Abstract
The present study focused on a brown macroalga (Halidrys siliquosa), with a particular emphasis on polyphenols and their associated biological activities. Two fractions were obtained by liquid/liquid purification from a crude hydroethanolic extract: (i) an ethyl acetate fraction and (ii) an aqueous fraction. Total phenolic contents and antioxidant activities of extract and both fractions were assessed by in vitro tests (Folin–Ciocalteu test, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, reducing power assay, superoxide anion scavenging assay, and β-carotene–linoleic acid system). For the most active fraction, i.e., the ethyl acetate fraction, the oxygen radical absorbance capacity (ORAC) value, antibacterial activities, and sunscreen potential (Sun Protection Factor and UV-A-Protection Factor) were tested in vitro. A high correlation found between antioxidant activities and total phenolic content was interpreted as the involvement of polyphenolic compounds in antioxidant mechanisms. Interestingly, the ethyl acetate fraction appeared to be a broad-spectrum UV absorber and showed a strong bactericidal activity against Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. In this fraction, four phenolic compounds (trifuhalols and tetrafuhalols and, for the first time, diphlorethols and triphlorethols) were identified using 1D and 2D nuclear magnetic resonance (NMR) and MS analysis. These findings are promising for the use of H. siliquosa, abundant in Brittany, as a valuable source of photoprotectant molecules for sunscreen and cosmetic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the consequences of global change is an increase in the ambient level of ultraviolet (UV) radiation, particularly in southern areas (resulting from ozone depletion) (Thomas et al. 2012). This phenomenon, in addition to chronic sun exposure behavior, induces various skin-related disorders in humans, such as premature aging and skin cancer development (Lautenschlager et al. 2007; Thomas et al. 2012; Saewan and Jimtaisong 2015). UV radiation mostly consists of UV-A radiation, which penetrates deep into the dermis and epidermis and is known to be responsible for the production of reactive oxygen species (ROS), which can cause DNA damage. UV-A is also known to cause premature skin aging by damaging the underlying structures of the dermis (Ichihashi et al. 2003; Lautenschlager et al. 2007). UV-B radiation, which represents a lesser proportion of the total UV radiation than UV-A, is known to be the solar light component the most inductive of skin cancer and can also cause DNA damage. It penetrates into the epidermis layer and causes some acute biological effects such as skin sunburn (Ichihashi et al. 2003; Lautenschlager et al. 2007). In order to lower the risk of skin diseases, topical sunscreen applications have been developed (Ichihashi et al. 2003; Lautenschlager et al. 2007; Sambandan and Ratner 2011). An optimal sunscreen combines UV-A and UV-B filters in order to provide broad-spectrum protection (Lautenschlager et al. 2007; Sambandan and Ratner 2011). Present UV filters are, however, based on chemical compounds and cause many side effects, such as irritant or allergic reactions, dermatitis, and, in a few cases, severe anaphylactic reactions (reviewed in Lautenschlager et al. 2007). Thus, biotechnological research is now focusing on natural compounds, such as phytochemicals, that show promising bioactivities for sunscreen applications (Saewan and Jimtaisong 2015), such as absorption and scattering properties and conservative actions, respectively, in sunscreen and other cosmetic products, and are suitable to replace some synthetic compounds. Among the organisms recognized as valuable sources of diverse bioactive chemicals, seaweeds remain an underexploited resource, especially in Brittany (Bourgougnon and Stiger-Pouvreau 2011). Indeed, marine brown algae synthesize polyphenols, known as phlorotannins, that present various chemical structures (Singh and Bharate 2006) and have a large spectrum of biological activities (Li et al. 2011) of interest for skin protection. For example, phenolic compounds could have some antimicrobial (Eom et al. 2012), antioxidative (Liu et al. 2011), anticancer, radioprotective, and anti-inflammatory properties (Kim et al. 2009; Kang et al. 2013). Moreover, in brown seaweeds, phlorotannins are of special interest regarding to the UV-radiation protective effect (Pavia and Brock 2000; Swanson and Druehl 2002; Schoenwaelder 2002; Huovinen and Gómez 2015).
Previous studies on development of innovative natural products for preventive skin health care have been done by our laboratory (Surget et al. 2015). Among brown macroalgae which grow along the coasts of Brittany (France) and can form dense forests in the subtidal zone, Halidrys siliquosa has been reported to have a high phenolic content (Stiger-Pouvreau et al. 2014) and potentially useful biological properties, such as strong antioxidant and interesting antitumor activities (Zubia et al. 2009). Moreover, this species belongs to the Sargassaceae, a family which is known to present a natural diversity in bioactive compounds and is usually used as a model system for chemotaxonomic studies (Kornprobst 2010; Stiger-Pouvreau et al. 2014).
The goal of this study was to show the sunscreen potential of phlorotannins from this marine brown alga, in particular to identify active compounds (phytochemicals) with several biological activities. It focused on antioxidative activities associated with antibacterial and photoprotection capabilities of the phenolic-enriched fraction. Moreover, the total phenolic content of this active fraction was quantified with the Folin–Ciocalteu assay and qualified by nuclear magnetic resonance (NMR) and MS analysis.
Materials and methods
Halidrys siliquosa (Linnaeus) Lyngbye (Ochrophyta, Phaeophyceae, Fucales, Sargassaceae) was collected on January 30, 2013, from the sheltered side of the Porsmeur site (N48° 28′ 51″; W4° 46′ 8″) near Lanildut (France). Apical and median parts of thalli were collected, while the holdfasts were left on place to minimize collection impact on the seaweed population. Immediately after collection, epiphytes were removed and parts of thalli were thoroughly washed with distilled water. The cleaned algae were cut into small fragments and homogenized to remove effects of intra- and inter-individual variability. Samples were freeze-dried for 72 h and ground into powder for extraction.
Crude extraction and liquid–liquid purification processes
As described below and in Fig. 1, two fractions (an ethyl acetate fraction (EAF) and an aqueous fraction (AqF)) were obtained from the purification of the hydroethanolic crude extract (CE).
Crude extraction
To obtain the CE, 30 g of powder of thalli was extracted with 300 mL of ethanol/water (50/50, v/v) mixture (EtOH 50) in the dark at 40 °C for 2 h under rotary agitation (200 rpm). Sample was centrifuged at 3000×g at 10 °C for 10 min, and supernatant was collected. Next, the pellet was again extracted twice with 300 mL EtOH 50 in the dark at 40 °C for 1 h under rotary agitation (200 rpm), and centrifugation was repeated. Finally, 900 mL of the supernatant was obtained, pooled, and evaporated at 40 °C under vacuum to reach 100 mL of CE.
Liquid–liquid purification process
CE was semi-purified in order to concentrate the phenolic compounds using a process already described (Stiger-Pouvreau et al. 2014). Briefly, the process consisted of three steps. The first step was three dichloromethane washings. The second step consisted in rinsing the aqueous phase (containing phlorotannins) with acetone and then with ethanol. Finally, the third step was three ethyl acetate rinses that permitted to obtain the aqueous (AqF) and the ethyl acetate (EAF) fractions. All further analyses were carried out on the CE and these two purified fractions.
Total phenolic content
Total phenolic content (TPC) was determined by spectrophotometry using an adapted Folin–Ciocalteu assay as described by Le Lann et al. (2008) and Zubia et al. (2009). The samples (100 μL) were mixed with 50 μL Folin–Ciocalteu reagent, 200 μL of 20 % sodium carbonate solution, and 650 μL distilled water. Then, the mixture was allowed to stand at 70 °C in the dark for 10 min. After a blue color was produced, the absorbance was read at 650 nm. The TPC was expressed in milligram per gram of dry weight (mg g−1 DW) from a standard curve of phloroglucinol (1,3,5-trihydroxybenzene). This analysis was carried out in triplicate for each extract.
Antioxidant assays
2,2-Diphenyl-1-picrylhydrazyl radical scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was determined by the method described by Zubia et al. (2009), modified from Fukumoto and Mazza (2000) and Turkmen et al. (2007). Briefly, 22 μL of CE and both fractions at various concentrations were mixed with 200 μL of a DPPH solution (25 mg L−1) prepared fresh daily. The reaction was allowed to develop for 60 min in the dark at room temperature, before absorbance was read at 540 nm with a multi-well spectrophotometer. Water was used as a negative control and ascorbic acid (vitamin C), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and 2(3)-t-butyl-4-hydroxyanisole (butylated hydroxyl-anisole (BHA)) as positive controls. This assay was done in triplicate for each sample. The percentage of DPPH inhibition was calculated using the equation published in Surget et al. (2015). For each sample, a curve of extract concentration against % of DPPH inhibition was generated to estimate the concentration of extract needed to cause a 50 % reduction of the initial DPPH concentration. IC50 was expressed in milligram per milliliter. A high IC50 is considered as indicative of a weak radical scavenging activity and vice versa.
Reducing power assay
Total antioxidant capacity of the seaweed extract was determined using the ferric reducing–antioxidant power assay, as described by Zubia et al. (2009) and slightly modified by Surget et al. (2015). Briefly, 25 μL of sample at different concentrations was mixed with 25 μL sodium phosphate buffer (0.2 M, pH 6.6) and 25 μL 1 % potassium ferricyanide [K3Fe(CN)6]. After homogenization and incubation at 50 °C for 20 min, the mixture was cooled down in an ice bath prior to the addition of 25 μL 10 % trichloroacetic acid, 100 μL deionised water, and 20 μL 0.1 % FeCl3·6H2O. After homogenization, the reaction was allowed to develop for 10 min at room temperature and the absorbance was then read at 620 nm on a multi-well spectrophotometer. An increase of the absorbance at 650 nm of the reaction mixture indicated that the molecules had some reducing power. Results are expressed as EC50 in milligram per milliliter. This value was obtained by interpolation of a regression linear curve. This assay was carried out in triplicate for each sample and for the positive controls (ascorbic acid, Trolox, and BHA, as described above).
Superoxide anion scavenging activity (nitroblue tetrazolium)
The superoxide anion scavenging assay was carried out according to Chua et al. (2008), as slightly modified by Tanniou et al. (2014). The reaction mixture consisted of 203 μL of 5 × 10−3 M Tris–HCl buffer (pH 7.5), 57 μL 5 mM hypoxanthine, 30 μL 0.33 mM nitroblue tetrazolium (NBT), and 13 μL of the extracts, positive controls (ascorbic acid, Trolox, and BHA) or negative controls (distilled water and ethanol). After incubation at 25 °C for 10 min, the reaction was started by adding 30 μL xanthine oxidase. The absorbance was measured every 3 min for 21 min at 560 nm. The inhibition ratio (%) was calculated from the equation published in Chua et al. (2008). Results were then expressed as IC50 in milligram per milliliter (the concentration of substrate that causes a 50 % inhibition).
β-Carotene–linoleic acid system test
The antioxidant activity of samples was measured by the β-carotene bleaching method in accordance with Kaur and Kapoor (2002) and Koleva et al. (2002) after slight modifications, as described in Le Lann et al. (2008) and Zubia et al. (2009). Briefly, 2 mL of a solution of β-carotene in chloroform (0.1 mg mL−1) was added to round-bottom flasks containing 20 mg linoleic acid and 200 mg Tween 40. After evaporation with a rotavapor, oxygenated distilled water (50 mL) was added and the mixture was shaken to form a liposome solution. This mixture was added to 12 μL of extracts, positive controls (α-tocopherol, Trolox, and BHA) or negative controls (distilled water and ethanol). The absorbance of the solution at 450 nm was measured immediately (t = 0 min) and after 2 h at 50 °C (t = 120 min). All samples were assayed in triplicate. Antioxidant activity was expressed through the antioxidant activity coefficient (AAC700 in mg per mL) described by Le Lann et al. (2008). Furthermore, the lower the AAC700 was, the stronger the antioxidant activity became.
Oxygen radical absorbance capacity
Oxygen radical absorbance capacity (ORAC) assay summarizes two results in a single value: the inhibition percentage and inhibition rapidity of peroxyl radicals by antioxidants, in competition with the substrate (Dudonné et al. 2009). The ORAC assay was performed by INVIVO LABS company (www.invivo-labs.com) on the most antioxidant fraction. The protocol was adapted from Cao et al. (1993). Results were expressed as micromole Trolox equivalent (Te) per milligram.
Antibacterial activity
Antibacterial assays were performed by IDEA LAB company (www.groupeideatests.com), Plouzané, France. The most antioxidant fraction was tested against bacteria at 5 mg mL−1 in 1 % EtOH. The three tested bacterial strains, referenced in the European Pharmacopoeia 7.0 - (2011), were as follows: Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6538), and Escherichia coli (ATCC 8739). According to the benchmarks used, a product is bactericidal if a reduction of 4 or 5 log (AFNOR and European standards) of the bacterial concentration is obtained.
Protective efficacy: sun protection factor and UV-A protection factor
The photoprotective efficiency of H. siliquosa extracts was determined in UV-A and UV-B ranges (respectively, 320–400 and 290–320 nm) using a previously described in vitro method (Couteau et al. 2007; El-Boury et al. 2007). Sun protection factor (SPF) is used as a universal indicator to give information about the capacity of a product to reduce UV-induced solar erythema. An in vitro method makes it possible to highlight the UV filter role of organic or inorganic substances, which could later be incorporated into a sunscreen formulation for topical application.
Concisely, extracts were incorporated in a basic oil in water (O/W) emulsion to finally obtain an emulsion at 10 % (w/w) of extracts. Fifty milligrams of prepared emulsion was spread using a cot-coated finger across the entire surface (25 cm2) of a polymethylmethacrylate (PMMA) plate (Europlast, France). After spreading, only 15 mg of the emulsion remained on the plate. Transmission measurements between 200 and 400 nm were measured using a spectrophotometer equipped with an integrating sphere (UV Transmittance Analyzer UV1000S, Labsphere, USA). Three plates were prepared for each extract to be tested, and nine measurements were carried out on each plate. SPF and UV-A protection factor (PF-UV-A) were calculated using the following equations:
where E λ is the erythemal spectral effectiveness at λ, S λ is the solar spectral irradiance at λ, and T λ is the spectral transmittance of the sample at λ (Diffey and Robson 1989).
Results are given as SPF and PF-UV-A. A higher SPF/PF-UV-A value indicates that a sample offers more protection against UV-B/UV-A by absorbance or reflection.
Structural elucidation of active molecules by nuclear magnetic resonance and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis
The most antioxidant fraction was analyzed by 1H and 13C NMR. The 1D 1H- and 13C-NMR heteronuclear multiple bond correlation (HMBC) and heteronuclear single-quantum correlation spectroscopy (HSQC) experiments were carried out on a Bruker Avance 500. Samples were dissolved in 750 μL MeOD. Homonuclear and heteronuclear NMR spectra were recorded at 25 °C. Chemical shifts were measured in parts per million using tetramethylsilane (TMS) as a chemical shift reference at 0 ppm.
Analysis by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF MS) was performed using a Voyager DE-STR MALDI-TOF mass spectrometer (Applied Biosystems). The most active fraction (EAF) of H. siliquosa was first diluted to 1 mg mL−1 in water. Then, 1 μL of this solution was diluted with matrix at 1/1, 1/10, 1/100, and 1/1000 and spotted onto the MALDI target. The matrix solution consisted of 2,5 dihydroxybenzoic acid (Sigma) 10 mg mL−1 in 50 % ACN and 0.1 % TFA. Spectra were acquired in positive ion reflector mode under 20-kV accelerating voltage, a delay time of 80 s, and a mass range of 100–5000 Da. Each spectrum was the sum of 10,000 single laser shots randomized over ten positions within the same spot (1000 shots/position) at a laser frequency of 20 Hz. External calibration was performed using the Peptide mix 4 calibration mixture (Laser Bio Labs).
Statistical analysis
The software R (v. 2.12.0) for Windows was used, with the RStudio (v. 0.95.263) integrated development environment. All laboratory analyses were performed in triplicate, and results were expressed as mean values ± standard deviation (SD). As data did not respect the requirements for ANOVA (homogeneity of variances, tested with the Bartlett’s test at the 0.05 significance level), Kruskal–Wallis tests were performed, at a significance level of 95 %, to reveal potential significant differences. In the case of significant differences between the data, non-parametric multiple comparisons (Behrens–Fisher test) were applied using the nmpc package. Moreover, to test the correlation between phenolic content and antioxidant activity, principal component analysis (PCA) was performed with the FactoMineR package and cluster analysis with the fpc and pvclust packages. Pvclust is designed to assess the uncertainty in hierarchical cluster analysis. For each cluster in the hierarchical clustering, quantities called p values are calculated via multi-scale bootstrap resampling. The p value of a cluster indicates how strongly the cluster is supported by the data. Clusters with p values larger than 95 % are strongly supported by data.
Results
Total phenolic content
The purification of the CE of H. siliquosa was efficient in generating a phenolic-enriched EAF, as shown in Table 1, which compares the total phenolic contents (TPCs) of the CE and semi-purified fractions. Phenolic compounds were strongly concentrated in EAF. Indeed, almost 98 % of this fraction (975.73 ± 19.26 mg g−1 DW) consisted of phenolic compounds. The last 2 % is probably constituted of traces of fatty acids and carbohydrates, as it is shown by the 1H NMR analysis (Fig. 2). Conversely, AqF showed lower TPC (164.19 ± 7.58 mg g−1 DW) than CE (182.47 ± 3.33 mg g−1 DW; Kruskal–Wallis test: p < 0.05; Behrens–Fisher test).
Antioxidant activities
Table 1 shows results of the antioxidant activities of the CE and both semi-purified fractions compared with positive controls, assessed by DPPH radical scavenging activity, reducing power assay, superoxide anion scavenging activity, and the β-carotene bleaching method.
The CE and both fractions showed high DPPH radical scavenging activities. EAF exhibited the lowest IC50 (0.020 ± 3.54 × 10−4 mg mL−1), close to that obtained for the positive controls (e.g., 0.010 ± 4.82 × 10−5 mg mL−1 for BHA). The other antioxidant assays confirmed the results obtained by the DPPH test. Indeed, in the reducing power assay, EAF showed a low EC50 compared with those of the positive controls (p < 0.05, Behrens–Fisher test; 0.058 ± 0.001 mg mL−1 and 0.074 ± 0.002 mg mL−1 for EAF and BHA, respectively). In the superoxide anion scavenging test, the IC50 displayed by EAF (0.664 ± 0.019 mg mL−1) was equal to the IC50 obtained for BHA (0.664 ± 0.022 mg mL−1). In the β-carotene bleaching method, EAF showed the lowest AAC700 (0.206 ± 0.001 mg mL−1, p < 0.05, Behrens–Fisher test). It should be noted, however, that this AAC700 value is significantly higher than those of the positive controls (e.g., 0.015 ± 0.001 mg mL−1 for BHA) (Table 1). Despite this last result, EAF showed strong antioxidant activities. Furthermore, whatever the antioxidant assay considered, AqF always showed the lowest activity by exhibiting a high IC50 (DPPH and superoxide anion scavenging tests), a high EC50 (reducing power assay), and a strong AAC700 (β-carotene bleaching method). These results meant that AqF showed a weak antioxidant activity.
The EAF of H. siliquosa showed an interesting ORAC value of 5.39 ± 1.08 μmol TE mg−1 (Table 1), which is comparable to the ORAC value measured for ascorbic acid (9.35 ± 0.63) mentioned by Huang et al. (2010).
Correlations between antioxidant activities and phenolic content
It is worth noting that the EAF, with marked antioxidant activities, had the highest phenolic content (975.73 ± 19.26 mg mL−1 DW) whereas the AqF, which showed low antioxidant activities, had the lowest phenolic content (164.19 ± 7.58 mg mL−1 DW). These observations were confirmed by statistical analysis. Cluster analysis revealed a significant association between TPC and antioxidant activities (100 %). Moreover, non-significant associations were found between the two scavenging tests (DPPH radical scavenging and superoxide anion scavenging assays, 70 %) and between the reducing power test and β-carotene bleaching method (61 %). The principal component analysis (PCA, Fig. 3) confirmed that antioxidant activities were correlated with the TPC of the samples. Moreover, PCA showed that TPC values were strongly negatively correlated with EC50 obtained with reducing power. This means that the higher the measured TPC of a sample, the lower its EC50 and the stronger its antioxidant activity. Other antioxidant assays were less correlated with TPC. PCA also confirmed that DPPH radical scavenging and superoxide anion scavenging assays were positively associated.
Bactericidal activities
Results of the antibacterial assays are reported in Table 2. The negative control (1 % EtOH) had no effect on bacterial growth, whatever the pathogen. In contrast, at a concentration of 5 mg mL−1, EAF showed a strong bactericidal activity. Indeed, this fraction showed a reduction higher than 5 log of the initial bacterial concentration with Pseudomonas aeruginosa and Escherichia coli (<1 UFC mL−1). Concerning Staphylococcus aureus, EAF showed a bactericidal activity with a reduction of 4.5 log of the initial bacterial concentration (75 UFC mL−1).
Photoprotective sunscreen activity
The absorption spectrum of the H. siliquosa EAF shows a band with a maximum at 376 nm. Moreover, the O/W emulsion manufactured with this phlorotannin-enriched fraction (Halidrys emulsion) had a SPF of 3.55 ± 0.29 and a PF-UV-A value of 2.20 ± 0.13.
Structural elucidation of active molecules
Nuclear magnetic resonance analysis
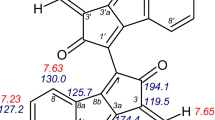
The 1H NMR spectrum of the EAF of H. siliquosa showed a broader distribution of the 1H signals between 5.8 and 6.3 ppm, characteristic of phlorotannins (Fig. 2). Observations from the HMBC experiments (Fig. 4 and Table 3) confirmed the presence of polyphenolic structures. Indeed, the HMBC spectrum showed characteristic carbon atom resonances at (1) 96.10 ppm, corresponding to quaternary methine groups; (2) between 124 and 132 ppm, corresponding to diaryl-ether bonds (ether-linked phloroglucinol units); (3) between 142 and 148 ppm for additional OH functions other than the 1,3,5 OH groups originally present in each phloroglucinol unit; and finally, (4) between 152 and 159 ppm, signals for phenolic carbons (Fig. 4 and Table 3). We can, therefore, hypothesize that fuhalol-type units, and also phlorethol-type units, are present. Moreover, it is worth noting the absence of signals between 100 and 105 ppm indicative of the absence of aryl-aryl carbons and, thus, the absence of fucol-type units in this purified phenolic sample.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
The MALDI-TOF analysis of the EAF of H. siliquosa revealed a molecular weight ranging from 222 to 763 Da (Table 4). It could be resumed that the EAF contained small polyphenolic compounds with no more than six phloroglucinol units (oligomers). The peak-to-peak mass increments observed at 273.06, 413.29, 523.36, and 537.38 Da could correspond to the formation of M+ Na adducts of (1) (250 Da), (3) (390 Da), and (4) (514 Da) (Table 4 and Fig. 5). Moreover, the peak-to-peak mass increment observed at 375.03 Da could correspond to the formation of M+ H adducts of (2) (374 Da). The peak-to-peak mass increments observed at 343.31 and 365.30 Da could correspond to the formation of M+ H and M+ Na adducts of a compound with a native mass of about 342 Da that seems not to be a phloroglucinol oligomer. Table 4 gives the experimental and calculated masses of the polyphenolic oligomers identified for the EAF sample (Fig. 5), together with their putative chemical structures. Masses of phlorethols and fuhalols were calculated using the respective chemical formula of a typical phlorethol and fuhalol (respectively, C6nH4n+2O3n and C6nH4n+2O3n+i , where n is the number of phloroglucinol units and i was the number of additional hydroxyl groups).
Discussion
The purification process used in the present study to purify phenolic compounds from H. siliquosa was highly efficient to isolating oligophlorotannins. This procedure was also described as efficient for other Sargassaceae species belonging to the genera Bifurcaria, Cystoseira, and Sargassum (Stiger-Pouvreau et al. 2014), for Fucaceae species such as Pelvetia canaliculata and Ascophyllum nodosum (Ar Gall et al. 2015), and also for halophytes (Surget et al. 2015). The preliminary structural analyses of the EAF, combined with data from the literature (McInnes et al. 1984; Cérantola et al. 2006), allowed the identification of four phenolic compounds: diphlorethols and triphlorethols and trifuhalols and tetrafuhalols. Until now, little information has existed on the chemical nature of phenolic compounds produced by H. siliquosa. Fuhalols had already been described in this species (Glombitza and Sattler 1973; Sattler et al. 1977), but as these studies were done on non-native compounds (peracetylated compounds), the true nature of the phlorotannins present in H. siliquosa was not fully explored. Moreover, the present study is the first time which identified phlorethol-type compounds in this species. Phlorethols are known to be present in other Sargassaceae species, such as in Sargassum muticum and some Cystoseira species from Brittany (see Stiger-Pouvreau et al. 2014 for a review). Moreover, the co-occurrence of fuhalols and phlorethols was already known in other Sargassaceae species, like Cystoseira tamariscifolia (Glombitza et al. 1975) and Sargassum spinuligerum (Keusgen and Glombitza 1995), but not in H. siliquosa. Additionally, the phenolic compounds identified in H. siliquosa in the present study were oligomers of phloroglucinol. These results are in line with previous data on Sargassaceae species, which produces low-molecular-weight phenolic compounds (Le Lann et al. 2012a; Le Lann et al. 2012b; Harnita et al. 2013; Jégou et al. 2015; Montero et al. 2016) rather than high-molecular-weight phlorotannins as observed in the Fucaceae and Laminariales (Wang et al. 2012; Shibata et al. 2015; Heffernan et al. 2015).
In order to characterize the antioxidant capacity of extracts and fractions of H. siliquosa, four fast, reliable, and classical biochemical methods were used. Three of them (DPPH, NBT, and reducing power tests) represent a single-electron transfer (SET) reaction, whereas the β-carotene bleaching method (BCBM) represents a hydrogen atom transfer (HAT) reaction (Huang et al. 2005). The antioxidant molecules can act as inhibitors of lipid oxidation through various different mechanisms in addition to free radical trapping, e.g., prevention of chain initiation, binding of transition metal ion catalysts, or peroxide decomposition (Frankel and Meyer 2000). Thus, simple in vitro tests are an approach to evaluate the antioxidant capacity of samples, and the combination of four of them made it possible to bypass the inability of a one-dimensional test of antioxidant capacity to accurately mirror the in vivo complexity of interactions between antioxidants in foods and biological systems (Frankel and Meyer 2000).
Among the tested samples, the EAF exhibited the highest antioxidant activity independent of what assay was used. Moreover, its antioxidant activity was equivalent to those displayed by the commercial antioxidants used as positive controls in this study (ascorbic acid, α-tocopherol, BHA, and Trolox) for the SET assays (DPPH, NBT, and reducing power). These results were reinforced by the ORAC value obtained for the EAF. Indeed, the ORAC value of the EAF (5.39 ± 1.08 μmol TE mg−1) was close to the ORAC values obtained for ascorbic acid in the literature (Huang et al. 2010; Ishimoto et al. 2012; Fujii et al. 2013). For the BCBM, the activity of the EAF is higher than those of commercial antioxidants. This test measures the activity of lipophilic molecules (Koleva et al. 2002; Le Lann et al. 2008), so the active compounds would therefore tend to be polar or faintly apolar compounds. Moreover, in the BCBM, the system is complex, and the emulsified lipid in use introduces additional variables liable to affect the oxidation process (Zubia et al. 2009).
To our knowledge, this study is the second to provide evidence for the existence of high antioxidant activity in this seaweed species, following a screening of antioxidant and antitumor activities of non-identified phlorotannins (Zubia et al. 2009). However, our paper is the first to identify active purified native phlorotannins from H. siliquosa. Moreover, the antioxidant activities exhibited by the extract and fractions of H. siliquosa were positively correlated with their phenolic contents. Additionally, Fujii et al. (2013) evaluated the antioxidative properties of phlorotannins isolated from the brown alga Eisenia bicyclis (Laminariales, Lessoniaceae). These authors found H-ORAC values of phloroglucinol (20.38 ± 1.11 μmol TE mg−1) and various eckols (H-ORAC values between 11.6 ± 1.2 and 20.62 ± 1.46 μmol TE mg−1). In the same way, in 2010, Parys et al. (2010) published ORAC values for phloroglucinol and fucophlorethols isolated from Fucus vesiculosus. Despite the unusual units used by these authors, their results showed that phloroglucinol was a little more active than fucophlorethols, meaning that oligomers of phloroglucinol could be more active than polymers for this test. Thus, our present study highlights, for the first time, the antioxidative potential of small phenolic compounds (oligofuhalols and oligophlorethols) from H. siliquosa. This enhances previous results on the high antioxidant potential of phenolic compounds from brown seaweeds. Further studies should now be carried out to isolate each phenolic compound and test them separately, to study potential synergy between phytochemicals. Furthermore, the marked correlation between the reducing power and the TPC could provide information on the antioxidant mechanism of phenolic compounds from H. siliquosa. Indeed, the reducing power determined in this work depends on the redox potentials of the compounds present in the sample. Therefore, it can be predicted that the phlorotannins present in the H. siliquosa EAF show low redox potential and, thus, a high antioxidant efficiency against free radicals (peroxyl or hydroxyl radicals) (Zhu et al. 2002).
The EAF of H. siliquosa also showed bactericidal activities against three bacterial strains: Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus. The antimicrobial activities of phenolic compounds from seaweeds are very well known (see Eom et al. 2012 for a review), including phenolic compounds from H. siliquosa (Sattler et al. 1977). The antibacterial activities of phlorotannins were reviewed by Li et al. (2011), but this is the first time that bactericidal activities have been reported for a phenolic-rich fraction from H. siliquosa. More specifically, phlorotannins from Ascophyllum nodosum showed bactericidal activities against Escherichia coli (Wang et al. 2009). The bactericidal activities of phenolic compounds seemed to be related to the number of hydroxyl groups (Smith et al. 2003; Wang et al. 2009) and to the degree of polymerization of phloroglucinol within phlorotannins (Nagayama et al. 2002). Moreover, Wang et al. (2009) suggested that condensed phlorotannins with high hydroxylation may inhibit bacterial growth by altering the cell membrane (Wang et al. 2009). The fuhalol compounds identified in the EAF of H. siliquosa have high hydroxyl functions. The oxidation potential of hydroxyl groups, well known in phlorotannins (Ragan and Glombitza 1986), could explain their bactericidal activity, by alteration of bacterial cell membranes. Because this work is a preliminary study, there is now a need to test the dose effect to identify the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of the EAF. Nevertheless, such fraction is interesting and could be used in cosmetic formula itself as natural conservators.
The SPF obtained for the Halidrys emulsion is similar to eight UV pure synthetic filters authorized in the European Union, like homosalate for example, tested in O/W emulsion with the same method (Couteau et al. 2007). While UV-B protection is imperative, UV-A protection is now recognized as being equally essential. Indeed, if UV-B is considered as “burning rays,” then UV-A can be considered as “aging rays” (Lautenschlager et al. 2007; Saewan and Jimtaisong 2015). With a maximal absorbance (376 nm) in the UV-A range (400–320 nm) and a PF-UV-A about 2.20 ± 0.13, the EAF of H. siliquosa exhibited an absorbance and a sunscreen activity similar to the synthetic filter Avobenzone (358 nm and 2.76 ± 0.31 for maximal absorbance and PF-UV-A, respectively) (Lohézic-Le Dévéhat et al. 2013). So, the EAF obtained from H. siliquosa is interesting because it appears to be a broad-spectrum UV absorber, useful to be included as a natural filter in solar cream for example.
The photoprotective capacities of plant extracts have been demonstrated in previous studies. Indeed, using a similar in vitro UV method, three sunscreen emulsions with ethyl acetate plant extract (10 wt%) were tested in vitro and authors obtained SPF and PF-UV-A values ranging from 6.00 ± 0.42 to 9.88 ± 1.66 and from 3.64 ± 0.07 to 6.96 ± 0.21, respectively (Jarzycka et al. 2013). Likewise, using the same protocol as the present study, one acetonic extract of the lichen Lasallia pustulata was found to have SPF and PF-UV-A maxima of 5.52 and 2.45, respectively (Lohézic-Le Dévéhat et al. 2013). Furthermore, usnic acid, extracted from the lichen Xanthoparmelia farinosa, was described as the best UV-B filter tested, with a protection factor similar to a commercial product called LSF 5 (4.1; Rancan et al. 2002). Among marine macrophytes, the EAF from a Salicornia ramosissima extract is an interesting candidate to provide an effective protective action over the whole UV-A–UV-B range, with large SPF and PF-UV-A values (Surget et al. 2015). Moreover, the photoprotection efficiency of seaweed extracts has been demonstrated for several species, as recently reviewed by Saewan and Jimtaisong (2015). For example, the photoprotective potential of extracts obtained from 11 commercial brown algae and 10 commercial red algae was evaluated against UV-B radiation (Guinea et al. 2012). In the same way, phlorotannins isolated and purified from Ecklonia cava were demonstrated as effective at protecting against UV-B radiation (Cha et al. 2012). Both of these studies used an in vivo test with a zebrafish (Danio rerio) embryo assay. In a more generalistic point of view, the common feature of UV-absorbing secondary metabolites is the presence of aromatic or conjugated bond structures as it is found in phlorotannins. Such molecules present a π-electron system, which is one of the most effective UV radiation absorbers (Cockell and Knowland 1999).
One other point that would be taken into consideration is the spatio-temporal variability of phenolic contents. Indeed, the phlorotannin pool strongly depends on sites, seasons, and phenology of algae (i.e., Parys et al. 2009; Le Lann et al. 2012a; Celis-Plá et al. 2016). So, knowledge of the chemical ecology of potentially exploitable species is essential to identify the best sites and seasons for the highest bioactivities.
In conclusion, this study emphasized, for the first time, the sunscreen potential and strong antioxidant and antibacterial capacities of a mix of four small phlorotannins, i.e., diphlorethols and triphlorethols and trifuhalols and tetrafuhalols, from the brown macroalga H. siliquosa. The antioxidant and sunscreen activities were found to be equivalent to several commercial antioxidant molecules and to some synthetic UV filters. Moreover, the correlation found between antioxidant activities and TPC supports the involvement of phenolic compounds in the antioxidant mechanisms. Furthermore, the EAF showed bactericidal activities against Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli, which represents interesting properties for proposing these compounds as natural preservatives in cosmetics for example. These findings therefore highlight the potential of this brown seaweed as a natural source of phytochemicals with several biological activities of interest to both the cosmetics and pharmaceutical industries. Nevertheless, it would be worth carrying out additional experiments in order to evaluate the photostability of compounds over time and to check for the absence of cytotoxicity so they can be used in formulations. Moreover, because our results suggest that bioactivities of the EAF are derived from the synergy between the active phlorotannins, other further studies need to be carried out to isolate and to separately test the bioactive compounds present in the active fraction in order to gain a better understanding of their mechanisms of action.
References
Ar Gall E, Lelchat F, Hupel M, Jegou C, Stiger-Pouvreau V (2015) Extraction and purification of phenols (phlorotannins) from brown algae. In: Stengel D, Connan S (eds) Natural products from marine algae: methods and protocols. Springer, New York, pp 131–143
Bourgougnon N, Stiger-Pouvreau V (2011) Chemodiversity and bioactivity within red and brown macroalgae along the French coasts, metropole and overseas departments and territories. In: Kim S-K (ed) Handbook of marine macroalgae. Wiley, Ltd, London, pp 58–105
Cao G, Alessio HM, Cutler RG (1993) Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med 14:303–311
Celis-Plá PSM, Bouzon ZL, Hall-Spencer JM et al (2016) Seasonal biochemical and photophysiological responses in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Mar Environ Res 115:89–97
Cérantola S, Breton F, Gall EA, Deslandes E (2006) Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot Mar 49:347–351
Cha S-H, Ko C-I, Kim D, Jeon Y-J (2012) Protective effects of phlorotannins against ultraviolet B radiation in zebrafish (Danio rerio). Vet Dermatol 23:51–e12
Chua M-T, Tung Y-T, Chang S-T (2008) Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour Technol 99:1918–1925
Cockell CS, Knowland J (1999) Ultraviolet radiation screening compounds. Biol Rev Camb Philos Soc 74:311–345
Couteau C, Pommier M, Paparis E, Coiffard LJM (2007) Study of the efficacy of 18 sun filters authorized in European Union tested in vitro. Pharm - Pharmazie 62:449–452
Diffey BL, Robson J (1989) A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J Soc Cosmet Chem 40:127–133
Dudonné S, Vitrac X, Coutière P, Woillez M, Mérillon JM (2009) Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem 57:1768–1774
El-Boury S, Couteau C, Boulande L, Paparis E, Coiffard LJM (2007) Effect of the combination of organic and inorganic filters on the sun protection factor (SPF) determined by in vitro method. Int J Pharm 340:1–5
Eom S-H, Kim Y-M, Kim S-K (2012) Antimicrobial effect of phlorotannins from marine brown algae. Food Chem Toxicol 50:3251–3255
European Pharmacopoeia 7.0 - 2011. https://www.edqm.eu. Accessed 27 Oct 2015
Frankel EN, Meyer AS (2000) The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J Sci Food Agric 80:1925–1941
Fujii Y, Tanaka R, Miyake H et al (2013) Evaluation for antioxidative properties of phlorotannins isolated from the brown alga Eisenia bicyclis, by the H-ORAC method. Food Nutr Sci 4:78–82
Fukumoto LR, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48:3597–3604
Glombitza K-W, Sattler E (1973) Trifuhalol, ein neuer triphenyldiäther aus Halidrys siliquosa (1). Tet Lett 14:4277–4280
Glombitza K-W, Rosener H-U, Müller D (1975) Bifuhalol und diphlorethol aus Cystoseira tamariscifolia. Phytochemistry 14:1115–1116
Guinea M, Franco V, Araujo-Bazán L, Rodríguez-Martín I, González S (2012) In vivo UVB-photoprotective activity of extracts from commercial marine macroalgae. Food Chem Toxicol 50:1109–1117
Harnita ANI, Santosa IE, Martono S, Sudarsono S, Widyarini S, Harren FJ (2013) Inhibition of lipid peroxidation induced by ultraviolet radiation by crude phlorotannis isolated from brown algae Sargassum hystrix v. buxifolium C. Agardh. Indones J Chem 13:14–20
Heffernan N, Brunton NP, FitzGerald RJ, Smyth TJ (2015) Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar Drugs 13:509–528
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Huang W-Y, Majumder K, Wu J (2010) Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chem 123:635–641
Huovinen P, Gómez I (2015) UV sensitivity of vegetative and reproductive tissues of two antarctic brown algae is related to differential allocation of phenolic substances. Photochem Photobiol 91:1382–1388
Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Horikawa T (2003) UV-induced skin damage. Toxicology 189:21–39
Ishimoto H, Tai A, Yoshimura M, Amakura Y, Yoshida T, Hatano T, Ito H (2012) Antioxidative properties of functional polyphenols and their metabolites assessed by an ORAC assay. Biosci Biotech Biochem 76:395–399
Jarzycka A, Lewińska A, Gancarz R, Wilk KA (2013) Assessment of extracts of Helichrysum arenarium, Crataegus monogyna, Sambucus nigra in photoprotective UVA and UVB; photostability in cosmetic emulsions. J Photochem Photobiol B 128:50–57
Jégou C, Kervarec N, Cérantola S, Bihannic I, Stiger-Pouvreau V (2015) NMR use to quantify phlorotannins. The case of Cystoseira tamariscifolia, a phloroglucinol-producing brown macroalga in Brittany (France). Talanta 135:1–6
Kang Y-M, Eom S-H, Kim Y-M (2013) Protective effect of phlorotannins from Eisenia bicyclis against lipopolysaccharide-stimulated inflammation in HepG2 cells. Environ Toxicol Pharmacol 35:395–401
Kaur C, Kapoor HC (2002) Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol 37:153–161
Keusgen M, Glombitza K-W (1995) Phlorethols, fuhalols and their derivatives from the brown alga Sargassum spinuligerum. Phytochemistry 38:975–985
Kim AR, Shin TS, Lee MS, Park JY, Park KE, Yoon NY, Kim JS, Choi JS, Jang BC, Byun DS, Park NK, Kim HR (2009) Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J Agric Food Chem 57:3483–3489
Koleva II, Van Beek TA, Linssen JPH, Groot AD, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13:8–17
Kornprobst JM (2010) Encyclopedia of marine natural products. Wiley-Blackwell, Weinheim
Lautenschlager S, Wulf HC, Pittelkow MR (2007) Photoprotection. Lancet 370:528–537
Le Lann K, Jégou C, Stiger-Pouvreau V (2008) Effect of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol Res 56:238–245
Le Lann K, Connan S, Stiger-Pouvreau V (2012a) Phenology, TPC and size-fractioning phenolics variability in temperate Sargassaceae (Phaeophyceae, Fucales) from Western Brittany: native vs. introduced species. Mar Environ Res 80:1–11
Le Lann K, Ferret C, VanMee E, Spagnol C, Lhuillery M, Payri C, Stiger‐Pouvreau V (2012b) Total phenolic, size-fractionated phenolics and fucoxanthin content of tropical Sargassaceae (Fucales, Phaeophyceae) from the South Pacific Ocean: spatial and specific variability. Phycol Res 60:37–50
Li Y-X, Wijesekara I, Li Y, Kim S-K (2011) Phlorotannins as bioactive agents from brown algae. Process Biochem 46:2219–2224
Liu H, Zhao L, Guo S, Xia Y, Zhou P (2011) Modification of fish skin collagen film and absorption property of tannic acid. J Food Sci Technol 51(6):1102–1109
Lohézic-Le Dévéhat F, Legouin B, Couteau C, Boustie J, Coiffard L (2013) Lichenic extracts and metabolites as UV filters. J Photochem Photobiol B 120:17–28
McInnes AG, Ragan MA, Smith DG, Walter JA (1984) High-molecular-weight phloroglucinol-based tannins from brown algae: structural variants. Hydrobiologia 116-117:597–602
Montero L, Sánchez-Camargo AP, García-Cañas V, Tanniou A, Stiger-Pouvreau V, Russo M, Rastrelli L, Cifuentes A, Herrero M, Ibáñez E (2016) Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J Chromatogr A 1428:115–125
Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T (2002) Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother 50:889–893
Parys S, Kehraus S, Pete R, Küpper FC, Glombitza KW, König GM (2009) Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur J Phycol 44:331–338
Parys S, Kehraus S, Krick A, Glombitza KW, Carmeli S, Klimo K, Gerhäuser C, König GM (2010) In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 71:221–229
Pavia H, Brock E (2000) Extrinsic factors influencing phlorotannin production in the brown alga Ascophyllum nodosum. Mar Ecol Process Ser 193:285–294
Ragan MA, Glombitza K-W (1986) Phlorotannins, brown algal polyphenols. Prog Phycol Res 4:129–241
Rancan F, Rosan S, Boehm K, Fernández E, Hidalgo ME, Quihot W, Rubio C, Boehm F, Piazena H, Oltmanns U (2002) Protection against UVB irradiation by natural filters extracted from lichens. J Photochem Photobiol B 68:133–139
Saewan N, Jimtaisong A (2015) Natural products as photoprotection. J Cosmet Dermatol 14:47–63
Sambandan DR, Ratner D (2011) Sunscreens: an overview and update. J Am Acad Dermatol 64:748–758
Sattler E, Glombitza K-W, Wehrli FW, Eckhardt G (1977) Antibiotica aus algen—XVI : Polyhydroxyphenyläther aus der Phaeophycee Halidrys siliquosa. Tetrahedron 33:1239–1244
Schoenwaelder MEA (2002) The occurrence and cellular significance of physodes in brown algae. Phycologia 41:125–139
Shibata T, Nagayama K, Sugiura S, Makino S, Ueda M, Tamaru Y (2015) Analysis on composition and antioxidative properties of phlorotannins isolated from Japanese Eisenia and Ecklonia species. Am J Plant Sci 06:2510–2521
Singh IP, Bharate SB (2006) Phloroglucinol compounds of natural origin. Nat Prod Rep 23:558–591
Smith AH, Imlay JA, Mackie RI (2003) Increasing the oxidative stress response allows Escherichia coli to overcome inhibitory effects of condensed tannins. Appl Environ Microbiol 69:3406–3411
Stiger-Pouvreau V, Jégou C, Cérantola S, Guérard F, Le Lann K (2014) Phlorotannins in Sargassaceae species from Brittany (France): Interesting molecules for ecophysiological and valorisation purposes. In: Nathalie Bourgougnon, Editor(s), Advances in Botanical Research, Academic Press. 71: 379–411
Surget G, Stiger-Pouvreau V, Le Lann K, Kervarec N, Couteau C, Coiffard LJM, Gaillard F, Cahier K, Guérard F, Poupart N (2015) Structural elucidation, in vitro antioxidant and photoprotective capacities of a purified polyphenolic-enriched fraction from a saltmarsh plant. J Photochem Photobiol B 143:52–60
Swanson AK, Druehl LD (2002) Induction, exudation and the UV protective role of kelp phlorotannins. Aquat Bot 73:241–253
Tanniou A, Vandanjon L, Incera M, Serrano Leon E, Husa V, Le Grand J, Nicolas JL, Poupart N, Kervarec N, Engelen A, Walsh R, Guerard F, Bourgougnon N, Stiger-Pouvreau V (2014) Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J Appl Phycol 26:1215–1230
Thomas P, Swaminathan A, Lucas RM (2012) Climate change and health with an emphasis on interactions with ultraviolet radiation: a review. Glob Change Biol 18:2392–2405
Turkmen N, Velioglu YS, Sari F, Polat G (2007) Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules 12:484–496
Wang T, Jónsdóttir R, Olafsdóttir G (2009) Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem 116:240–248
Wang T, Jónsdóttir R, Liu H, Gu L, Kristinsson HG, Raghavan S, Ólafsdóttir G (2012) Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J Agric Food Chem 60:5874–5883
Zhu QY, Hackman RM, Ensunsa JL, Holt RR, Keen CL (2002) Antioxidative activities of oolong tea. J Agric Food Chem 50:6929–6934
Zubia M, Fabre MS, Kerjean V, Le Lann K, Stiger-Pouvreau V, Fauchon M, Deslandes E (2009) Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem 116:693–701
Acknowledgments
This work was co-financed with the support of the European Union ERDF-Atlantic Area Programme, MARMED project no. 2011-1/164 and the project RIV-ALG (n° 13006760), financed by CBB Development, and the region Bretagne (France). Authors from LEMAR would like to thank the Labex Mer initiative, a State Grant from the French Agence Nationale de la Recherche (ANR) in the “Investissements d’avenir” Program (reference ANR-10-LABX-19-01, Labex Mer). The first author thanks Alexandre Canton for his assistance during the extraction and purification process and Helen McCombie for reviewing English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. All authors contributed significantly to this work and are in agreement with the content of the manuscript.
Authors’ contributions
Klervi Le Lann, Valérie Stiger-Pouvreau and Laurence Coiffard conceived and designed the experiments. Klervi Le Lann, Gwladys Surget, Céline Couteau, Stéphane Cérantola, and Fanny Gaillard performed the experiments. Klervi Le Lann analyzed the data. Mayalen Zubia, Fabienne Guérard, Nathalie Poupart, and Valérie Stiger-Pouvreau contributed materials and analysis tools. Klervi Le Lann, Valérie Stiger-Pouvreau, Fabienne Guérard, Gwladys Surget, Stéphane Cérantola, Mayalen Zubia, and Laurence Coiffard wrote the paper.
Rights and permissions
About this article
Cite this article
Le Lann, K., Surget, G., Couteau, C. et al. Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys siliquosa . J Appl Phycol 28, 3547–3559 (2016). https://doi.org/10.1007/s10811-016-0853-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0853-0