Abstract
The present study aims to investigate antioxidant activity and the phenolic content in five terrestrial cyanobacterial strains isolated from the Fruška Gora mountain forest ecosystem (Serbia). The antioxidant activity of crude ethanolic extracts was assessed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing ability of plasma (FRAP) assay. All the tested cyanobacterial strains belonging to Phormidium, Nostoc, Oscillatoria, Anabaena, and Calothrix genera exhibited antioxidant activity. The ethanolic extracts from Calothrix M2 showed strong inhibition against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals (half maximal inhibitory concentration (IC50) = 30.72 ± 3.31 μg mL−1) whereby Phormidium M1 expressed the highest radical potential in FRAP assay (22.48 ± 2.18 mg ascorbic acid equivalents (AAE) g−1). Furthermore, the presence and content of 15 phenolics in ethanolic extracts of the tested cyanobacterial strains were identified using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The richest strain in polyphenolics was Phormidium M1 which contained 12 compounds, mostly phenolic acids and flavonoid glycosides, followed by Nostoc M1 and Oscillatoria M2. The noticeable amount of phenolic acids (quinic acid 502 μg g−1; gallic acid 84.9 μg g−1; vanillic acid 50 μg g−1) was observed in Phormidium M1 indicating that phenolic compounds could contribute to the antioxidant activity observed in FRAP assay. LC-MS/MS analysis showed that the tested terrestrial cyanobacterial species could be regarded as a promising new source of bioactive phenolic compounds. Since no significant correlation was observed between phycocyanin content and antioxidant activity, other compounds present in the crude extracts contributed to antioxidant capacities in DPPH and FRAP assay. This study highlights the potential of terrestrial cyanobacterial strains inhabiting forest ecosystems as an interesting source of compounds with antioxidant potential for further researches and exploitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are a group of photosynthetic prokaryotes comprising more than 150 genera and 2200 species that play diverse yet significant roles in aquatic and terrestrial ecosystems (Woese 1987). As a result of obstinate survival in assorted habitats, cyanobacteria produce a range of secondary metabolites, each with specialized functions in order to compete successfully in different environments (Rastogi and Sinha 2009). Moreover, because of their phototrophic life and permanent exposure to high oxygen and radical stresses, they have a high capability for production of numerous efficient protective chemicals against oxidative and radical stressors (Tsao and Deng 2004). These microorganisms exhibit adaptive responses to oxidative stresses by stimulation of their intrinsic antioxidant defense system (Srivastava et al. 2005) based on mediator compounds such as ascorbic acid, reduced glutathione, tocopherols, carotenoids, and phycocyanin (Guedes et al. 2013). Among these compounds different biological activities (such as anti-inflammatory, anti-hepatotoxicity, anti-cancerogenic activity) of phycocyanin are well documented due to its radical scavenging-antioxidant properties (Soni et al. 2008). As a major antenna pigment, phycocyanin is present in all cyanobacteria (Gantar et al. 2012; Simeunović et al. 2013), and because of the linear tetrapyrrole prosthetic group commonly called bilins, phycocyanin has the ability to scavenge reactive oxygen species (ROS) and to act as an antioxidant (Soni et al. 2008).
Another group of compounds which are particularly considered as one of the most important classes of natural antioxidants are phenolic compounds (Machu et al. 2015) due to their structural requirements of free radical scavengers (Kepekçi and Saygideger 2012). Chemically, polyphenols can be divided into several classes, such as phenolic acids (hydroxybenzoic acids, hydroxycinnamic acids), flavonoids (flavones, flavonols, flavanones, flavanonols, flavanols, anthocyanins), isoflavonoids (isoflavones, coumestans), stilbenes, lignans, and phenolic polymers (Manach et al. 2004). They represent a ubiquitous and widespread class of secondary metabolites produced from the shikimate, acetate, and potentially the proline-linked pentose phosphate pathways in plants (Kepekçi and Saygideger 2012). Due to the fact that microalgae are considered to be the first evolutionary photoautotrophic organisms, they carry out many of the same fundamental biochemical processes that produce phenolic compounds which can be also found in higher plants (Duval et al. 1999). However, little information is available concerning the presence of phenolic compound in microalgae (Goiris et al. 2012; Kepekçi et al. 2013). The occurrence of phenolic compounds in cyanobacteria also is less documented than in higher plants (Santoyo et al. 2006; Colla et al. 2007). and information about specific phenols in cyanobacteria has not been sufficiently examined (Klejdus et al. 2009). although they are capable of producing relatively complex polyphenols (Goiris et al. 2014).
Having in mind the significance of cyanobacteria as potent producers of various bioactive compounds with antioxidant activity and regarding the fact that terrestrial cyanobacteria inhabiting Serbian forests are still unexplored in this respect, it is worthwhile to investigate the antioxidant activity as well as phenolic content of these novel isolated cyanobacteria. The aim of this study was to determinate the antioxidant activity of terrestrial cyanobacterial strains using two different in vitro antioxidant activity tests. Because cyanobacteria can produce a wide range of antioxidant compounds, an important goal of this study was the identification and quantification of active compounds such as phenols by chromatography and mass spectrometry in selected cyanobacterial strains. Another aim of this study was to establish the relationship between phycobiliprotein pigment phycocyanin (PC), known for its ability to scavenge free radicals, and antioxidant activity. Furthermore, the results of this study could also give insight in the metabolic activity and phenolic content of different cyanobacterial genera originating from forest environments which are still insufficiently explored and highlight the most potent strains.
Material and methods
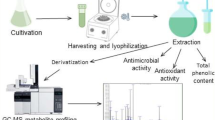
In this study, five cyanobacterial strains belonging to five different genera including Anabaena, Nostoc, Calothrix, Oscillatoria, and Phormidium were used to investigate the antioxidant activity. Tested cyanobacterial strains were isolated from the forest ecosystem in the Vojvodina region of Serbia (Fruška Gora mountain, area Morović) and have been cultivated in the laboratory within the Culture Collection of Cyanobacteria—NSCCC at the Department of Biology and Ecology in Novi Sad. The strains were grown photoautotrophically in 250-mL Erlenmeyer flasks containing the mineral, nutrient medium BG-11 with NaNO3 as nitrogen source in concentration of 2 g L−1 and in BG-11 medium without nitrogen (Rippka et al. 1979). Heterocystous cyanobacterial strains Anabaena M2, Nostoc M1, and Calothrix M2 were grown in medium BG-11 without nitrogen. Non-heterocystous strains Oscillatoria M2 and Phormidium M1 were grown in nutrient medium BG-11 supplied with NaNO3 as a nitrogen source. The cultures were incubated at 22–24 ± 1 °C under the illumination of cool white fluorescent light (50 μmol photons m−2 s−1). The used mode of dark and light period was 12-h light and 12-h dark. The pigment content, antioxidant activity, and phenolic content were determined on the 21st day of cultivation in the stationary phase of growth.
Preparation of the cyanobacterial extracts
Lyophilized biomass obtained after the 21st day of cultivation was used for the preparation of cyanobacterial extracts which were further applied in two antioxidant tests, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing ability of plasma (FRAP) method, and used for the identification of phenolic compounds. The extraction was achieved by suspension of lyophilized biomass in 70 % ethanol (1 g of biomass added to 100 mL of ethanol), followed by homogenization of the samples (1 g biomass was mixed for 60 min). The further destruction of the cell wall and the release of intracellular content were performed by sonication of the samples from 2 to 3 min in an ultrasonic cleaning bath (Ultrasons, J.P. Selecta, s.a., Spain) at room temperature, atmospheric pressure, and 50/60 Hz. The cell debris was removed by centrifugation at 10,000×g for 10 min. After centrifugation, supernatants of all tested cyanobacterial strains were separated. In the end, for each strain, five different concentrations of extracts were made in order to determine the half maximal inhibitory concentration (IC50) values in the case of DPPH method and milligram of ascorbic acid equivalents per gram in the case of FRAP method.
Determination of phycobiliproteins
The content of phycobiliprotein pigment, phycocyanin, was determined using the method described by Bennett and Bogorad (1973). Cyanobacterial strains were grown in Erlenmeyer vessels where the inoculum of 2 mL was inoculated in 200 mL of medium. After 21 days incubation the cultures were lyophilized. The lyophilized biomass was used for phycocyanin extraction, and pigment concentration was calculated according to dry biomass. The quantitative extraction of biliprotein was achieved by suspension in 5 mL 0.01 M sodium phosphate buffer (pH 7.0). Further destruction of the cell wall and releasing of intracellular content was performed by sonication for 10 min with cycles of 30 s at room temperature, atmospheric pressure, and 50/60 Hz. The cell debris was removed by centrifugation at 10,000×g for 15 min at room temperature. After centrifugation, all supernatants were separated. The concentration of phycocyanin (PC) was calculated spectrophotometrically (PC (mg mL−1) = (A615-0.474 × A652)/5.34). Sodium phosphate buffer was used as a blank.
DPPH• free radical scavenging assay
Antioxidant activity of all extracts was measured according to the method described by Espin et al. (2000). For each cyanobacterial strain, five different concentrations were tested (from 30 to 200 μg mL−1 in the reaction mixtures). As a positive control, synthetic antioxidant 3,5-di-tert-butyl-4-hydroxytoluene (BHT) was used. The procedure followed was the following: 15.7 mg of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was weighed and dissolved in absolute ethanol. This solution was stored at 4 °C in the dark. For antioxidant activity, the working solution of 90 μM DPPH was prepared on the day of measurement (22 mL of primary DPPH solution dissolved to a volume of 100 mL in methanol). A volume of 10 μL of the sample was transferred into a 96-well plate with 60 μL of freshly prepared DPPH reagent. In the control, the exact amount of the extract was substituted with solvent, and in the blank, only methanol and extract were mixed. The plate was left to stand in the dark at room temperature for 30 min. The absorbance was measured at 515 nm using a Multiskan GO (Thermo Scientific) plate reader.
The antioxidant activity of the extracts was expressed as a concentration of the extract that inhibited the DPPH radical formation by 50 % (IC50). Corresponding inhibition-concentration curves were drawn using Origin software, version 8.0, and IC50 values were determined. All of the results are expressed as mean ± SD of the three different working trials.
Ferric reducing ability of plasma assay
The ferric reducing/antioxidant power (FRAP) assay was according to Benzie and Strain (1996) for 96-well microplates. Cyanobacterial extracts were prepared in the concentrations ranging from 29 to 116 μg mL−1, whereas ascorbic acid ranging from 0 to 38.91 μg mL−1 was used to create a standard curve. The reduction potential of cyanobacterial extracts was calculated using the calibration curve of the standard solution of ascorbic acid (R 2 = 0.99). Synthetic antioxidant BHT was used as a positive control.
The working FRAP reagent was produced by mixing 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) solution and 20 mM FeCl3·6H2O in a 10:1:1 ratio prior to use and heated to 37 °C. The 300 mM acetate buffer was prepared by mixing 0.31 g of sodium acetate (C2H3NaO2) with 1.6 mL glacial acetic acid (C2H4O2) and brought to 100 mL with dH2O. The TPTZ solution was prepared by mixing 10 mM TPTZ with 40 mM HCl.
A volume of 10 μL of the sample was transferred into a 96 well plate with 225 μL of freshly prepared FRAP reagent. Due to the coloration of the extracts, it was necessary to prepare a control (blank) which consisted of 10 μL of 70 % ethanol (which was used as extraction agent) added to 225 μL of FRAP reagent and 22.5 μL of dH2O. The absorbance at 593 nm was determinate after 6 min incubation in dark at room temperature. All the samples were made in triplicate and mean values of reducing power were expressed as milligram of ascorbic acid equivalents (AAE) per gram of dry weight, calculated according to the standard calibration curve.
HPLC-MS/MS determination of the phenolic compounds
For HPLC-tandem mass spectrometry (MS/MS) determination of the phenolic profile, the method of Orčić et al. (2014) was used. All extracts were diluted with mobile phase solvents A (0.05 % aqueous formic acid) and B (methanol), premixed in 1:1 ratio, to obtain the final concentration 2 mg mL−1. Fifteen working standards, ranging from 1.53 to 25.0 × 103 ng mL−1, were prepared by serial 1:1 dilutions of standard mixture with solvents A and B (1:1). Samples and standards were analyzed using an Agilent Technologies 1200 Series high-performance liquid chromatograph coupled with Agilent Technologies 6410A Triple Quad tandem mass spectrometer with electrospray ion source and controlled by Agilent Technologies MassHunter Workstation software - Data Acquisition (ver. B.03.01). Five microliters was injected into the system, and compounds were separated on Zorbax Eclipse XDB-C18 (50 mm × 4.6 mm, 1.8 μm) rapid resolution column held at 50 °C. Mobile phase was delivered at flow rate of 1 mL min−1 in gradient mode (0 min 30 % B, 6 min 70 % B, 9 min 100 % B, 12 min 100 % B, re-equilibration time 3 min). Eluted compounds were detected by ESI-MS, using the ion source parameters as follows: nebulization gas (N2) pressure 276 kPa, drying gas (N2) flow 9 L min−1 and temperature 350 °C, capillary voltage 4 kV, and negative polarity. The data were acquired in dynamic MRM mode, using the optimized compound-specific parameters (retention time, precursor ion, product ion, fragmentor voltage, collision voltage) given in Orčić et al. (2014). For all the compounds, the peak areas were determined using Agilent MassHunter Workstation software - Qualitative Analysis (ver. B.03.01.). Calibration curves were plotted and samples concentrations calculated using the OriginLabs Origin Pro (ver. 8.0) software.
Standards and reagents
Deionized water was produced using a Millipore water purification system. Reference standards of the phenolic compounds were obtained from Sigma-Aldrich Chem (Germany), Fluka Chemie (Switzerland), or ChromaDex (USA). HPLC gradient grade methanol was from J. T. Baker (The Netherlands) and formic acid from Merck (Germany). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was from Fluka Chemie (Switzerland). Ascorbic acid and 2,4,6-tripyridyl-s-triazine (TPTZ) were from Merck. 3,5-di-tert-Butyl-4-hydroxytoulene (BHT) was from Sigma-Aldrich (Germany).
Statistical analyses
Corresponding inhibition-concentration curves were drawn using Origin software, version 8.0 and IC50 values (concentration of extracts that inhibited DPPH) were determined. All the results are expressed as mean value ± SD of the three different trails. The data were subjected to analysis of variance (one-way ANOVA), and the means were compared by the least significant difference test. A probability value of P = 0.05 was considered significant. Pearson’s correlation and regression analyses were carried out using StatSoft to evaluate the relationship between the antioxidant capacity and the phycocyanin content.
Results and discussion
In vitro antiradical activity tests
In the present study, antioxidant activity was observed in the case of all the five terrestrial cyanobacterial strains belonging to Nostoc, Anabaena, Calothrix, Oscillatoria, and Phormidium genera. Since ethanolic extracts of all the tested strains expressed radical scavenging ability towards DPPH radicals as well as reducing power, the obtained data indicates that antiradical in vitro activity was a strain-specific property (Table 1).
The antiradical activity was determined by the changes in the absorbance value of the sample extract solution when it has reduced 2,2-diphenyl-1-picrylhydrazyl (DPPH˙) free radicals. Among the tested genera, the level of DPPH activity differed quite significantly (ANOVA, F = 66.689, P = 0.00). In particular, ethanolic extracts of Calothrix M2 exhibited the most effective scavenging ability on DPPH radicals with IC50 = 30.72 ± 3.31 μg mL−1 (Table 1). The effective scavengers of DPPH radical were also ethanolic extracts of Phormidium M1 and Anabaena M2 (IC50 = 47.62 ± 7.59 μg mL−1, IC50 = 50.54 ± 4.74 μg mL−1). However, the extracts of two cyanobacterial strains, Oscillatoria M2 and Nostoc M1, showed a poorer activity towards DPPH radical (IC50 = 93.25 ± 8.63 μg mL−1, IC50 = 102.47 ± 7.29 μg mL−1). The ability of extracts and BHT to neutralize DPPH radical increased in the following order: BHT < Calothrix M2 < Phormidium M1 < Anabaena M2 < Oscillatoria M2 < Nostoc M1, indicating a lower activity of the extracts of the tested cyanobacterial strains with regards to the synthetic antioxidant BHT (IC50 = 9.8 ± 1.00 μg mL−1). Compared to the highest radical scavenging activity observed for water fractions of Nostoc sp. (41.81 ± 2.56), Nostoc muscorum (45.24 ± 2.78) and Anabaena cylindrica (84.91 ± 4.89) (Hajimahmoodi et al. 2010). the ethanolic extracts of the strain Calothrix M2 were more potent. In addition, compared to the three Synechocystis strains (IC50 = 54.59 ± 2.2 μg mL−1, IC50 = 56.79 ± 1.8 μg mL−1, IC50 = 65.16 ± 1.4 μg mL−1), Leptolyngbya sp. BASO700 (IC50 = 69.05 ± 1.2 μg mL−1) and Oscillatoria sp. BASO703 (IC50 = 78.43 ± 2.4 μg mL−1) (Babaoğlu Aydaş et al. 2013), the ability to neutralize DPPH radical was higher in the case of three strains (Calothrix M2, Phormidium M1, and Anabaena M2) tested in this study.
In the case of FRAP method, the antiradical activity was calculated based on the reduction of a ferric-tripyridyl triazine complex to its ferrous-blue-colored form in the presence of antioxidants. The reducing power of extracts (Table 1), determinated as ascorbic acid equivalent (AAE) per gram of dry weight (dw), was not significantly different among the tested cyanobacterial strains (ANOVA, F = 0.95, P = 0.47). The most potent strain was Phormidium M1 (22.48 ± 2.18 mg AAE g−1) followed by Oscillatoria M2 (22.04 ± 2.35 mg AAE g−1) whereby Anabaena M2 (21.93 ± 3.88 mg AAE g−1) and Nostoc M1 (21.50 ± 2.21 mg AAE g−1) expressed a similar activity. In contrast to the results obtained in the DPPH method, the strain with the lowest reducing ability was Calothrix M2 (16.88 ± 2.33 mg AAE g−1). Compared to the synthetic antioxidant BHT (25.32 ± 2.50 mg AAE g−1), the tested strains expressed lower reduction capacity. The recorded values were in similar range with the results obtained by Kepekçi and Saygideger (2012) for Arthrospira (Spirulina) platensis methanol extracts (25.63 ± 0.35 μM AAE g−1, 35.57 ± 1.03 μM AAE g−1). In the study (Pandey and Pandey 2008). the maximum antioxidant capacity (120.35 μM AAE g−1) was observed in aqueous extract of immobilized cell cultures of Nostoc lobatus supplied with P and Fe indicating the importance of supplements on the enhanced production of antioxidants.
Moreover, in the present study, antiradical activities of the tested strains were different in DPPH and FRAP methods which is probably due to the presence of diverse groups of antioxidant compounds in the crude extracts. According to Hajimahmoodi et al. (2010) in DPPH assay, some other functional components could be effective compared to FRAP assay given that the FRAP assay can react with iron (II) and SH-group-containing antioxidants (Benzie and Strain 1996) and DPPH method uses organic radicals (Chandrasekar et al. 2006). Furthermore, in order to extensively characterize the antioxidant potential of the extracts, the total antioxidant activity should be evaluated by different methods due to the fact that antioxidant activity can be manifested in a wide range of actions (Beara et al. 2012) and different antioxidant mechanisms should be taken into account (Goiris et al. 2012). Thus, different antioxidant activity assays should be performed additionally for all the tested strains in order to extensively characterize their antioxidant capacity.
Determination of phenolic profile
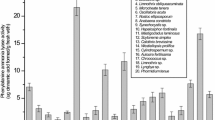
An important goal of this study was to identify and quantify particular phenols present in ethanolic extracts of the tested cyanobacterial strains regarding the fact that these compounds can act as antioxidant agents. In the selected cyanobacterial strains, high-performance liquid chromatography coupled with triple-quadrupole mass spectrometer (HPLC-MS/MS) was used to analyze the contents of flavonoids and phenolic acids in the tested strains. A total of 15 phenolic compounds were identified and quantified (Fig. 1) using the method optimized for quantification of 45 phenols, previously developed by Orčić et al. (2014).
The results showed that phenolic composition was significantly different among the strains (Table 2). Ethanolic extract of Phormidium M1 was the richest in phenolic compounds compared to other tested strains. Moreover, it should be noted that this strain expressed the highest reducing power in FRAP assay indicating that phenolic compounds could potentially contribute to the observed antioxidant activity. In this strain, 12 phenolic compounds were identified. The most dominant phenolic acids found in Phormidium M1 were quinic acid (502 μg g−1 dw), followed by gallic acid (84.9 μg g−1 dw) and vanillic acid (50 μg g−1 dw). Compared to the results obtained by Babaoğlu Aydaş et al. (2013) for Synechocystis sp. BASO444 (7.4 ± 1.3 μg g−1) and Synechocystis sp. BASO673 (9.0 ± 1.4 μg g−1), the determined amount of gallic acid was significantly higher in Phormidium M1. Moreover, in comparison to Synechocystis sp. BASO444 (0.1 ± 0.03 μg g−1) and Synechocystis sp. BASO673 (0.1 ± 0.03 μg g−1) (Babaoğlu Aydaş et al. 2013), Phormidium M1 also contained a higher amount of p-coumaric acid (1.13 μg g−1 dw). In this strain, protocatechuic acid (5.21 μg g−1 dw) and 5-O-caffeoylquinic acid (6.11 μg g−1 dw) were also present, whilst the contents of two phenolic acids (gentisic acid 1.73 μg g−1 dw; p-coumaric acid 1.13 μg g−1 dw) were markedly lower compared to other identified phenolic acids. Concerning the amount of phenolic compounds, microalgae and cyanobacteria generally contain phenolic acids or aldehydes at microgram levels per gram of lyophilized sample (Klejdus et al. 2009).
Besides phenolic acids, Onofrejová et al. (2010) pointed out that several classes of flavonoids (isoflavonoids, flavanones, flavonols, and dihydrochalcones) can also be found in microalgae and cyanobacteria. The study showed that regarding flavonoid glycosides, five of them were detected in Phormidium M1 where hyperoside (34.5 μg g−1 dw) and quercetin 3-O-glucoside (26.6 μg g−1 dw) were identified in higher amounts compared to the other glycosides (luteolin 7-O-glucoside, quercitrin, rutin) (Table 2). On the other hand, in the case of Calothrix M2, Oscillatoria M2, and Nostoc M1, only one flavonoid glycoside compound was identified in each strain (luteolin 7-O-glucoside in Calothrix M2 and Oscillatoria M2; rutin in Nostoc M1), and in Anabaena M2, no flavonoid glycosides were detected.
Nevertheless, ethanolic extracts of Oscillatoria M2 contained high amount of vanillic acid (59.3 μg g−1 dw) whereby the p-hydroxybenzoic acid was present only in Nostoc M1 (10.6 μg g−1 dw). Furthermore, quinic acid was found in both strains, the highest being in Nostoc M1 (33 μg g−1 dw), whilst the content in Oscillatoria M2 was lower (12.6 μg g−1 dw). Compared to three tested strains (Oscillatoria M2, Phormidium M1, and Nostoc M1) ethanolic extracts of two strains (Anabaena M2 and Calothrix M2) were not rich in phenolic compounds since only two or one phenolic compounds were identified in these two strains. Regarding the phenolic profile of four freshwater cyanobacterial species (A. platensis, Anabaena doliolum, Cylindrospermum sp., Nostoc sp.), most of the identified benzoic acid derivatives in these species (Klejdus et al. 2009) were present in some of the tested strains in this study (p-hydroxybenzoic acid in Nostoc M1; protocatechuic acid in Phormidium M1 and Oscillatoria M2; vanillic acid in Phormidium M1 and Oscillatoria M2). Besides benzoic acid derivatives, cinnamic acid derivatives are very important as basic precursors in the phenyl-propanoid pathway for the synthesis of polyphenolic compounds (Dixon et al. 2002). However, cinnamic acid derivatives as well as hydroxyl benzaldehydes found in freshwater cyanobacterial strains (Klejdus et al. 2009) were not detected. Cinnamic acid and p-coumaric acid are very important as they represent crucial precursors in the synthesis of different bioactive phenols in photosynthetic organism (Onofrejová et al. 2010). In the present study, the cinnamic acid was absent in all the tested cyanobacterial extracts whereby p-coumaric acid was identified in Anabaena M2 and Phormidium M1. Compared to the phenolic compounds identified among different Arthrospira (Spirulina) maxima extracts (Abd El-Baky et al. 2009), three phenolic acids (gallic acid, p-OH-benzoic acid, vanillic acid) were detected in the following strains: Phormidium M1, Nostoc M1, Phormidium M1, and Oscillatoria M2. In comparison to the phenolic profile of freshwater cyanobacteria A. doliolum and three algae (Onofrejová et al. 2010). four identified phenolic acids were also identified in the present study (protocatechuic acid, Phormidium M1 and Oscillatoria M2; p-hydroxybenzoic acid, Nostoc M1; vanillic acid, Phormidium M1 and Oscillatoria M2; p-coumaric acid, Anabaena M2 and Phormidium M1). However, phenolic acids such as 2,3-dihydroxybenzoic, syringic, caffeic, salicylic, and chlorogenic acids found in the previous study (Onofrejová et al. 2010) were not detected in the cyanobacterial extracts examined in this one.
According to previously published results, freshwater microalgae contained higher amount of phenols compared to cyanobacteria species (Klejdus et al. 2009) owing to the fact that as eukaryotic organisms algae are more evolutionary advanced than cyanobacteria and probably have more developed phenol-based metabolic pathways (Onofrejová et al. 2010). Yet, bearing in mind that studies concerning phenol profile are mainly focused on freshwater cyanobacteria and microalgae, the obtained results are of great value and can contribute to further research of the terrestrial cyanobacteria.
Furthermore, with the purpose of establishing the correlation between phycocyanin and antioxidant activity, the phycobiliprotein contents were measured on day 21 of cultivation (Fig. 2). Since phycobiliproteins have been described as a strong antioxidant, such as in the case of PC (Bhat and Madyastha 2000; Hirata et al. 2000; Piñero Estrada et al. 2001; Benedetti et al. 2004; Huang et al. 2007; Gantar et al. 2012). the relationships between antioxidant activity and phycobiliprotein content were determined by Pearson correlation and regression analysis (Table 3). The correlation coefficients (R 2) between PC content and the antioxidant capacities did not indicate a significant correlation at the 95 % confidence level (P < 0.05). Statistically, no significant differences were found between PC content and antioxidant activity observed in DPPH method (Pearson correlation coefficient P = 0.965) as well as in FRAP method (Pearson correlation coefficient P = 0.278). These results suggest that other components present in the extracts contributed to the antioxidant activity. Thus, not only PC, but also other compounds should be considered when using crude cyanobacterial extracts as a source of natural antioxidants. According to Pumas et al. (2011) the antioxidative activities from cell-free extracts of cyanobacteria are the co-responsibility of the PBPs, phenolic compounds, as well as other antioxidative substances.
Since algae are complex matrices of compounds, therefore, in many extracts, antioxidant activity would not be closely connected to a specific compound but to a mixture of compounds. Thus, microalgal biomass may be considered as multi-component antioxidant systems, which are generally more effective due to additive or synergistic interactions between the different antioxidant components (Gouveia et al. 2006). Moreover, the additive effects of phytochemicals, the bioactive plant compounds, are known to be responsible for potent antioxidant activity of plants as they represent complex mixture of bioactive components (Liu 2003). Regarding the fact that algal extracts contain a complex mixture of antioxidants in which a specific antioxidant can be regenerated by another antioxidant (Goiris et al. 2012). the antioxidant activity cannot be explained according to the analysis of a specific algae component (Plaza et al. 2010). Furthermore, individual antioxidants may act by multiple mechanisms in a single system or by a different single mechanism depending on the reaction system (Singh and Singh 2008). For instance, not only α- and β-carotene but also other carotenoids (such lycopene, zeaxanthin, lutein, echinenone, astaxanthin, and canthaxanthin), well described in cyanobacteria, show antioxidant activity against radicals and interactions between them probably contribute to the antioxidant activity of each fraction (Böhm et al. 2002). Besides carotenoids, other compounds such as polyunsaturated fatty acids and polysaccharides including exopolymers may also play an important role in radical scavenging activity (Chen 1996; Chen et al. 2005; Parwani et al. 2014).
Based on the presented results, it should be noted that phenolic compounds, known as good antioxidants, may have an impact on radical scavenging activity in the case of FRAP method as the most potent strains (Phormidium M1 and Oscillatoria M2) in the aforementioned method were the richest in phenolic compounds. These results are in agreement with some of the previously published results that phenolic compounds could contribute to the antioxidant activity considering phenolic compounds as major contributors to the antioxidant capacities (Hajimahmoodi et al. 2010; Kepekçi and Saygideger 2012). Nevertheless, additional studies regarding antioxidant activity and identification of other compounds present in the crude extracts should be performed. In addition, the impact of other factors on antioxidant activity and phenolic production should also be investigated.
In conclusion, the data of this study showed that the tested terrestrial cyanobacterial strains belonging to Nostoc, Anabaena, Calothrix, Oscillatoria, and Phormidium genera possess antioxidant activity. These results indicate their potential as a source of various compounds showing antioxidant activity as each strain expressed different activities regarding the applied method. Furthermore, determination of phenolic profile gave an insight into the part of metabolic capacity of the tested strains, whereby Phormidium M1 was shown to be a promising source of phenolic acids such as quinic acid. Moreover, it should be noted that studies regarding phenolic profile of terrestrial cyanobacterial strains originating from forest ecosystems are still scarce. Therefore, this study could provide an interesting overview of metabolic possibilities in this extremely interesting group of microorganisms which could be a source of bioactive natural compounds. Keeping in mind that the tested terrestrial strains are isolated from habitats characterized by harsh environmental conditions, it is possible that the phenolic production as well as antioxidant activity would be increased by combining other factors as a consequence of their diverse metabolic strategies. These results indicate that it is necessary to carry out additional and more detailed research of the tested strains as well as to study the influence of other environmental stressors on their antioxidant activity and phenolic composition. The present findings could also serve as a starting point for further research on identification of the other active compounds showing antioxidant activity. Overall, these findings suggest that the tested cyanobacterial strains may be a rich source of phenolic compounds which makes them an interesting topic in further research, especially concerning their biological activity. As well as this, their content could implicate different numerous potential applications.
References
Abd El-Baky HH, El Baz FK, El-Baroty GS (2009) Production of phenolic compounds from Spirulina maxima microalgae and its protective effects. Afr J Biotechnol 8:7059–7067
Babaoğlu Aydaş S, Ozturk S, Aslım B (2013) Phenylalanine ammonia lyase (PAL) enzyme activity and antioxidant properties of some cyanobacteria isolates. Food Chem 136:164–169
Beara I, Lesjak M, Četojević-Simin D, Orčić D, Janković T, Anačkov G, Mimica-Dukić N (2012) Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of endemic Plantago reniformis G. Beck. Food Res Int 49:501–507
Benedetti S, Benvenuti F, Pagliarani S, Francogli S, Scoglio S, Canestrari F (2004) Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci 75:2353–2362
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 239:70–76
Bhat VB, Madyastha KM (2000) C-phycocyanin: a potent peroxyl radical scavenger in vivo and in vitro. Biochem Biophis Res Commun 275:20–25
Böhm V, Puspitasari-Nienaber NL, Ferruzzi MG, Schwartz SJ (2002) Trolox equivalent antioxidant capacity of different geometrical isomers of α-carotene, β-carotene, lycopene, and zeaxanthin. J Agric Food Chem 50:221–226
Chandrasekar D, Madhusudhana K, Ramakrishna S, Diwan PV (2006) Determination of DPPH free radical scavenging activity by reversed-phase HPLC: a sensitive screening method for polyherbal formulations. J Pharm Biomed 40:460–464
Chen F (1996) High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol 14:421–426
Chen F, Li HB, Wong RNS, Ji B, Jiang Y (2005) Isolation and purification of the bioactive carotenoid zeaxanthin from the microalga Microcystis aeruginosa by high-speed counter-current chromatography. J Chromatogr A 1064:183–186
Colla LM, Reinehr CO, Reichert C, Costa JAV (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresource Technol 98:1489–1493
Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MS, Wang L (2002) The phenylpropanoid pathway and plant defence—a genomics perspective. Mol Plant Pathol 3:371–390
Duval B, Shetty K, Thomas WH (1999) Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J Appl Phycol 11:559–566
Espin JC, Soler-Rivas C, Wichers HJ (2000) Characterisation of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agric Food Chem 48:648–656
Gantar M, Simović D, Djilas S, Gonzalez WW, Miksovska J (2012) Isolation, characterization and antioxidative activity of C-phycocyanin from Limnothrix sp. strain 37-2-1. J Biotechnol 159:21–26
Goiris K, Muylaert K, Fraeye I, Foubert I, de Brabanter J, de Cooman L (2012) Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J Appl Phycol 24:1477–1486
Goiris K, Muylaert K, Voorspoels S, Noten B, De Paepe D, Baart GJE, De Cooman L (2014) Detection of flavonoids in microalgae from different evolutionary lineages. J Phycol 50:483–492
Gouveia L, Raymundo A, Batista AP, Sousa I, Empis J (2006) Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. Eur Food Res Technol 222:362–367
Guedes AC, Amaro HM, Gião MS, Malcata FX (2013) Optimization of ABTS radical cation assay specifically for determination of antioxidant capacity of intracellular extracts of microalgae and cyanobacteria. Food Chem 138:638–643
Hajimahmoodi M, Faramarzi MA, Mohammadi N, Soltani N, Oveisi MR, Nafissi-Varcheh N (2010) Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J Appl Phycol 22:43–50
Hirata T, Tanaka M, Ooike M, Tsunomura T, Sakaguchi M (2000) Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J Appl Phycol 12:435–439
Huang Z, Guo BJ, Wong RNS, Jiang Y (2007) Characterization and antioxidant activity of selenium-containing phycocyanin isolated from Spirulina platensis. Food Chem 100:1137–1143
Kepekçi RA, Saygideger SD (2012) Enhancement of phenolic compound production in Spirulina platensis by two-step batch mode cultivation. J Appl Phycol 24:897–905
Kepekçi RA, Polat S, Çelik A, Bayat N, Saygideger SD (2013) Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem 141:1972–1979
Klejdus B, Kopecký J, Benešová L, Vacek J (2009) Solid-phase/supercritical-fluid extraction for liquid chromatography of phenolic compounds in freshwater microalgae and selected cyanobacterial species. J Chromatogr A 1216:763–771
Liu RH (2003) Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 78:517S–520S
Machu L, Misurcova L, Ambrozova JV, Orsavova J, Mlcek J, Sochor J, Jurikova T (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20:1118–1133
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Onofrejová L, Vašíčková J, Klejdus B, Stratil P, Mišurcová L, Kráčmar S, Kopecký J, Vacek J (2010) Bioactive phenols in algae: the application of pressurized-liquid and solid-phase extraction techniques. J Pharm Biomed 51:464–470
Orčić DZ, Francišković MM, Bekvalac KJ, Svričev EĐ, Beara IN, Lesjak MM, Mimica-Dukić NM (2014) Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem 143:48–53
Pandey U, Pandey J (2008) Enhanced production of biomass, pigments and antioxidant capacity of a nutritionally important cyanobacterium Nostochopsis lobatus. Bioresource Technol 99:4520–4523
Parwani L, Bhatnagar M, Bhatnagar A, Sharma V (2014) Antioxidant and iron-chelating activities of cyanobacterial exopolymers with potential for wound healing. J Appl Phycol 26:1473–1482
Piñero Estrada JE, Bermejo Bescós P, Villar del Fresno AM (2001) Antioxidant activity of different fractions of Spirulina platensis protean extract. Il Farmaco 56:497–500
Plaza M, Santoyo S, Jaime L, Reina GGB, Herrero M, Señoráns FJ, Ibáñez E (2010) Screening for bioactive compounds from algae. J Pharm Biomed 51:450–455
Pumas C, Vacharapiyasophon P, Peerapornpisal Y, Leelapornpisid P, Boonchum W, Ishii M, Khanongnuch C (2011) Thermostablility of phycobiliproteins and antioxidant activity from four thermotolerant cyanobacteria. Phycol Res 59:166–174
Rastogi RP, Sinha RP (2009) Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol Adv 27:521–539
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Santoyo S, Herrero M, Javier F, Cifuentes A, Ibanez E, Jaime L (2006) Functional characterization of pressurized liquid extracts of Spirulina platensis. Eur Food Res Technol 224:75–81
Simeunović J, Bešlin K, Svirčev Z, Kovač D, Babić O (2013) Impact of nitrogen and drought on phycobiliprotein content in terrestrial cyanobacterial strains. J Appl Phycol 25:597–607
Singh S, Singh RP (2008) In vitro methods of assay of antioxidants: an overview. Food Rev Int 24:392–415
Soni B, Trivedi U, Madamwar D (2008) A novel method of single step hydrophobic interaction chromatography for the purification of phycocyanin from Phormidium fragile and its characterization for antioxidant property. Bioresource Technol 99:188–194
Srivastava AK, Bhargava P, Rai LC (2005) Salinity and copper-induced oxidative damage and changes in the antioxidative defence systems of Anabaena doliolum. World J Microbiol Biotechnol 21:1291–1298
Tsao R, Deng Z (2004) Separation procedures for naturally occurring antioxidant phytochemicals. J Chromatogr B 812:85–99
Woese CR (1987) Bacterial evolution. Microbiol Rev 51:221–271
Acknowledgments
This study has been supported by the funding of the Ministry of Education and Science of the Serbian Government (project number: III 43002) which is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babić, O., Kovač, D., Rašeta, M. et al. Evaluation of antioxidant activity and phenolic profile of filamentous terrestrial cyanobacterial strains isolated from forest ecosystem. J Appl Phycol 28, 2333–2342 (2016). https://doi.org/10.1007/s10811-015-0773-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0773-4