Abstract
Semi-continuous algal cultivation was completed in outdoor flat-panel photobioreactors (panels) and open raceway ponds (raceways) from February 17 to May 7, 2015 for side-by-side comparison of areal productivities at the Arizona Center for Algae Technology and Innovation in Mesa, AZ, USA. Experiments used two strains of Scenedesmus acutus (strains LB 0414 and LB 0424) to assess productivity, areal density, nutrient removal, and harvest volume across cultivation systems and algal strains. Panels showed an average biomass productivity of 19.0 ± 0.6 g m−2 day−1 compared to 6.62 ± 2.3 g m−2 day−1 for raceways. Photosynthetic efficiency ranged between 1.32 and 2.24 % for panels and between 0.30 and 0.68 % for raceways. Panels showed an average nitrogen consumption rate of 38.4 ± 8.6 mg N L−1 day−1. Cultivation in raceways showed a consumption rate of 3.8 ± 2.5 and 7.1 ± 4.2 mg N L−1 day−1 for February/March and April/May, respectively, due to increase in biomass productivity. Excess nutrients were required to prevent a decrease in productivity. Daily biomass harvest volumes between 18 and 36 % from panels did not affect culture productivity, but density decreased with increased harvest volume. High cultivation temperatures above 30 °C caused strain LB 0414 to lyse and crash. Strain LB 0424 did not show any difference in biomass productivity when peak temperatures reached 34, 38, or 42 °C, but showed decreased productivity when the peak temperature during cultivation was 30 °C. Using algal strains with different temperature tolerances can generate increased annual biomass productivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High productivity in algal cultures is obtained through semi-continuous cultivation methods. This entails removing a percentage of the culture volume at a set frequency. Previous research studies in both flat-panel (panel) photobioreactors and raceway ponds (raceways) that removed a portion of the culture every 1–2 days were able to achieve maximum growth rates beyond 50 and 40 g m−2 day−1, respectively (Hu et al. 1998a; Moheimani and Borowitzka 2006, 2007). However, these are optimal values under optimal environmental conditions. Moheimani and Borowitzka (2006) showed average productivities for different months of the year with growth during the winter below 3.2 g m−2 day−1 compared to greater than 30 g m−2 day−1 during the summer. The lower growth during winter can be attributed to less light and sub-optimal culture temperatures that can cause photoinhibition (Vonshak et al. 2001; Crowe et al. 2012; Ras et al. 2013). The effect of sub-optimal morning temperatures reduces culture productivity year-round. Slegers et al. (2013) predicted that controlling the temperature of open ponds would increase the yearly productivity by 5–10 g m−2 day−1. However, considering the energy being lost to the environment with a high surface area to volume, the required energy for heating would significantly outweigh the benefits of increased biomass productivity.
Cultivation in panels also has significant issues with temperature limiting productivity; however, the concern is high peak temperatures, which requires cooling to prevent culture crashes. This is attributed to the shorter path length in panels compared to that in raceways, which decreases the heat capacity of the culture and consequently reduces the duration of sun exposure before the culture reaches peak temperature (Hindersin et al. 2013; Zemke et al. 2013; Grobbelaar 2013). Therefore, reducing temperatures in photobioreactors is a major energy cost, and to minimize this energy consumption, it would be advantageous to have seasonal algal crop rotation with algal strains that can tolerate different temperatures.
Raceways versus panels (areal density vs volumetric)
Previous research has shown that the optimal productivity for panels occurs with a light path between 1 and 10 cm, while raceways are operated between 10 and 30 cm in depth, with 15 cm being the most common (Hu et al. 1998b; Richmond and Cheng-Wu 2001; Moheimani and Borowitzka 2006; Grobbelaar 2013). However, these cultivation systems can also be assessed based on areal density and productivity. This is accomplished by assessing the amount of biomass per unit surface area and eliminates the differences in volume, which provides the best method for assessing photosynthetic efficiency of the photosynthetically active radiation (PAR) flux. Previous research has shown that optimal growth occurs in raceways when the areal density of the culture is between 40 and 150 g m−2 depending on culture depth and the species being cultivated (Grobbelaar et al. 1995; Hartig et al. 1988). Hartig et al. (1988) concluded that the optimal areal density was between 40 and 45 g m−2, with a respective volumetric density of 0.45 g L−1 in raceways operated at 10 cm. In addition, Grobbelaar et al. (1995) determined that by reducing the depth below 1 cm, along with high mixing rates, the cascade reactor increased the optimal areal density close to 150 g m−2, which was a volumetric density greater than 15 g L−1 in a system with a path length below 1 cm. This is a significant reduction in water required for cultivation, which significantly reduces the energy expenditure during harvesting (Borowitzka 1999). However, less effort has been put into these system designs while most research focused on raceways (Buchanan et al. 2013).
Little comparative research has been reported on cultures grown in both panels and raceways; however, previous studies have assessed the use of tubular photobioreactors. Torzillo et al. (2012) cultivated Phaeodactylum tricornutum in an outdoor tubular reactor alongside a circular pond for comparison of the two systems. The results showed that the open pond had an increase of 27 % productivity compared to the photobioreactor. However, it is important to note that the tubular photobioreactor utilized a centrifugal pump for culture flow and mixing, which has a significant amount of shear and can cause a drastic decrease in productivity (Torzillo et al. 2003). This is why many cultivation systems utilize aeration for mixing or airlifts to minimize shear stress (Molina et al. 2001; Sánchez Mirón et al. 2000). Raes et al. (2014) compared Tetraselmis sp. in an open raceway pond and the Biocoil helical photobioreactor and showed that this alga in semi-continuous cultivation achieved productivities of 3 and 0.85 g m−2 day−1, respectively. The growth rate in the raceways was comparable to that obtained in previous studies; however, the study had significantly lower productivities for photobioreactors than previously reported (Hu et al. 1998a; Moheimani and Borowitzka 2007; Eustance et al. 2015b). It is important to remember that flat-panel photobioreactors are not generally considered feasible for large-scale cultivation of algae, except for the production of high-value products. However, they provide a valuable research tool by creating a benchmark for achievable productivity.
The purpose of this study was to provide a definitive side-by-side comparison of flat-panel photobioreactors with raceway ponds for two Scenedesmus acutus strains in an outdoor environment during the spring growing season (end of February through the beginning of May 2015) on the Arizona Center for Algae Technology and Innovation field site in Mesa, AZ, USA. Culture density, productivity, and nitrogen consumption were measured along with ambient conditions to provide information on biomass production under semi-continuous cultivation.
Materials and methods
All experiments were carried out using two strains of Scenedesmus acutus, strain LB 0414 and strain LB 0424, cultured in outdoor raceway ponds and flat-panel photobioreactors (panels) at the Arizona Center for Algae Technology and Innovation (AzCATI) field site in Mesa, AZ, USA. Strain LB 0414 has a maximum temperature tolerance of 30 °C, while strain LB 0424 has a heat tolerance exceeding 45 °C. Raceway experiments were completed in three identical ponds each consisting of two channels 6.1 m long and 1.7 m wide, with two ends each with a radius of 1.78 m providing a total area of 30.37 m2. Raceways were operated with an average cultivation depth of 7.5 cm and a volume of 2300 L. Raceways were operated at shallower depths to reduce cultivation volume and assess productivities in systems with less light attenuation associated with culture depth. Furthermore, Chiaramonti et al. (2013) highlighted that decreasing depth from 20 to 5 cm significantly increased the specific head loss, but showed a 50 % decrease in energy consumption based on moving a smaller amount of water. This further alleviates energy consumption by decreasing the harvesting volume. Velocity of raceways was set to an average linear flow of 25 cm s−1. During the day, CO2 was added through a Sweetwater diffusion stone measuring 30 cm long and 5 cm wide at a rate of 5 L min−1. Inoculating cultures for raceways and panel experiments were grown in 55-L flat-panel photobioreactors with Modified BG-11 Medium. The panels had a north–south facing exposure measuring 46 in (1.17 m) in width by 46 in (1.17 m) in height and approximately 1.5 in (3.8 cm) in depth (thickness) or path length. The panels had an internal volume of 60 L; however, the reactors were filled with approximately 55 L of medium to account for the volume required for aeration. Aeration was provided by small drilled holes (∼1/32 in (0.8 mm)) in 0.5 in (1.3 cm) PVC located at the bottom of the reactor at a rate of approximately 0.5 vvm. During the day, CO2 was added to the aeration line to provide a concentration of 1.5 % CO2 (v/v). The reactors contained an internal 0.5-in (1.3 cm) stainless steel cooling line connected to an evaporative cooling system.
The composition of the Modified BG-11 Medium (1/4 BG-11) utilized in the photobioreactors was 375 mg L−1 NaNO3, 9.58 mg L−1 K2HPO4, 1.31 mg L−1 citric acid monohydrate, 5.24 mg L−1 of ammonium ferric citrate, 0.71 mg L−1 H3BO3, 0.445 mg L−1 MnCl2·4H2O, 0.06 mg L−1 ZnSO4·7H2O, 0.10 mg L−1 Na2MoO4·2H2O, 0.02 mg L−1 CuSO4·5H2O, and 0.1 mg L−1 Co(NO3)2·6H2O. Because these experiments utilized tap water, magnesium sulfate, calcium chloride, and sodium carbonate were not added to the Modified BG-11 Medium. Tap water used averaged 180 mg Na L−1, 75 mg Ca L−1, 70 mg S L−1, 24 mg Mg L−1, and 8 mg K L−1 with a hardness of 280 mg L−1 as CaCO3. During the second part of experimentation in April, the nitrogen source was changed from NaNO3 to NH4HCO3 at 348.8 mg L−1, to provide the same nitrogen concentration.
For raceway experiments, approximately 75 to 100 L of culture was transferred to the raceways, where cultures were grown in Modified BG-11 Medium with industrial fertilizers replacing the nitrate and phosphate sources: Ammonium iron citrate was used for an iron source. Nitrate, phosphate, and iron were added at a concentration of 1/6 of the original concentration of Modified BG-11 Medium (1/6 BG-11 Medium), while the “micronutrients” were added at a concentration 1/50 that of Modified BG-11 (1/50 bg-11) as represented by the following concentration values. The cultures utilized calcium nitrate 15.5-0-0 (YaraLiva Tropicote) for a nitrogen source at 264 mg L−1 (41.2 mg N L−1), and Blooming and Rooting 9-58-8 (Ferti-lome) for a phosphorus source at 4.69 mg L−1 (1.13 mg P L−1). Micronutrients were added at reduced concentration compared to those for panels, at a concentration of 0.057 mg L−1 H3BO3, 0.036 mg L−1 MnCl2·4H2O, 0.0048 mg L−1 ZnSO4·7H2O, 0.008 mg L−1 Na2MoO4·2H2O, 0.0016 mg L−1 CuSO4·5H2O, and 0.008 mg L−1 Co(NO3)2·6H2O.
Calculating areal productivity
The calculation of areal productivity for panels was accomplished by multiplying the volumetric biomass productivity by the culture volume of 55 L and dividing by the surface area of one side of the reactor 1.34 m2. The array of reactors at AzCATI are set up with the spacing between rows to be equivalent to the reactor height. Therefore, it is assumed that the surface area of the vertical panel is equivalent to the ground space being taken up.
The calculation of areal productivity for raceways was accomplished by multiplying the volumetric biomass productivity by the culture volume of 2300 L and dividing by the surface area of 30.37 m2.
This study reports the results of areal productivity, as the main limitation in algal growth is the utilization of sunlight (flux). Furthermore, by utilizing areal productivity, it provides the capability to compare outdoor productivity in different systems. The utilization of area eliminates the differences in volumes and path lengths associated with different cultivation systems that prevent direct comparisons of outdoor productivity. Volumetric productivity in the described raceways will be always less than those for panels, even at the same areal biomass productivity as they have a longer path length. As outdoor cultivation hinges on the ability to increase areal productivity, it is important to compare systems using direct metrics for areal parameters.
Monitoring and control
Temperature and pH were continuously monitored using a Neptune Apex controller (Neptune Systems, LLC.). The Apex also controlled solenoid valves to turn aeration on and off at programmed intervals during the night. In addition, solenoid valves were attached to the cooling lines of the first four reactors to allow cultures to achieve higher temperature with the same cooling water. Ambient conditions were measured with an Argus weather station capable of recording ambient temperature, relative humidity, and light intensity (Argus Control Systems, LLC.). For part of the experiment, PAR was measured on the front and backside of the photobioreactors using Li-190 sensors attached to a Li-1400 Logger (Li-COR Biosciences).
Analytical
Biomass density
Ash-free dry weight (AFDW) was measured in duplicate to assess growth performance. Glass filters (VWR 696 glass microfiber 1.2 μm) were ashed for 4 h at 500 °C prior to initial weighing. Duplicate samples were collected by filtering 10 to 20 mL per filter (depending on culture density). Samples were placed into an oven at 60 °C overnight. The filters with biomass were weighed to determine dry cell weight and then ashed at 500 °C for 4 h to determine the AFDW and ash content of the biomass.
Nitrogen
Nitrogen levels as nitrate and ammonium were monitored using flow injection analysis with a Lachat QuikChem 8500 with samples being analyzed in duplicate.
Results
Experimentation was completed during two periods: February 17 to March 27, 2015 and April 2 to May 7, 2015. During these periods, flat-panel photobioreactors (panels) and open raceway ponds (raceways) were operated under semi-continuous growth conditions with different variables being changed as highlighted in Table 1 for February 17 to March 27 and Table 3 for April 2 to May 7.
February 17 to March 27 experimentation
Initial experimentation was completed in panels and raceways during February 17 to March 27, 2015. During this time frame, cultivation was accomplished using S. acutus strain LB 0414. Figure 1 shows the light intensity (Fig. 1a), ambient and culture temperature (Fig. 1b), nitrogen concentration (Fig. 1c), and volumetric ash-free dry weight (AFDW) for cultures in panels (Fig. 1d) and raceways (Fig. 1e). For both panels and raceways, cultures were operated in a semi-continuous manner, and at the first point of harvest indicated by points 1 (Fig. 1d) and 9 (Fig. 1e), respectively, nutrients were added back for only the volume of culture removed. For panels, this caused significant nutrient limitation and reduced culture productivity to 10.9 ± 4.9 and 10.2 ± 4.7 g m−2 day−1 by removing either 36 or 29 % of the culture volume, respectively, with nutrients consumed within 24 h (Fig. 1c). This is significantly lower than when providing nitrogen sufficient cultures by adding nutrients based on the entire cultivation volume and not the volume being removed, which was 19.7 ± 3.9 and 18.7 ± 3.7 g m−2 day−1 for 36 and 29 % being removed, respectively (Table 2). In raceways, cultivation was initially tested to determine if tap water would provide enough micronutrients for growth. Initial growth showed minimal effects from not adding the micronutrients (Fig. 1e), although this is attributable to the high levels of micronutrients found in the inoculum. This is based on the lack of growth from the second harvest when micronutrients were not added (Fig. 1e, point 10) up to when micronutrients were added at a concentration of 1/50 BG-11 (Fig. 1e, point 11) and began to show renewed growth. This was corrected in later experimentation by adding micronutrients when adding nitrogen, phosphorus, and iron. In the raceways, the macronutrients were added at a concentration of 1/6 BG-11 Medium to prevent nutrient limitation, while the micronutrients were adjusted to 1/50 BG-11 Medium levels for the entire volume, since the concentration found in BG-11 Medium contained excessive levels of micronutrients.
Light intensity (a), temperature (b), nitrogen concentration (c), and AFDW (d, e) for cultivation occurring between February 17 and March 27, 2015 using strain LB 0414. Average±S.D. for raceway ponds (black solid line/filled triangle) is reported for two separate experiments. Average±S.D. for panels is reported for removing 29 % of culture (gray dash line/filled circle) and 36 % of culture (black dot line/filled square). Numerical identification of key changes is indicated in the figure and follows the descriptions found in Table 1
Due to culture temperature issues in raceways, highlighted as point 15 on Fig. 1e, the culture exhibited significant amounts of cell lysis as peak culture temperatures exceeded 30 °C (Fig. 1b). This caused significant culture loss and growth inhibition resulting in cessation of experimentation at point 16 (Fig. 1e).
Once panels were provided with sufficient nutrient levels at point 3 (Fig. 1d), semi-continuous cultivation was accomplished by removing either 29 or 36 % of the culture volume daily. In an attempt to reduce energy consumption, aeration was turned off at night starting at point 4 and ending at point 5. Figure 1d shows that cultivation had a drop in the peak culture density; this may be attributed to cloudy weather as shown by the lower light intensity on days 22 and 23 (Fig. 1a). Furthermore, Table 2 shows that productivity without aeration was not significantly different as aerated cultures had a biomass productivity of 22.5 ± 4.5 and 18.2 ± 5.8 g m−2 day−1 for 29 and 36 % removed, respectively, while eliminating aeration at night had a productivity of 20.0 ± 3.9 and 19.8 ± 1.9 g m−2 day−1, respectively. Due to operating in outdoor conditions, environmental factors including temperature and light intensity have a significant impact on productivity and account for the large standard deviation values being reported.
To further evaluate nutrient requirements being added to panels, the concentration of BG-11 Medium added was reduced from 1/4 to 1/6 between the points of 5 and 6 on Fig. 1d. As shown by the decrease in daily peak AFDW, the culture was not harvested on day 31 to allow the operating density to increase and prevent photoinhibition from occurring. Table 2 shows that the average productivity during this timeframe dropped to 17.2 ± 2.5 and 16.6 ± 2.5 g m−2 day−1 for harvesting volumes of 36 and 29 %, respectively.
April 2 to May 7 experimentation
From April 2 to May 7, a second set of semi-continuous experiments was completed in panels and raceways. However, cultivation in raceways was switched from strain LB 0414 to strain LB 0424, a thermotolerant strain of S. acutus able to accommodate warmer spring temperatures. In panels, condition 1 (Cond 1) and condition 2 (Cond 2) utilized strain LB 0424, while condition 3 (Cond 3) utilized strain LB 0414 (Table 3).
Figure 2 shows the light intensity (Fig. 2a), ambient and culture temperature (Fig. 2b), nitrogen concentration (Fig. 2c), and AFDW for cultures in panels (Fig. 2d) and raceways (Fig. 2e) throughout the cultivation time frame. The initial conditions for the panels were set with all panels receiving 1/4 BG-11 for the full culture volume and harvesting 29 % of the culture volume every day once the desired culture density was achieved. In addition, Cond 1, 2, and 3 had peak operating temperatures of 34, 38, and 27 °C, respectively. The results show that both strains had nearly identical operating densities. Furthermore, strain LB 0414 in Cond 3 showed a productivity of 18.6 ± 3.7 g m−2 day−1 (Table 4), which was comparable to the overall productivity for strain LB 0414 operated during experimentation in March with 18.7 ± 3.7 g m−2 day−1.
Light intensity (a), temperature (b), nitrogen concentration (c), and AFDW (d, e) for cultivation occurring between April 2 and May 7, 2015 using strain LB 0414 and strain LB 0424. Average±S.D. for raceway ponds (black solid line/filled triangle) is reported for growth with strain LB 0424. Average±S.D. for panels is reported for strain LB 0424 in condition 1 (gray dash line/filled circle) and condition 2 (black dot line/filled square), with strain 0414 grown in condition 3 (gray dash dot line/filled diamonds). Numerical identification of key changes is indicated in the figure and follow the descriptions found in Table 3
To determine the effect of reducing daily harvesting volume, Cond 3 at point 19 reduced the volume of culture being removed from 29 to 18 % and was maintained at that removal rate until point 23 (Fig. 2d). During this timeframe, the peak culture density increased from 1.63 ± 0.13 to 2.33 ± 0.14 g L−1 and did not show a significant change in culture productivity (Table 4).
At point 20 for Cond 1 and 2 and point 21 for Cond 3, the cultures were switched from NaNO3 to NH4HCO3 to determine if culture productivity changed with different nitrogen source (Fig. 2d). Based on the data in Table 4, neither culture showed any significant change in culture productivity with change in nitrogen source. At point 22, all three conditions were switched from continuous aeration at night to intermittent sparging at a frequency of 1 min per 30 min. This was done to minimize ammonia toxicity and volatility at night when culture pH values increased due to the external CO2 source being turned off as was shown in previous research (Eustance et al. 2015a). This had no effect on the cultures’ productivity.
At point 23 (Fig. 2d), all three conditions were changed. Cond 1 had the culture volume being removed increased from 29 to 36 %, Cond 2 had the peak culture temperature decreased from 38 to 30 °C, and Cond 3 had the culture volume being removed increased from 18 to 29 %. By reducing the peak operating temperature in Cond 2, strain LB 0424 showed a small decrease in the biomass productivity from points 23 to 34, which decreased further from points 24 to 25 (Table 4). Furthermore, at point 24 (Fig. 2d), Cond 1 had the peak temperature increased from 34 to 42 °C and showed a small increase in productivity, which highlights this strain’s capability to grow over a very large temperature range without having a significant change in culture productivity.
The raceways operated between April 2 and May 7, 2015 utilized strain LB 0424. At point 27 (Fig. 1e), 43 % of the culture was removed every other day until point 28, where 65 % of the culture was removed. This was accomplished once, since removing over 50 % of the culture was challenging due to the shallow depth of the raceways. At point 30, the cultures had 50 % of the culture removed every 4 days. This provided a large operating range of areal densities to determine if there was a peak productivity associated with any of the culture densities. Based on Fig. 4 and Table 4, the range in which the culture was operated showed no drastic effect on the biomass productivity, which averaged 8.7 ± 2.3 g m−2 day−1.
Discussion
Raceways versus panels (areal vs volumetric density)
Previous research has suggested that the optimal areal density for cultures is between 40 and 150 g m−2 depending on culture depth and the species being cultivated (Hartig et al. 1988; Grobbelaar et al. 1995). Hartig et al. (1988) concluded that the optimal areal density was between 40 and 45 g m−2 for ponds operated at 15 to 30 cm. In addition, Grobbelaar et al. (1995) determined that by reducing the depth and with the high mixing rates, the cascade reactor increased the optimal areal density close to 150 g m−2. This is similar to the results shown in the current study, where increasing the operating areal density above 100 g m−2 (Figs. 3 and 4) in the flat-panel photobioreactors (panels) had little impact on the productivity suggesting that cultures with high levels of mixing can operate at higher culture densities. Comparatively, raceways operated at a depth of 7.5 cm have significantly less mixing, which contributed to the limited productivity independent of the areal density as shown in Figs. 3 and 4. Both figures show no correlation between areal density and areal productivity. However, it is important to note that the cultures were operated within a range of 20 to 70 g m−2 and may have needed an areal density greater than 100 g m−2 to show a decrease in productivity.
Areal density and areal productivity for experiments completed between February 17 and March 27, 2015 using strain LB 0414. Average±S.D. for raceway ponds (black solid line/filled triangle) is reported for two separate experiments. Average±S.D. for panels is reported for removing 29 % of culture (gray dash line/filled circle) and 36 % of culture (black dot line/filled square)
Areal productivity and areal density for experiments completed between April 2 and May 7, 2015 using strain LB 0414 and strain LB 0424. Average±S.D. for raceway ponds (black solid line/filled triangle) is reported for growth with strain LB 0414. Average±S.D. for panels is reported for strain LB 0424 in condition 1 (gray dash line/filled circle) and condition 2 (black dot line/filled square), with strain LB 0414 grown in condition 3 (gray dash dot line/filled diamonds)
The growth rate of raceways compared to panels for semi-continuous cultivation consistently showed that panels outperformed raceways with an average productivity of 19.0 ± 0.6 g m−2 day−1 compared to 6.62 ± 2.3 g m−2 day−1, respectively. However, it is important to note that panels are used to provide an idea of the maximum productivity a culture may be able to achieve in raceways.
Temperature concerns
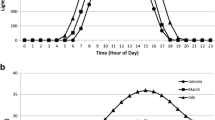
One of the biggest concerns and reasons for a decrease in culture productivity in raceways compared to panels, or the value achieved in panels compared to the theoretical limit of 77 g m−2 day−1 is the diurnal temperature fluctuation (Melis 2009). This is shown in Fig. 5, which highlights two different days during April 2015 and provides culture and ambient temperatures along with light intensity. Figure 5 shows that during the first few hours of the day, the cultures are at sub-optimal temperatures. Previous research has shown that sub-optimal temperatures cause photoinhibition and reduced enzyme activity, which reduces culture productivity (Vonshak et al. 2001; Ras et al. 2013). Furthermore, Fig. 5 showed that raceways required more time to reach the desired operating range compared to panels and helps explain the lower productivity achieved. Furthermore, the average morning temperature, defined as the time between 7 a.m. and 10 a.m., was 11.2 ± 1.9 °C in the raceways from February 17 to March 8, while from April 2 to May 7, the average was 14.8 ± 3.1 °C. However, these values are both significantly lower than the values found in panels, which had averages of 18.0 ± 2.7 and 20.3 ± 3.4 °C for the same time frames. By decreasing the culture path length/depth, the system has a decreased heat capacity and will, therefore, increase in temperature faster, which minimizes time spent with sup-optimal morning conditions but will reach higher peak temperatures. In panels, this can be combated by utilizing an evaporative cooler and stainless steel cooling lines suspended in the culture media; however, the raceways did not and typically do not have temperature control. One of the main cited reasons for raceways to be operated at a depth of greater than 15 cm, other than hydraulic limitations, is to minimize temperature fluctuations and peak daytime temperatures, which can lead to culture crashes (Oswald 1988; Moheimani and Borowitzka 2007). This was shown in Fig. 1e at point 15, where strain LB 0414 in raceways began to reach undesirable temperatures at which the culture began to lyse and crash. Research has shown that cultures tend to have 4 to 5 °C range between the optimal temperature and the point where cultures begin to die from high temperatures (Ras et al. 2013; Buchanan et al. 2013). This has drastic effects on culture productivity if the temperature spikes unexpectedly but may be minimized by rotating algal crops to accommodate the different temperature ranges as was done in this study by switching from strain LB 0414 to strain LB 0424.
Actual and preferred temperature ranges for strain LB 0414 and strain LB 0424 at two different time points during cultivation in April 2015. Strain LB 0424 in raceways (black solid line) and panels in condition 1 (gray dash line) and condition 2 (black dot line/filled square), and strain LB 0414 grown in condition 3 (gray dash dot line). Ambient temperature (gray dotted line) and light intensity (black dash dot line) are shown for comparison
Aeration at night
Previous research has shown that limiting aeration at night can decrease lipid accumulation but has little effect on log phase cultures (Eustance et al. 2015a). To determine the long-term effect of limiting aeration at night, cultures were operated with no aeration from points 4 to 5 (Fig. 1d), which showed no significant difference in the culture biomass productivity (Table 2). Cultures were switched from nitrate to ammonium at points 20 for Cond 1 and 2, and 21 for Cond 3 (Fig. 2d), and to avoid ammonia toxicity and volatility, and were switched to an intermittent sparging frequency of 1 min per 30 min for the remainder of experimentation at point 22 (Fig. 2d). Table 4 shows that the change to intermittent sparging at night had no significant impact on growth as was previously shown in Eustance et al. (2015a). Furthermore, Cuello et al. (2014) showed that stopping the paddle wheel at night in semi-continuous raceways did not statistically change biomass productivity compared to constant mixing, which had the advantage of decreasing energy consumption by 37 %.
Nitrogen usage
Cultivation in panels in March showed an average nitrogen consumption rate of 36.4 ± 10.7 mg N L−1 day−1 (1.49 ± 0.44 g N m−2 day−1), while cultivation in April and May showed a consumption rate of 39.4 ± 7.2 mg N L−1 day−1 (1.61 ± 0.29 g N m−2 day−1). These values correspond to a consumption of between 77 and 87 mg N g−1 biomass. However, cultures required excess nitrogen to prevent a reduction in growth rate, as cultures that received approximately 60 mg N L−1 day−1 had a higher growth rate than cultures that received approximately 40 mg N L−1 day−1. However, the consumption rate of nitrogen may be skewed as nitrogen was most likely removed during the time required for a culture to mix. This is further exemplified in both Figs. 1c and 2c, as the maximum nitrogen concentration did not continue to increase over time but reached a plateau that corresponded to the culture density. This suggests that the cultures were able to remove higher levels than that measured from the medium and were able to remove closer to 60 mg N L−1, since this was the amount being added to the cultures.
Cultivation in raceways showed lower nitrogen consumption rates due to lower biomass productivities. During cultivation in February and March, the average consumption rate was 3.8 ± 2.5 mg N L−1 day−1 (0.29 ± 0.19 g N m−2 day−1), while growth in April and May had a consumption rate of 7.1 ± 4.2 mg N L−1 day−1 (0.54 ± 0.32 g N m−2 day−1). This corresponded to a consumption rate between 64 and 76 mg N g−1biomass, which is similar to that determined for panels. This also shows that due to the slower growth rate in raceways, the overall nitrogen consumption rate decreased as well.
Productivity and photosynthetic efficiency
Tables 2 and 4 show productivity and photosynthetic efficiency values for panels and raceways operated between February 17 and May 7, 2015. At the beginning of March, the average solar irradiance for cultivation was 5.5 ± 0.5 kWh m−2 day−1, and by the end of experimentation in May, the value had increased to 7.1 ± 0.7 kWh m−2 day−1. However, during this time frame, the average biomass productivity in panels did not significantly change. This is attributed to a change in the angle of incidence of the sun, which increases in the summer and therefore provides a smaller fraction of the total light to a vertical surface. From March 11 to March 27 and April 23 to May 7, PAR was measured on the front and backside of the panels. During these times, the average solar irradiance was 5.7 ± 1.0 kWh m−2 day−1 for March and 6.9 ± 0.8 kWh m−2 day−1 for April. When these values are converted to PAR based on the value found in Melis (2009) of 5 kWh m−2 day−1 being equivalent to approximately 35 mol photons m−2 day−1, the corresponding average for March was 39.8 ± 6.7 mol photons m−2 day−1 and for April was 48.5 ± 5.3 mol photons m−2 day−1. However, the combined PAR for the front and backside of the panels was 36.2 ± 4.9 mol photons m−2 day−1 in March and 30.8 ± 1.3 mol photons m−2 day−1 in April. This shows that the panels were receiving less light at the surface and maintaining the same level of productivity. However, when calculating the photosynthetic efficiency (PE) as shown in Tables 2 and 4, PE decreased throughout experimentation for cultures grown in panels from 1.90 ± 0.07 % (points 3–4) initially to 1.46 ± 0.05 % for cultivation between points 17 and 25. Panels did not show an increase in PE due to the vertical orientation, which decreased the amount of usable light. When reconsidering PE based on the PAR actually reaching the surfaces of the panel, the PE increased from 2.0 ± 0.0 to 2.3 ± 0.2 % for March 11–27 and April 22 to May 7, respectively, which indicates that more favorable culture conditions allowed increased daily productivity despite having less usable light. Assessing raceways, which utilize a horizontal surface, showed an increase in both biomass productivity and PE as experimentation proceeded toward higher sun angles. The average PE for raceways during April and May experimentation was 0.66 ± 0.02 %, which increased from 0.36 ± 0.08 % for cultivation in February and March. This also shows that factors other than solar irradiance alone increased biomass productivity which is most likely due to the increase in morning temperatures as was previously discussed. The values achieved for PE are significantly lower than the theoretical range of 8–10 % suggested by Melis (2009). However, the values for panels are closer to the range suggested by Park et al. (2011) of 1.3 to 2.4 % and suggested that the additional drop was due to cultures experiencing light saturation beyond levels of 200 μmol photons m−2 s−1.
Previous research studies that have assessed the productivity of raceways typically utilize smaller units with a surface area of 1–4 m2 compared to the current study, which utilized raceways with a surface area of 30.37 m2 (Moheimani and Borowitzka 2006; Raes et al. 2014; Torzillo et al. 2012). This is significant as it affects the distance the culture travels between paddle wheel points. With smaller raceways, the culture interacts with the paddle wheel more frequently, which significantly increases the mixing rate and surface interactions. Raes et al. (2014) showed the change in the Reynolds number in a 1-m2 raceway with a drop from 3250 to 1730 over the distance of 3 m, or the distance the culture traveled before encountering the paddle wheel again. This suggests a significant change in the Reynolds number over a small distance, which indicates that scaling information found in a 1-m2 raceway may overestimate the productivity of larger systems by not accounting for decreased mixing and paddle wheel interaction. The Arizona Center for Algae Technology and Innovation field site hosts raceways with a surface area of 4.22 and 30.37 m2 with maximum fluid traveling lengths of 9.4 and 17.8 m, respectively, which highlights how a small change in area of a raceway can drastically change the distance between paddle wheels. This is critical as Oswald (1988) notably suggested that the distance between paddle wheels in large-scale cultivation will most likely be between 493 to 2120 m depending on the cultivation depth. This would drastically decrease the level of mixing and surface interaction and will decrease productivity, which is rarely considered or discussed when reporting productivity values or in the estimation of large-scale cultivation productivity. Further work is needed to evaluate the effect of paddle wheel interactions and Reynolds number on productivity in larger systems.
Conclusions
-
Flat-panel photobioreactors had an average biomass productivity of 19.0 ± 0.6 g m−2 day−1 compared to 6.62 ± 2.3 g m−2 day−1 for open raceway ponds.
-
Cultivation in panels and raceways for March showed an average nitrogen consumption rate of 36.4 ± 10.7 and 3.8 ± 2.5 mg N L−1 day−1, respectively. Cultivation in April and May showed a consumption rate of 39.4 ± 7.2 and 7.1 ± 4.2 mg N L−1 day−1 for panels and raceways, respectively. All of these numbers correspond to a nitrogen consumption rate of 64 to 87 mg N g biomass−1.
-
Excess nutrients were required to prevent decreased biomass productivity
-
Cultivation temperatures above 30 °C caused strain LB 0414 to lyse and the culture to crash.
-
Strain LB 0424 did not show any statistical difference in biomass productivity when the peak temperature reached 34, 38, or 42 °C, but had lower productivity when the peak temperature was 30 °C, which is the maximum temperature for strain LB 0414.
-
The reduction in aeration or sparging of cultures to a frequency of 1 min per 30 min at night did not impact biomass productivity.
-
Photosynthetic efficiency observed was between 1.23 and 2.24 % for panels and between 0.30 and 0.68 % for raceways.
-
Photosynthetic efficiency increased in raceways from February to March, which suggests that increased culture temperature improved biomass productivity.
References
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Buchanan AN, Bolton N, Moheimani N, Svoboda IF, Grant T, Batten D, Cheng NN, Borowitzka MA, Fallowfield HJ (2013) Algae for energy and feed: a wastewater solution. A Review. Pork CRC, Canberra, p 203
Chiaramonti D, Prussi M, Casini D, Tredici MR, Rodolfi L, Bassi N, Zittelli GC, Bondioli P (2013) Review of energy balance in raceway ponds for microalgae cultivation: re-thinking a traditional system is possible. Appl Energy 102:101–111
Crowe B, Attalah S, Agrawal S, Waller P, Ryan R, Van Wagenen J, Chavis A, Kyndt J, Kacira M, Ogden KL, Huesemann M (2012) A comparison of Nannochloropsis salina growth performance in two outdoor pond designs: conventional raceways versus the ARID pond with superior temperature management. Int J Chem Eng 2012:9
Cuello M, Cosgrove J, Randhir A, Vadiveloo A, Moheimani N (2014) Comparison of continuous and day time only mixing on Tetraselmis suecica (Chlorophyta) in outdoor raceway ponds. J Appl Phycol. doi:10.1007/s10811-014-0420-5
Eustance E, Wray JT, Badvipour S, Sommerfeld MR (2015a) The effects of limiting nighttime aeration on productivity and lipid accumulation in Scenedesmus dimorphous. Algal Res 10:33–40
Eustance E, Wray JT, Badvipour S, Sommerfeld MR (2015b) The effects of cultivation depth, areal density and nutrient level on lipid accumulation of Scenedesmus acutus in outdoor raceway ponds J Appl Phycol. doi:10.1007/s10811-015-0709-z
Grobbelaar JU (2013) Mass production of microalgae at optimal photosynthetic rates. In: Dubinsky Z (ed) Photosynthesis. InTech, pp 357–371. doi:10.5772/55193
Grobbelaar JU, Nedbal L, Tichy L, Setlik L (1995) Variation in some photosynthetic characteristics of microalgae cultured in outdoor thin-layered sloping reactors. J Appl Phycol 7:175–184
Hartig P, Grobbelaar JU, Soeder CJ, Groeneweg J (1988) On the mass-culture of microalgae—areal density as an important factor for achieving maximal productivity. Biomass 15:211–221
Hindersin S, Leupold M, Kerner M, Hanelt D (2013) Irradiance optimization of outdoor microalgal cultures using solar tracked photobioreactors. Bioprocess Biosyst Eng 36:345–355
Hu Q, Faiman D, Richmond A (1998a) Optimal tilt angles of enclosed reactors for growing photoautotrophic microorganisms outdoors. J Ferment Bioeng 85:230–236
Hu Q, Kurano N, Kawachi M, Iwasaki I, Miyachi S (1998b) Ultrahigh-cell-density culture of a marine green alga Chlorococcum littorale in a flat-plate photobioreactor. Appl Microbiol Biotechnol 49:655–662
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177:272–280
Moheimani NR, Borowitzka MA (2006) The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J Appl Phycol 18:703–712
Moheimani NR, Borowitzka MA (2007) Limits to productivity of the alga Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. Biotechnol Bioeng 96:27–36
Molina E, Fernandez J, Acien FG, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131
Oswald WJ (1988) Large-scale algal culture systems (engineering aspects). In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal biotechnology. Cambridge University Press, Cambridge, pp 357–394
Park JBK, Craggs RJ, Shilton AN (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol 102:35–42
Raes EJ, Isdepsky A, Muylaert K, Borowitzka MA, Moheimani NR (2014) Comparison of growth of Tetraselmis in a tubular photobioreactor (Biocoil) and a raceway pond. J Appl Phycol 26:247–255
Ras M, Steyer J-P, Bernard O (2013) Temperature effect on microalgae: a crucial factor for outdoor production. Rev Environ Sci Biotechnol 12:153–164
Richmond A, Cheng-Wu Z (2001) Optimization of a flat plate glass reactor for mass production of Nannochloropsis sp. outdoors. J Biotechnol 85:259–269
Sánchez Mirón A, García Camacho F, Contreras Gómez A, Grima EM, Chisti Y (2000) Bubble-column and airlift photobioreactors for algal culture. AICHE J 46:1872–1887
Slegers PM, Lösing MB, Wijffels RH, van Straten G, van Boxtel AJB (2013) Scenario evaluation of open pond microalgae production. Algal Res 2:358–368
Torzillo G, Pushparaj B, Masojidek J, Vonshak A (2003) Biological constraints in algal biotechnology. Biotechnol Bioprocess Eng 8:338–348
Torzillo G, Faraloni C, Silva AM, Kopecký J, Pilný J, Masojídek J (2012) Photoacclimation of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown outdoors in photobioreactors and open ponds. Eur J Phycol 47:169–181
Vonshak A, Torzillo G, Masojidek J, Boussiba S (2001) Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant Cell Environ 24:1113–1118
Zemke P, Sommerfeld M, Hu Q (2013) Assessment of key biological and engineering design parameters for production of Chlorella zofingiensis (Chlorophyceae) in outdoor photobioreactors. Appl Microbiol Biotechnol 97:5645–5655
Acknowledgments
The authors would like to acknowledge the Arizona Center for Algae Technology and Innovation for access to facilities and equipment, and its staff for their intellectual and technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eustance, E., Badvipour, S., Wray, J.T. et al. Biomass productivity of two Scenedesmus strains cultivated semi-continuously in outdoor raceway ponds and flat-panel photobioreactors. J Appl Phycol 28, 1471–1483 (2016). https://doi.org/10.1007/s10811-015-0710-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0710-6