liquid cultures and on plates.
The data reported here are consistent with the known targets of antibiotic actions and their suitability as selection markers in transformation. Ampicillin, which inhibits peptidoglycan synthesis of the prokaryotic cell wall, is expected to be harmless to eukaryotic algae, and our results are in agreement with this. Kanamycin, streptomycin, paromomycin, gentamycin, nourseothricin, and chloramphenicol represent another group of antibiotics that act by blocking prokaryotic translation. It was not surprising therefore that this group inhibited Nannochloropsis growth since ribosomes in mitochondria and chloroplasts are of prokaryotic origin (Gray 1983, 1988; Manuell et al. 2007; Bullerwell 2011). However, the different sensitivities of Nannochloropsis strains towards this group of antibiotics (see Fig. 1) suggest that penetration into organelles may vary between strains or their intracellular stabilities may differ.
The nature of the chloroplast outer membrane is determined by the primary or secondary endosymbiotic origin of the species. Nannochloropsis species chloroplasts were generated by secondary endosymbiosis, as for the diatom P. tricornutum. The Nannochloropsis organellar genome has recently been sequenced and was found to be similar to chloroplasts and mitochondria in Phaeodactylum (Wei et al. 2013). In contrast, C. reinhardtii, another widely studied algal model, was generated by a single endosymbiotic event. Comparisons of antibiotic sensitivities of these three algae, however, do not correlate with their endosymbiotic origins. For instance, both nourseothricin and paromomycin had rather limited effects on the two Nannochloropsis species (Table 1), but they are used as standard antibiotics for selection of Phaeodactylum and Chlamydomonas transformants (Zaslavskaia et al. 2000; Sizova et al. 2001).
The last group of antibiotics tested in this study, zeocin, hygromycin, gentamycin, geneticin, and nourseothricin, are active against both prokaryotic and eukaryotic cells or only against eukaryotes (cycloheximide). Nevertheless, with the exception of zeocin and cycloheximide, the effects of these antibiotics on Nannochloropsis growth were heterogeneous and strain dependent. Even zeocin, which is very effective against all three Nannochloropsis strains, was much less effective against other algae. Reported concentrations used to select transformants were 10 (C. reinhardtii) to 100 times (P. tricornutum) higher than the lethal concentrations observed for Nannochloropsis (Apt et al. 1996; Zaslavskaia et al. 2000; Kovar et al. 2002; Sineshchekov et al. 2002). These data therefore show that regardless of the mechanism of antibiotic activity or the location (organelles, nucleus), the effects of antibiotics vary between different algal species, including related strains. Cell chemistry and properties of the cell wall are likely therefore to be crucial factors affecting antibiotic tolerance.
In algae, the cell wall can be a barrier to transformation, and there are reports that transformation may only be achieved after eliminating or weakening the cell wall (Jarvis and Brown 1991; Kim et al. 2002; Chen et al. 2008). Although two Nannochloropsis species have been transformed by electroporation without the need for enzymatic cell permeabilization (Radakovits et al. 2012; Vieler et al. 2012), others required pretreatment with 4% (w/v) hemicellulase and 2% (w/v) driselase (Chen et al. 2008).
Our permeability assay showed that the N. limnetica cell wall can be readily degraded with lysozyme. This finding agrees with Gerken et al. (2013), where lysozyme caused growth inhibition in Nannochloropsis sp. NANNP2 and was the most effective enzyme for degrading the cell wall of Chlorella vulgaris (Gerken et al. 2013). Nevertheless, the pronounced effect of this enzyme on integrity of the cell wall of N. limnetica was remarkable, given the minimal degradation caused in other freshwater algae, and that in C. vulgaris, lysozyme increased cell permeability by only 8% (Gerken et al. 2013). In the latter case, it was proposed that lysozyme removes a protective outer layer allowing other enzymes to reach their target substrates (Gerken et al. 2013). Therefore, to obtain higher levels of permeabilization, a combination with other enzymes was necessary. Chen et al. (2008) showed that a combination of 6% hemicellulase and 3% driselase efficiently digested the N. oculata cell wall, leading to >85% protoplast production after 1 h of treatment. However, protoplast formation in N. limnetica was much lower (˂20%) using the same cocktail and lysozyme alone was better than a mixture of enzymes. These results are in agreement with the results of other researchers showing that due to the complexity and variety of algal cell walls, each species often requires a specific enzymatic treatment (Mazalova et al. 2011).
The methods established in this study (sensitivity to antibiotics and protoplast production) are now being used by our group in trials to achieve genetic transformation of N. limnetica. pSELECT100 plasmid (kindly provided by Prof. Christoph Benning, Michigan State University), conferring resistance to hygromycin under the control of N. oceanica endogenous promoter, is being used to transform this freshwater algae by electroporation and biolistic transformation. Electroporation is being tested in normal and permeabilized cells. Same plasmids have been previously used to transform N. oceanica (Vieler et al. 2012) and N. gaditana (our group, not published). Unfortunately, no transformants have been obtained yet in N. limnetica. However, there is still a lot of space to test and optimize the transformation of this alga by using different plasmids, a N. limnetica endogenous promoter controlling the expression of the antibiotic resistance gene or an Agrobacterium-mediated transformation system.
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11:36–42
Adarme-Vega TC, Lim DK, Timmins M, Vernen F, Li Y, Schenk PM (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Factories 11:96
Aguirre AM, Bassi A, Saxena P (2013) Engineering challenges in biodiesel production from microalgae. Crit Rev Biotechnol 33:293–308
Anami S, Njuguna E, Coussens G, Aesaert S, van Lijsebettens M (2013) Higher plant transformation: principles and molecular tools. Int J Develop Biol 57:483–494
Apt KE, Grossman A, Kroth-Pancic P (1996) Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Molec Gen Genet 252:572–579
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Brookes JD, Geary SM, Ganf GG, Burch MD (2000) Use of FDA and flow cytometry to assess metabolic activity as an indicator of nutrient status in phytoplankton. Mar Freshw Res 51:817–823
Bullerwell CE (ed) (2011) Organelle genetics: evolution of organelle genomes and gene expression. Springer, Dordrecht
Chen HL, Li SS, Huang R, Tsai HJ (2008) Conditional production of a functional fish growth hormone in the transgenic line of Nannochloropsis oculata (Eustigmatophyceae). J Phycol 44:768–776
Coll JM (2006) Methodologies for transferring DNA into eukaryotic microalgae. Span J Ag Res 4:316–330
Dunahay TG, Jarvis EE, Dais SS, Roessler PG (1996) Manipulation of microalgal lipid production using genetic engineering. Appl Biochem Biotech 57:223–231
Gerken HG, Donohoe B, Knoshaug EP (2013) Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 237:239–253
Gong YM, Hu HH, Gao Y, Xu XD, Gao H (2011) Microalgae as platforms for production of recombinant proteins and valuable compounds: progress and prospects. J Ind Microbiol Biot 38:1879–1890
Gouveia L, Oliveira A (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biot 36:269–274
Gray MW (1983) The bacterial ancestry of plastids and mitochondria. Bioscience 33:693–699
Gray MW (1988) Organelle origins and ribosomal-RNA. Biochem Cell Biol 66:325–348
Ho SH, Ye XT, Hasunuma T, Chang JS, Kondo A (2014) Perspectives on engineering strategies for improving biofuel production from microalgae—a critical review. Biotechnol Adv 32:1448–1459
Hu HH, Gao KS (2006) Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol Lett 28:987–992
Jarvis EE, Brown LM (1991) Transient expression of firefly luciferase in protoplasts of the green alga Chlorella ellipsoidea. Curr Genet 19:317–321
Kaye Y, Grundman O, Leu S, Zarka A, Zorin B, Didi-Cohen S, Khozin-Goldberg I, Boussiba S (2015) Metabolic engineering toward enhanced LC-PUFA biosynthesis in Nannochloropsis oceanica: overexpression of endogenous Δ12 desaturase driven by stress-inducible promoter leads to enhanced deposition of polyunsaturated fatty acids in TAG. Algal Res 11:387–398
Kilian O, Benemann CSE, Niyogi KK, Vick B (2011) High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc Natl Acad Sci USA 108(52):21265–21269. doi:10.1073/pnas.1105861108
Kim D, Kim Y, Cho J, Bae J, Hur S, Hwang I, Choi T (2002) Stable integration and functional expression of flounder growth hormone gene in transformed microalga Chlorella ellipsoidea. Mar Biotechnol 4:63–73
Kovar J, Zhang J, Funke R, Weeks D (2002) Molecular analysis of the acetolactate synthase gene of Chlamydomonas reinhardtii and development of a genetically engineered gene as a dominant selectable marker for genetic transformation. Plant J 29:109–117
Krienitz L, Wirth M (2006) The high content of polyunsaturated fatty acids in Nannochloropsis limnetica (Eustigmatophyceae) and its implication for food web interactions, freshwater aquaculture and biotechnology. Limnologica 36:204–210
Leon R, Fernandez E (2007) Nuclear transformation of eukaryotic microalgae—historical overview, achievements and problems. Adv Exp Med Biol 616:1–11
Li Y, Han D, Hu G, Sommerfeld M, Hu Q (2010) Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol Bioeng 107:258–268
Lubián LM, Montero O, Moreno-Garrido I, Huertas IE, Sobrino C, González-del Valle M, Parés G (2000) Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J Appl Phycol 12:249–255
Ma YB, Wang ZY, Yu CJ, Yin YH, Zhou GK (2014) Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresource Technol 167:503–509
Manuell AL, Quispe J, Mayfield SP (2007) Structure of the chloroplast ribosome: novel domains for translation regulation. PLoS Biol 5:1785–1797
Mazalova P, Sarhanova P, Ondrej V, Poulickova A (2011) Quantification of DNA content in freshwater microalgae using flow cytometry: a modified protocol for selected green microalgae. Fottea 11:317–328
Mehrabi R, Mirzadi GA, da Silva G, Steinberg G, Kema G, de Wit P (2015) Flexible gateway constructs for functional analyses of genes in plant pathogenic fungi. Fungal Genet Biol 79:186
Mühlroth A, Li K, Rokke G, Winge P, Olsen Y, Hohmann-Marriott MF, Vadstein O, Bones AM (2013) Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar Drugs 11:4662–4697
Pereira CMP, Hobuss CB, Maciel JV, Ferreira LR, Del Pino FB, Mesko MF, Jacob-Lopes E, Neto PC (2012) Biodiesel derived from microalgae: advances and perspectives. Quim Nov. 35:2013–2018
Qin S, Lin H, Jiang P (2012) Advances in genetic engineering of marine algae. Biotechnol Adv 30:1602
Radakovits R, Jinkerson R, Darzins A, Posewitz M (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486
Radakovits R, Eduafo PM, Posewitz MC (2011) Genetic engineering of fatty acid chain length in Phaeodactylum tricornutum. Metabol Eng 13:89–95
Radakovits R, Jinkerson RE, Fuerstenberg SI, Tae H, Settlage RE, Boore JL, Posewitz MC (2012) Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. NatCommun 3:686. doi:10.1038/ncomms1688
Ramazanov A, Ramazanov Z (2006) Isolation and characterization of a starchless mutant of Chlorella pyrenoidosa STL-PI with a high growth rate, and high protein and polyunsaturated fatty acid content. Phycol Res 54:255–259
Recht L, Zarka A, Boussiba S (2012) Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl Microbiol Biotechnol 94:1495–1503
Rodolfi L, Chini ZG, Bassi N, Padovani G, Biondi N, Bonini G, Tredici M (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Sato T (1968) A modified method for lead staining of thin sections. J Electron Microsc 17:158–159
Schatz D, Eisenstadt D, Gressel J, Ufaz S, Chen O, Eckstein DJ (2013) Genetic transformation of algal and cyanobacteria cells by microporation. USA Patent 83677392
Sineshchekov OA, Jung K-H, Spudich JL (2002) Two rhodopsins mediate phototaxis to low-and high-intensity light in Chlamydomonas reinhardtii. Proc Nat Acad Sci 99:8689–8694
Singh NK, Dhar DW (2011) Microalgae as second generation biofuel. A review. Agron Sustain Dev 31:605–629
Sizova I, Fuhrmann M, Hegemann P (2001) A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene 277:221–229
Vieler A, Wu GX, Tsai CH, Bullard B, Cornish AJ, Harvey C, Reca IB, Thornburg C, Achawanantakun R, Buehl CJ, Campbell MS, Cavalier D, Childs KL, Clark TJ, Deshpande R, Erickson E, Ferguson AA, Handee W, Kong Q, Li XB, Liu BS, Lundback S, Peng C, Roston RL, Sanjaya SJP, TerBush A, Warakanont J, Zauner S, Farre EM, Hegg EL, Jiang N, Kuo MH, Lu Y, Niyogi KK, Ohlrogge J, Osteryoung KW, Shachar-Hill Y, Sears BB, Sun YN, Takahashi H, Yandell M, Shiu SH, Benning C (2012) Genome, functional gene annotation and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet 8(11):e1003064
Wei L, Xin Y, Wang DM, Jing XY, Zhou Q, Su XQ, Jia J, Ning K, Chen F, Hu Q, Xu J (2013) Nannochloropsis plastid and mitochondrial phylogenomes reveal organelle diversification mechanism and intragenus phylotyping strategy in microalgae. BMC Genomics 14:534
Zaslavskaia LA, Lippmeier JC, Kroth PG, Grossman AR, Apt KE (2000) Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J Phycol 36:379–386
Acknowledgments
This research was supported by the Czech National Program of Sustainability I (LO1416). We thank Kominist Asmamaw Anley for the assistance with the antibiotic tests. L.B. was suported by FEI & Czechoslovak Microscopy Society’s grant 2014 awarded for young scientists.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 2985 kb)
Rights and permissions
About this article
Cite this article
Noda, J., Mühlroth, A., Bučinská, L. et al. Tools for biotechnological studies of the freshwater alga Nannochloropsis limnetica: antibiotic resistance and protoplast production. J Appl Phycol 29, 853–863 (2017). https://doi.org/10.1007/s10811-016-1001-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-1001-6