Abstract
Microalgae can stimulate antioxidant defense systems as adaptive responses to oxidative stress. Therefore, these organisms can be a potential source of natural antioxidants. In this work, forty-two strains of microalgae and cyanobacteria were selected within major groups held in the Coimbra Collection of Algae (ACOI). The antioxidant capacity of ethanolic extracts was determined by two spectrophotometric methods: the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay and the 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl (DPPH) assay. Raspberry extract was used as a reference for comparison purposes. The ABTS assay showed an antioxidant capacity range of 16.61 ± 0.15 to 258.20 ± 0.65 mg Trolox (TE) (100 g)−1 fresh biomass (FW). High antioxidant capacity was observed in Eustigmatophyceae and Chlorophyta, with high results achieved for Vischeria helvetica ACOI 299, Characiopsis aquilonaris ACOI 2424, and Micrasterias radiosa var. elegantior ACOI 1568. The DPPH assay revealed that the eustigmatophytes Characiopsis sp. ACOI 2428, Characiopsis minima ACOI 2426, and V. helvetica ACOI 299, the cryptophyte Cryptomonas pyrenoidifera ACOI 1850, and the chlorophyte Mychonastes homosphaera ACOI 1850 had the highest scavenging activity. Cyanophytes revealed low antioxidant capacity, and mucilagineous strains of different taxa remained undetermined. The assessment of these strains and the broadening of a screening survey of the ACOI Culture Collection are expected to reveal very promising antioxidant-producing strains that may be applied in the field of human nutrition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although oxygen is required for aerobic life, it can promote oxidative stress (Halliwell 2007), which is triggered by an inbalance between the production of reactive oxygen species (ROS) and the antioxidant defenses (Halliwell 1994). This inbalance may lead to oxidative damage of tissues and consequent disorders, such as cancer and neurodegenerative diseases (Ndhlala et al. 2010). ROS are produced from molecular oxygen as a result of normal cellular metabolism. If they contain one or more unpaired electrons, the molecule becomes reactive, and the ROS are termed free radicals (Birben et al. 2012). ROS include superoxide anion (O2 −•), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2) (Carocho and Ferreira 2013). The human body has a natural antioxidant defense mechanism composed of biological antioxidants that may be enzymatic (e.g., catalase) or non-enzymatic such as radical scavengers (e.g., the water-soluble vitamin C and the lipid-soluble vitamin E) and quenchers (e.g., β-carotene) (Benzie 2000; Huang et al. 2005; Ndhlala et al. 2010). The most accepted definition of a biological antioxidant is “any substance that, when present at low concentrations compared with those of the oxidizable substrate, considerably delays or inhibits oxidation of the substrate” (Gutteridge 1995). Although these defense mechanisms are quite effective, they are incomplete because the human body cannot produce some important antioxidants, which therefore must be taken in the diet (Benzie 2000). Antioxidants include different classes of compounds, namely vitamins (vitamin C and E), carotenoids (carotenes and xanthophylls), and polyphenols (flavonoids, phenolic acids, lignans, and stilbenes) (Oroian and Escriche 2015).
Antioxidant assays are based on the mechanism of reaction and are classified as hydrogen atom transfer (HAT)-based assays and electron transfer (ET)-based assays (Prior et al. 2005; Apak et al. 2013). The HAT-based assays measure the capability of an antioxidant to quench free radicals by H-atom donation and involve a synthetic free radical generator, an oxidizable molecular probe, and an antioxidant (Huang et al. 2005). Oxygen radical absorbance capacity (ORAC), total radical-trapping antioxidant parameter (TRAP), total oxidant scavenging capacity (TOSC), chemiluminescence (CL), photochemiluminescence (PCL), croton or β-carotene bleaching by LOO•, and low-density lipoprotein (LDL) oxidation are HAT-based methods. The ET-based assays measure the ability of an antioxidant to transfer one electron in order to reduce any compound. Ferric reducing antioxidant power (FRAP) and cupric reduction assay (CUPRAC) are ET-based methods (Prior et al. 2005). The 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays including Trolox equivalent antioxidant capacity (TEAC), the 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl (DPPH) assay, and total phenolics assay are in principle ET-based assays (Apak et al. 2013). However, some authors consider that these assays use HAT and ET mechanisms simultaneously. The dominating mechanism is determined by the antioxidant structure and properties, solubility, partition coefficient, and system solvent (Prior et al. 2005). The colorimetric assays ABTS and DPPH are the more widely used because they are simple to perform and do not require special equipment other than a spectrophotometer. These are desirable characteristics, especially when the nature of the compounds present in the extract is unknown, and there is an interest to determine the antioxidant capacity of the whole extract. Furthermore, the ABTS method is widely used and can be used to evaluate both water and lipid soluble antioxidants (Floegel et al. 2011). In contrast, some methods are not suitable for screening purposes. For example, the ORAC assay does not measure the activity of the lipidic fraction of the extract (Pinchuk et al. 2012).
Microalgae are an extremely diverse group of organisms, and their full potential is yet to be explored. These organisms can synthesize complex organic compounds and subsequently accumulate and/or secrete many primary and secondary metabolites of interest (Guedes et al. 2011). They can also exhibit adaptive responses to oxidative stress, via stimulation of their antioxidant defense system (Srivastava et al. 2005; Li et al. 2007; Chacón-Lee and González-Mariño 2010; Hajimahmoodi et al. 2010; Goiris et al. 2012). Algal antioxidants include enzymes, fat-soluble compounds, such as carotenoids and vitamin E (α-tocopherol), and water-soluble compounds, such as other vitamins, phycobiliproteins, and polyphenols (Shalaby 2015).
The Coimbra Collection of Algae (ACOI), at the Department of Life Sciences, University of Coimbra, Portugal, holds 4000 strains of microalgae and cyanobacteria, and it is the largest of its kind in the world (Santos and Santos 2004). The collection includes a plethora of different taxa, many of which are rare and unique. This taxonomic diversity of these algae also reflects a great diversity in the chemical composition of the strains and makes them highly attractive to prospect for bioactive compounds such as antioxidants. Although the first steps are being taken toward a realistic commercialization of new antioxidants from microalgae, a critical point for research is the thorough search for new “all-star” strains that may add to the diversity of sources for the market. In this study, we present a screening process for antioxidant capacity in extracts of 42 ACOI strains of diverse microalgae and cyanobacteria. For this purpose, two different colorimetric assays were performed, ABTS and DPPH, and raspberry extracts were included, in order to provide a reference value of reknown antioxidant capacity.
Materials and methods
Strains
Forty-two strains of microalgae of 33 different genera were selected from the ACOI (acoi.ci.uc.pt), comprising 10.2 % of the ACOI genera (314 in total). These genera represent most of the classes held at ACOI, with the exception of Synurophyceae and some Chlorophyta. A percentage of 3.5 % of the studied genera are chlorophytes, 2.2 % are eustigmatophytes, and 1.6 % cyanobacteria. The remaining 2.9 % of studied genera belong to other classes (Fig. 1). The strains belong to nine different higher rank taxa namely Cyanophyceae, Xanthophyceae, Eustigmatophyceae, Cryptophyceae, Chrysophyceae, Haptophyceae, Euglenophyceae, Rhodophyceae, and Chlorophyta (Table 1).
Culture conditions
For each strain, a mother culture was established in 250-mL Erlenmeyer flasks by diluting 100 mL of a dense culture with fresh culture medium 1:1 (v/v) (Table 1). This culture was maintained for 5 days under a light intensity of 11 μmol photons m−2 s−1, a photoperiod of 16:8 h of light/dark, and a temperature of 23 °C. Cultures to be tested were established in 300-mL Erlenmeyer flasks with an inoculum of 125 mL of the mother culture diluted with fresh medium 1:1 (v/v). The cultures were provided with air bubbling, 0.5–1 L min−1, and cultivated for 10 days.
Preparation of the ethanolic extracts
Cultures were centrifuged at 3260×g for 15 min at room temperature and the supernatant was discarded. Algal extracts were obtained by adding a volume of ethanol (Sigma-Aldrich, Germany, absolute p.a.) to the pellet to obtain a final concentration of 10 mg mL−1 and resuspended. Extraction was by subjecting the mixture to an ultrasound bath treatment (35 kHz, 240 W, 1 % liquid detergent added to the water), for 30 min in dim light. The extract was kept at −4 °C overnight until analysis.
Fresh red raspberry fruits and raspberry ketone powder capsules (Natiris S.A., Portugal) were purchased in the supermarket and used as references. Reference extracts were prepared by using the same method, and ethanol was added to a final concentration of 10 mg mL−1. In the case of raspberry ketone powder, an extract of 1 mg mL−1 was also prepared. Each microalgal extract was analyzed by both methods in the same day, and the whole study was conducted over a 5-month period. In each analysis, a fresh raspberry extract was prepared and analyzed in addition to the microalgal extracts. The absorption spectrum was obtained for each extract at 400–700 nm. All tests were performed under dim light.
The ABTS assay expressed as equivalent to ascorbic acid (AEAC) and to Trolox (TEAC)
The antioxidant potential (radical scavenging capacity) of intracellular extracts of all strains was evaluated by using the ABTS assay optimized for microalgae (Guedes et al. 2013a). The cation ABTS•+ (Sigma-Aldrich) solution was diluted with ultra-pure water in order to achieve an absorbance of 0.700 ± 0.020 at 734 nm. The 6-min reaction was started by adding a volume of algal extract to 1 mL of ABTS•+ and was followed by absorbance reading at 734 nm.
Ascorbic acid (Sigma-Aldrich) and Trolox (97.0 % pure, Sigma-Aldrich) were used as standards. The quantitative results of antioxidant content for all extracts were expressed as milligrams equivalent to ascorbic acid (AE) or to Trolox (TE) per 100 g of fresh biomass (FW) (mg AE (100 g)−1 or mg TE (100 g)−1) (Table 2) and obtained through calibration curves where X-axis stands for concentration and Y-axis stands for percent of inhibition (PI). Standard solutions of ascorbic acid and Trolox were prepared in distilled water and ranged from 1 to 500 mg L−1. The PI was calculated according to Guedes et al. (2013a):
where Abs ABTS denotes the initial absorbance of diluted ABTS•+ and Abs sample denotes the absorbance of the sample after 6 min of reaction.
The 2.2-diphenyl-1-picrylhydrazyl assay
The DPPH• assay was based on the method of Brand-Williams et al. (1995), with some modifications. DPPH• solution 0.06 mM was prepared by dissolving 4.8 mg of DPPH• in 200 mL of methanol. Each extract was diluted with ethanol in order to obtain four different concentrations to be tested: 3, 5, and 10 mg mL−1. The 15-min reactions were started by adding 0.2 mL of extract to 1.8 mL DPPH•. The absorbance at 515 nm was immediate determined. A blank was performed by reading the absorbance of a DPPH• solution that was prepared by adding 0.2 mL ethanol to 1.8 mL DPPH•. The antioxidant capacity of the extracts and references was determined by the PI and IC50 value. For each algal extract, the PI was calculated according to Mishra et al. (2012):
where Abs DPPH . denotes the initial absorbance of methanolic DPPH• at 0.06 mM and Abs sample denotes the absorbance of the sample (10 mg mL−1 diluted extract reaction with DPPH• after 15 min).
For the determination of IC50 for each strain, the concentrations 3, 5, 7, and 10 mg mL−1 (X-axis) were plotted against the corresponding PI and calculated by linear regression (r 2 ≥ 0.980) by using the following formula:
where b is the Y-intercept and m is the slope of the linear equation. Values are expressed with the standard error (SE) XY as IC50 ± SE xy.
Statistical analysis
The absorbance measurements were performed in r ≥ 3, and the results are expressed as mean ± standard deviation (SD) of mean or IC50 value ± standard error of XY (SE xy). The significant differences between ascorbic acid equivalent antioxidant capacity (AEAC) and TEAC were tested by a Student’s t test for dependent samples (p < 0.05). The AEAC and TEAC data were subjected to analysis of covariance (ANCOVA), where the considered covariate was the day of assay. Significant differences were assessed by post hoc Dunnett’s test for multiple comparisons (p < 0.05) with the reference value (fresh red raspberry). For the DPPH• data, the correlation of the IC50 values with the % inhibition was obtained by a non-parametric Spearman’s rank correlation coefficient. Statistical analysis was performed by using Statistica software package Statsoft Statistica v7.0.61.0 EN.
Results
The ABTS assay expressed as equivalent to ascorbic acid (AEAC) and to Trolox (TEAC)
Antioxidant capacity evaluated by the ABTS assay for fresh raspberry was 174.37 ± 4.37 mg AE (100 g)−1 and 224.8 ± 7.19 mg TE (100 g)−1. These values fall within the data obtained for the microalgal extracts (Table 2). The values obtained for the raspberry ketone extracts were very high and impossible to measure for the concentration of 10 mg mL−1 (data not shown) and still very high for a concentration of 1 mg mL−1 (2828.50 ± 36.61 mg AE (100 g)−1 and 3028.22 ± 40.38 mg TE (100 g)−1), compared to the values obtained for the microalgae. Raspberry ketone extracts were not used as reference for the ABTS assay.
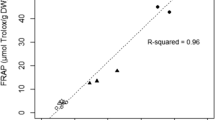
The range of values for the antioxidant capacity obtained for all microalgal strains was 4.65 ± 0.14 to 195.03 ± 0.40 mg AE (100 g)−1 and 16.61 ± 0.15 to 258.20 ± 0.65 mg TE (100 g)−1 (Table 2). For most strains, there is consistently higher values expressed as TE compared to AE (p < 0.05). For simplicity, the Trolox values are used throughout the discussion of results. Ten strains show antioxidant capacity similar to that of fresh red raspberry, corresponding to 23.8 % of the studied strains. These strains belong to Eustigmatophyceae and Chlorophyta. Two eustigmatophyte extracts produced the best results in the study and had values higher than the reference. These eustigmatophyte strains are Vischeria helvetica ACOI 299 and Characiopsis aquilonaris ACOI 2424 with 258.20 ± 0.65 and 225.24 ± 0.32 mg TE (100 g)−1, respectively. The chlorophyte Micrasterias radiosa var. elegantior ACOI 1568 was the third best extract of the study, with 214.34 ± 0.16 mg TE (100 g)−1.
Lower values were observed for strains of Cyanophyceae, Rhodophyceae, and Euglenophyceae and some strains of other classes within the Chlorophyta. The studied cyanophytes showed very low values of antioxidant capacity, with Ammatoidea normanii ACOI 948 achieving the highest value of 38.90 ± 0.0.27 mg TE (100 g)−1. All rhodophytes showed low antioxidant capacity, including Porphyridium strains, with Porphyridium aerugineum achieving 67.95 ± 0.36 mg TE (100 g)−1.
Different classes of Chlorophyta strains were studied, and a wide range of results were obtained. Mychonastes homosphaera ACOI 217 and Chlorella vulgaris ACOI 879 showed high values of antioxidant capacity, 174.30 ± 0.46 and 147.99 ± 0.97 mg TE (100 g)−1, respectively. On the other hand, Haematococcus pluvialis extracts prepared from cysts (“red phase”) and motile cells (“green phase”) of H. pluvialis ACOI 3380 had low antioxidant capacity. The red phase extract showed the lowest value, 20.30 ± 0.18 mg TE (100 g)−1, compared with 89.77 ± 0.25 mg TE (100 g)−1 for the green phase extract. Also, a very low absorbance spectrum was observed for the red phase extract, compared to the spectrum of V. helvetica ACOI 299, the extract with the highest value (Fig. 2).
Euglena cantabrica ACOI 1095 was the only studied euglenophyte and showed low antioxidant capacity (86.99 ± 0.08 mg TE (100 g)−1) compared to the reference (Table 2).
Phragmonema sordidum ACOI 969, Chlorobotrys sp. ACOI 3672ni, Interfilum paradoxum ACOI 590, and Aphanocapsa muscicola ACOI 615 showed undetectable antioxidant capacity and very low absorbance spectra (Fig. 2).
DPPH assay
For the DPPH method, both reference extracts, fresh raspberry and raspberry ketones, at 10 mg mL−1 concentration, showed similar IC50 values of ∼20 mg mL−1 needed to reduce 50 % of the DPPH• radical (Table 3). Fresh red raspberry showed slightly lower antioxidant capacity with an IC50 of 19.95 ± 0.21 (31.51 ± 0.005 % inhibition), compared to raspberry ketones, with an IC50 of 17.70 ± 0.52 (30.19 ± 0.006 % inhibition). The Characiopsis sp. ACOI 2428 extract had the best result for the IC50 of algal extracts, 44.27 ± 0.39 mg mL−1 (Table 3). No strain showed an IC50 value lower than the raspberry reference extracts. Considering the wide range of IC50 values obtained in the study, two thresholds were defined: one for determining the best strains (IC50 < 100 mg mL−1) and another for the extracts with the lower antioxidant capacity (IC50 > 700 mg mL−1).
Five strains showed the highest values of DPPH• scavenging activity and fell inside the threshold of <100 mg mL−1 extract necessary to inhibit 50 % DPPH• radical. Three strains are in the class Eustigmatophyceae: Characiopsis sp. ACOI 2428 (IC50 44.27 ± 0.39 mg mL−1), Characiopsis minima ACOI 2426 (IC50 80.26 ± 0.48 mg mL−1), and V. helvetica ACOI 299 (IC50 86.11 ± 0.11 mg mL−1). High scavenging activity was also achieved with the cryptophyte Cryptomonas pyrenoidifera ACOI 217 and the chlorophyte M. homosphaera ACOI 1850 with an IC50 of 66.03 ± 0.41 and 80.08 ± 0.61 mg mL−1, respectively.
The extracts with the lowest antioxidant capacity (IC50 > 700 mg mL−1) were the chlorophyte H. pluvialis ACOI 3380 (red phase) with IC50 1421.60 ± 0.27 mg mL−1, the eustigmatophyte Chlorobotrys sp. ACOI 3672ni with IC50 802.48 ± 0.15 mg mL−1, and two cyanophytes. The studied cyanophytes showed low to intermediate DPPH• scavenging activity, with A. muscicola ACOI 615 and A. normanii ACOI 948 with low values of IC50, 1034.39 ± 1.22 and 732.07 ± 0.48 mg mL−1, respectively.
Discussion
Microalgal antioxidants belong to a variety of chemical families with opposite polarities; however, most antioxidants are non-polar. The choice of an extraction solvent for antioxidant analysis must be authorized for use in the food industry, and ethanol has been widely employed (Guedes et al. 2013a). Different antioxidant molecules in the microalgal ethanolic extracts may act through different mechanisms. Furthermore, each assay provides an estimate of the antioxidant capacity of all extracted molecules, which may not reflect the antioxidant capacity of pure compounds and is dependent upon time of reaction, method, and the complexity of reaction kinetics. For these reasons, it is advisable to use at least two assays for the antioxidant capacity evaluation of natural extracts (Özgen et al. 2006; Gülçin et al. 2011). The published data on the evaluation of antioxidant capacity in plant and microalgal extracts is fragmented thus making the comparison of results a very difficult task. The ABTS and DPPH methodologies are poorly described, and the data analysis and units are very diverse (Goiris et al. 2012; Guedes et al. 2013b). This lack of consistency is most noticeable in the DPPH assay, in which the results are presented either as % inhibition (Cerón et al. 2007; Šavikin et al. 2009), radical scavenging activity (RSA) (Aremu et al. 2014; Choochote et al. 2014), EC50 (Hu et al. 2008; Souza et al. 2014), or IC50 (Šavikin et al. 2009; Chaudhuri et al. 2014). Furthermore, different authors use different calculation formulae, with no cited reference or the omission of the formula. In some cases, after a long chain of cited references, the DPPH method is tracked back to the first description of this assay by Brand-Williams et al. (1995). The antioxidant capacity from the DPPH assay is often expressed as % inhibition (Natrah et al. 2007; Hajimahmoodi et al. 2010; Custódio et al. 2012; Aremu et al. 2014; Maadane et al. 2015). Our results show that this value is important for calculating the IC50, but it is not informative by itself regarding the antioxidant capacity of an algal extract. This is very well reflected in Table 3, in which the many values obtained for the microalgal extracts are around 10 % inhibition and range from 2.68 ± 0.002 to 17.70 ± 0.038 % inhibition, whereas the IC50 values range from 44.27 ± 0.39 to 1421.60 ± 0.79 mg mL−1 extract necessary to inhibit 50 % DPPH• radical. Furthermore, for each strain, the IC50 and corresponding % inhibition values do not correlate fully (p < 0.05 and ρ (Spearman) = −0.73), so we consider the IC50 as the indicator of antioxidant capacity of algal extracts assessed by DPPH•. A recent study by Maadane et al. (2015) also shows discrepant values of RSA% with the corresponding values of IC50 for each strain, although the authors do not discuss this discrepancy. The five best values of RSA% reported in this study do not correspond to the five best values of IC50.
The screening of microalgae as possible new sources of antioxidants for nutrition is most commonly performed in close datasets. The antioxidant capacity is compared between strains in each published study with no comparison of the values obtained from microalgal extracts with those values from food. Red raspberry is known for its antioxidant properties and a good candidate for providing this external reference. Several studies reveal that the antioxidant properties are due to a high phenolic content from ketones, ellagic acid, vitamin C, flavonoids, and anthocyanins (Liu et al. 2002; Kim and Padilla-Zakour 2004; Çekiç and Özgen 2010; Sariburun et al. 2010; Gülçin et al. 2011; Zafrilla et al. 2001). Red raspberry is a widely consumed fruit, either fresh or in supplement capsules, so these were tested as external references. Some variation was expected in fresh red raspberry extract, since different fruits were used every time extracts were prepared. Despite the fact that the chosen brand of raspberry distributor company was constant throughout the study, production, processing, and storage influence the antioxidant capacity of foods (Kalt 2005). In fact, the ABTS•+ determinations show high SD, but the IC50 value calculated in the DPPH assay does not show this effect.
The results from microalgal extracts showed a large variation of values among the strains in the same class of algae. For example, among the studied eustigmatophytes, V. helvetica ACOI 299 had the highest ABTS•+ scavenging value in the whole study, while the Chlorobotrys extracts showed undetectable or a very low value. This effect is also present in lower rank taxa, since C. pyrenoidifera ACOI 1847 shows a low value of 30.44 ± 0.10 mg TE (100 g)−1 and another strain of the same species displays a higher value of 110.42 ± 0.61 mg TE (100 g)−1. Other authors also report this variation within different strains of the same taxa, namely the same species or family (Li et al. 2007; Goiris et al. 2012).
The 14 Eustigmatophyceae extracts provide a broad survey of the antioxidant capacity within this class, which is known for oleaginous genus Nannochloropsis (Gouveia and Oliveira 2009; Zakariah et al. 2015). ABTS•+ and DPPH• scavenging activity was low to intermed for this genus (Custódio et al. 2012; Goiris et al. 2012; Guedes et al. 2013b; Maadane et al. 2015). In previous reports for DPPH• scavenging activity for 1 mg mL−1 algal extracts (expressed as RSA%) of Nannochloropsis oculata and Nannochloropsis gaditana, the measured activity was, respectively, 7 to 60-fold lower than the positive control BHT (Custódio et al. 2012; Maadane et al. 2015). Our results for ACOI microalgal extracts show the same tendency, in that no strain achieved a value as low as the raspberry extract reference, with the best result achieving a 2-fold higher value of 44.27 ± 0.39 mg mL−1 (Table 3). Based on these reports for Nannochloropsis, it was expected that other eustigmatophyte extracts would not show high antioxidant capacity. However, the results for Characiopsis and Vischeria indicate the opposite trend (Tables 2 and 3), and Pseudostaurastrum, Goniochloris, and Dioxys strains also showed high values. Based on statistical analysis, these values were considered different from fresh red raspberry (p < 0.05). Eustigmatos and Chlorobotrys strains were the exception to this result.
Among all studied taxa, the chlorophyte strains also show high antioxidant capacity, as previously reported (Li et al. 2007; Goiris et al. 2012; Guedes et al. 2013b). Micrasterias radiosa var. elegantior ACOI 1568 produced the highest value for the surveyed strains, a surprising result considering that desmids are a poorly studied group of algae for biotechnological purposes. The widely studied genus Chlorella was included in this study, and C. vulgaris ACOI 879 showed high values of antioxidant capacity by the ABTS assay. Previous reports for C. vulgaris also showed some strains with medium to high antioxidant capacity (Goiris et al. 2012). The C. vulgaris ACOI 879 strain was recently included in a study of the ABTS•+ scavenging activity of microalgae, and medium to low antioxidant capacity for this strain was reported in comparison to all other surveyed strains (Guedes et al. 2013b). However, the values were expressed as mg L−1 equivalent to ascorbic acid μg−1 chlorophyll a, which hampers a direct comparison with our determination for this strain, because our results are expressed as mg AE (100 g)−1 FW.
Haematococcus pluvialis is a well-known and studied chlorophyte due to the rich content of the carotenoid astaxanthin, especially in the cyst cells (red phase) (Kobayashi and Sakamoto 1999). Therefore, this organism should have a high antioxidant capacity. Surprisingly, both extracts prepared from red phase and motile cells (green phase) of H. pluvialis ACOI 3380 have low antioxidant capacity. Goiris et al. (2012) found similar results, with low activity of both extracts, specially the red phase. On the other hand, high antioxidant capacity was reported for a H. pluvialis strain, but no specifications regarding the phase of culture were provided (Guedes et al. 2013b). Haematococcus pluvialis cells have a strong, rigid sporopollenin cell wall (Damiani et al. 2006) that may act as an obstacle during the extraction process treatment. This may have accounted for a low presence of intracellular content in the extract rather than a real inability to scavenge the ABTS•+ radical. This is confirmed by very low absorbance spectrum for the H. pluvialis ACOI 3380 extract in comparison to V. helvetica ACOI 299, the extract with the best result (Fig. 2). This low value was also observed by other authors that applied mild extraction techniques (Mendes-Pinto et al. 2001) such as ultrasound treatment (used in the present study) or grinding (Goiris et al. 2012). Guedes et al. (2013b) used a high-speed homogenizer at 14,000 rpm for 30 min for disruption of H. pluvialis. This severe treatment possibly facilitated extraction of the cell contents and may explain the higher antioxidant capacity.
Chlorophytes and eustigmatophytes produce high amounts of carotenoids such as β-carotene, violaxanthin, and neoxanthin (Takaichi 2011), and recent studies reveal high antioxidant capacity of these carotenoids (Müller et al. 2011). These results may justify the antioxidant capacity of strains belonging to these taxa, but fractionation would be needed to determine which group of compounds were responsible for the radical scavenging during the assays.
The cryptophyte C. pyrenoidifera ACOI 1850 extracts revealed interesting antioxidant activity and produced the second best result for the DPPH assay and intermediate ABTS•+ scavenging compared to the reference (Tables 2 and 3). This study is the first record of promising antioxidant capacity for a strain of this species.
Low to intermediate values of ABTS•+ and DPPH• scavenging activity were determined for some strains of other classes within the Chlorophyta and also strains in the Rhodophyceae and Cyanophyceae. Li et al. (2007) revealed that extracts of Nostoc and Anabaena have antioxidant capacity in the range of chlorophytes such as Chlorella and Chlamydomonas. However, some reports claim that the levels of antioxidant activity found in extracts of microalgae are greater than in cyanobacteria (Guedes et al. 2013b). Our results also show this tendency. Furthermore, a study of 11 cyanophyte strains revealed low to medium antioxidant capacity of intracellular extracts but high antioxidant capacity of the extracellular extract (Hajimahmoodi et al. 2010). Based on these findings, there is a possibility that these organisms may expel outwards the compounds with antioxidant activity. Possible explanations for low antioxidant capacity found in ACOI cyanophytes may include an inefficient biomass extraction due to the presence of a tight mucilaginous sheath around the cell, which acts as an obstacle. Also, if in some strains antioxidants are excreted to the extracellular component of the culture, these compounds could not be detected in intracellular extracts.
Porphyridium (Rhodophyceae) is a genus known by its biotechnologically interesting biocompounds (Klein et al. 2012). Nevertheless, previous studies reveal a low antioxidant capacity of both marine (Goiris et al. 2012) and freshwater (Guedes et al. 2013b) strains. We also confirmed this low antioxidant capacity in our determinations for the freshwater strains P. aerugineum ACOI 329 and P. sordidum ACOI 1767. Therefore, antioxidant properties of intracellular ethanolic extracts may not be an interesting side of the numerous applicabilities of Porphyridium. Euglena cantabrica ACOI 1095 (Euglenophyceae) was the only studied euglenophyte and showed low antioxidant capacity compared to the reference (Tables 2 and 3). In contrast, other in vitro assays considered Euglena tuba as an excellent source of natural antioxidant (Chaudhuri et al. 2014). These results indicate the general trend that different species of the same genus may reveal very different antioxidant capacity.
Aphanocapsa muscicola ACOI 615, P. sordidum ACOI 969, Chlorobotrys sp. ACOI 3672ni, and I. paradoxum ACOI 590 extracts showed undetectable antioxidant capacity. All the strains are characterized by the presence of a dense mucilagineous sheath around the cell that may hinder the extraction of the intracellular content. Low absorbance spectra obtained for these extracts contrasts with high absorbance spectra found in antioxidant-rich extracts which reinforces this possibility (Fig. 2).
An overall comparison of the results obtained from both assays reveals that the best results were achieved in algal extracts of strains from the Eustigmatophyceae and some Chlorophyta and the lowest results in strains of Cyanophyceae and some Chlorophyta. However, a comparative analysis reveals different results at lower taxa level. The best antioxidant scavenging activity of ABTS•+ is from V. helvetica ACOI 299 and of DPPH• is in Characiopsis sp. ACOI 2428. The lowest activity for ABTS•+ was found with Gloeococcus minor ACOI 937-A extract, and for DPPH•, it was with H. pluvialis ACOI 3380 red phase. Another example of conflicting results for both assays was observed in P. sordidum ACOI 969 and I. paradoxum ACOI 590 extracts, which showed undetectable activity in the ABTS assay but had intermediate activity values of IC50 (DPPH assay). These results were also reported by Shalaby and Shanab (2013). Because both assays evaluate the total antioxidant capacity of polar to non-polar compounds present in ethanolic extracts and both function through HAT and ET mechanisms simultaneously (Prior et al. 2005), comparable results were expected for each strain. The high variety of compounds with antioxidant activity present in each extract causes difficulties in the interpretation of the antioxidant capacity results in microalgal extracts (Marxen et al. 2007). In fact, besides its primary function of reacting with the radical, the antioxidant molecules may also be involved in unpredictable interactions (synergistic or antagonistic) with other compounds present in the extract (Pérez-Jiménez et al. 2008). There are also some drawbacks of the radical scavenging assays. Since the reaction may not be complete when the absorbance is measured, an underestimation of the antioxidant capacity may occur (Pérez-Jiménez et al. 2008; Maadane et al. 2015). In addition, the choice of solvent may affect the assay efficiency.
Studies by Maadane et al. (2015) revealed that carotenoids are important contributors to the antioxidant capacity in microalgal biomass. On the other hand, in some cases, the highest antioxidant capacity was found in the hot water fraction of water-ethanol extracts with low carotenoid content, which indicates that carotenoids are not the only contributors. Phenolic compounds may be the molecules responsible for the effect in this case (Goiris et al. 2012; Choochote et al. 2014). The DPPH assay has been regarded as a limited evaluator of antioxidant capacity in living systems because the radical used for determinations is quite stable, unlike radicals present in living organisms (Pérez-Jiménez et al. 2008). The major drawback of the DPPH assay in our study was the wavelength used for absorbance reading. If DPPH• is mixed with a solution of carotenoids, a dark purple brown color is produced which interferes with the absorbance readings at 515 nm, the absorbance maximum wavelength of DPPH• (Müller et al. 2011). Because most microalgal extracts contain high amounts of carotenoids, the interference of the extract color with the reaction absorbance measurement at 515 nm causes an emission spectrum overlap and inviabilizes the assay. In the case of the raspberry reference extracts, the differences between the two methods (Tables 2 and 3) are not explained by this interference since the extracts were almost colorless. Regarding the ABTS assay, the activity was so high in the raspberry ketone extract (10 mg mL−1) that it was impossible to measure. However, for DPPH•, this extract showed IC50 similar to fresh red raspberry. There may not be a single biological/chemical explanation for the differences obtained in both assays. A previous DPPH assay on 14 strains failed to obtain the determinations of RSA; all values were negative (Natrah et al. 2007). The authors claim that none of the microalgal extracts tested were good radical scavengers. However, three of the tested strains belong to Scenedesmus, Chlorella, and Nannochloropsis. These were previously genera studied and reported to have radical scavenging activity. Based on this example, there is a good possibility that the general problem with the diversity of calculation formulae and scarcity of method descriptions for data treatment in radical scavenging assays may be affecting the results.
The tested strains represent 1 % of the ACOI collection. Many of these strains showed relevant antioxidant activity up to the level of fresh raspberry, a fruit commonly used as a natural antioxidant. The most promising algal taxa revealed in this study are Eustigmatophyceae and some Chlorophyta strains, with high antioxidant capacity of most studied genera. The antioxidant capacity remains undetermined for several strains that display characteristics requiring tailor-made assays.
A potential use of microalgal-derived radical antioxidants may be projected in the future as part of commercial antioxidant product development. Studies for the evaluation of antioxidant capacity of microalgal extracts are expanding, so the standardization of the notations is crucial for interlaboratorial comparisons and for the progress toward the development of efficient estimation methods.
References
Apak R, Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçlü K (2013) Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl Chem 85:957–998
Aremu AO, Masondo NA, Stirk WA, Ördög V, Staden JV (2014) Influence of culture age on the phytochemical content and pharmacological activities of five Scenedesmus strains. J Appl Phycol 26:407–415
Benzie IFF (2000) Evolution of antioxidant defence mechanisms. Eur J Nutr 39:53–61
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28:25–30
Carocho M, Ferreira ICFR (2013) A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol 51:15–25
Çekiç Ç, Özgen M (2010) Comparison of antioxidant capacity and phytochemical properties of wild and cultivated red raspberry (Rubus idaeus L.). J Food Compos Anal 23:540–544
Cerón MC, García-Malea MC, Rivas J, Acien FG, Fernandez JM, Del Río E, Guerrero MG, Molina E (2007) Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl Microbiol Biot 74:1112–1119
Chacón-Lee TL, González-Mariño GE (2010) Microalgae for “healthy” foods—possibilities and challenges. Compr Rev Food Sci F 9:655–675
Chaudhuri D, Ghate NB, Deb S, Panja S, Sarkar R, Rout J, Mandal N (2014) Assessment of the phytochemical constituents and antioxidant activity of a bloom forming microalgae Euglena tuba. Biol Res 47:1–11
Choochote W, Suklampoo L, Ochaikul D (2014) Evaluation of antioxidant capacities of green microalgae. J Appl Phycol 26:43–48
Custódio L, Justo T, Silvestre L, Barradas A, Duarte CV, Pereira H, Barreira L, Rauter AP, Alberício F, Varela J (2012) Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem 131:134–140
Damiani MC, Leonardi PI, Pieroni OI, Cáceres EJ (2006) Ultrastructure of the cyst wall of Haematococcus pluvialis (Chlorophyceae): wall development and behaviour during cyst germination. Phycologia 45:616–623
Floegel A, Kim D, Chung S, Koo S, Chun OK (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal 24:1043–1048
Goiris K, Muylaert K, Fraeye I, Foubert I, Brabanter JD, Cooman LD (2012) Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J Appl Phycol 24:1477–1486
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as a source of high added-value compounds—a brief review of recent work. Biotechnol Prog 27:597–613
Guedes AC, Amaro HM, Gião MS, Malcata FX (2013a) Optimization of ABTS radical cation assay specifically for determination of antioxidant capacity of intracellular extracts of microalgae and cyanobacteria. Food Chem 138:638–643
Guedes AC, Gião MS, Seabra R, Ferreira ACS, Tamagnini P, Moradas-Ferreira P, Malcata FX (2013b) Evaluation of antioxidant activity of cell extracts from microalgae. Mar Drugs 11:1256–1270
Gülçin I, Topal F, Çakmakçl R, Bilsel M, Gören AC, Erdogan U (2011) Pomological features, nutritional quality, polyphenol content analysis, and antioxidant properties of domesticated and 3 wild ecotype forms of raspberries (Rubus idaeus L.). J Food Sci 76:585–593
Gutteridge JMC (1995) Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 41:1819–1828
Hajimahmoodi M, Faramarzi MA, Mohammadi N, Soltani N, Oveisi MR, Nafissi-Varcheh N (2010) Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J Appl Phycol 22:43–50
Halliwell B (1994) Free radicals, antioxidants and human disease: curiosity, cause or consequence. Lancet 344:721–724
Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35:1147–1150
Hu C, Lin J, Lu F, Chou F, Yang D (2008) Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem 109:439–446
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agr Food Chem 53:1841–1856
Kalt W (2005) Effects of production and processing factors on major fruit and vegetable antioxidants. J Food Sci 70:11–19
Kim DO, Padilla-Zakour IO (2004) Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: cherry, plum, and raspberry. J Food Sci 69:395–400
Klein BC, Walter C, Lange HA, Buchholz R (2012) Microalgae as natural sources for antioxidative compounds. J Appl Phycol 24:1133–1139
Kobayashi M, Sakamoto Y (1999) Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol Lett 21:265–269
Li H, Cheng K, Wong C, Fan K, Chen F, Jiang Y (2007) Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem 102:771–776
Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH (2002) Antioxidant and antiproliferative activities of raspberry. J Agr Food Chem 50:2926–2930
Maadane A, Merghoub N, Ainane T, Arroussi HE, Benhima R, Amzazi S, Bakri Y, Wahby I (2015) Antioxidant activity of some Moroccan marine microalgae: Pufa profile, carotenoids and phenolic content. J Biotechnol 215:13–19
Marxen K, Vanselow KH, Lippemeier S, Hintze R, Ruser A, Hansen U (2007) Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors 7:2080–2095
Mendes-Pinto MM, Raposo MFJ, Bowen J, Young AJ, Morais R (2001) Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: effects on astaxanthin recovery and implications for bioavailability. J Appl Phycol 13:19–24
Mishra K, Ojha H, Chaudhury NK (2012) Estimation of antiradical properties of antioxidants using DPPH• assay: a critical review and results. Food Chem 130:1036–1043
Müller L, Fröhlich K, Böhm V (2011) Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging. Food Chem 129:139–148
Natrah FMI, Yusoff FM, Shariff M, Abas F, Mariana NS (2007) Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J Appl Phycol 19:711–718
Ndhlala ER, Moyo M, Van Staden J (2010) Natural antioxidants: fascinating or mythical biomolecules? Molecules 15:6905–6930
Oroian M, Escriche I (2015) Antioxidants: characterization, natural sources, extraction and analysis. Food Res Int 47:10–36
Özgen M, Reese RN, Tulio AZ, Scheerens JC, Miller AR (2006) Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agr Food Chem 54:1151–1157
Pérez-Jiménez J, Arranz S, Tabernero M, Díaz-Rubio ME, Serrano J, Goňi I, Saura-Calixto F (2008) Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res Int 41:274–285
Pinchuk I, Shoval H, Dotan Y, Lichtenberg D (2012) Evaluation of antioxidants: scope, limitations and relevance of assays. Chem Phys Lipids 165:638–647
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agr Food Chem 53:4290–4302
Santos LMA, Santos MF (2004) The Coimbra Culture Collection of Algae (ACOI). Nova Hedwigia 79:39–47
Sariburun E, Şahin S, Demir C, Türkben C, Uylaşer V (2010) Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J Food Sci 75:328–335
Šavikin K, Zdunić G, Janković T, Tasić S, Menković N, Stević T, Đorđević B (2009) Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Food Hum Nutr 64:212–217
Shalaby EA (2015) Algae as a natural source of antioxidant active compounds. In: Dubey NK (ed) Plants as a source of natural antioxidants. CAB International, United Kingdom, pp. 129–147
Shalaby EA, Shanab SMM (2013) Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J Mar Sci 45:556–564
Souza VR, Pereira PAP, Silva TLT, Lima LCO, Pio R, Queiroz F (2014) Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem 156:362–368
Srivastava AK, Bhargava P, Rai LC (2005) Salinity and copper-induced oxidative damage and changes in the antioxidative defence systems of Anabaena doliolum. World J Microb Biot 21:1291–1298
Takaichi S (2011) Carotenoids in algae: distributions, biosynthesis and functions. Mar Drugs 9:1101–1118
Zafrilla P, Ferreres F, Tomás-Barberán FA (2001) Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J Agr Food Chem 49:3651–3655
Zakariah NA, Rahman NA, Hamzad F, Jahi TM, Ismail A (2015) Nannochloropsis oculata algae as biofuels: a review. In: Saha B (ed) Environmental science and sustainable development: international conference on environmental science and sustainable development. World Scientific Publishing, Singapore, pp. 217–222
Acknowledgments
We thank Prof. Dr. José Paulo Sousa for advice on statistical analysis of the data and Prof. Dr. Karen Fawley for the English review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Raquel Amaral was supported by Portuguese Science and Technology Agency (FCT) through PhD funding SFRH/BD/73359/2010 under POPH/QREN financing program.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Assunção, M.F.G., Amaral, R., Martins, C.B. et al. Screening microalgae as potential sources of antioxidants. J Appl Phycol 29, 865–877 (2017). https://doi.org/10.1007/s10811-016-0980-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0980-7