Abstract
Two commercially available seaweed products, derived from Ascophyllum nodosum (An) and Ecklonia maxima (Em), were evaluated for their potential as control agents for the root-knot nematodes, Meloidogyne chitwoodi and Meloidogyne hapla. The effects of both products on hatching, host location and penetration by second-stage juveniles (J2) were examined. Continuous exposure of M. chitwoodi egg masses to 50 and 100 % An significantly reduced the final percentage hatch, but this result could not be confirmed. In a bioassay with pluronic gel, more J2 of M. chitwoodi and M. hapla were found within the 0.5-cm vicinity of a tomato root tip after 24- and 6-h pre-exposures to Em, respectively. On agar plates, J2 of M. chitwoodi pre-exposed to An or Em showed less attraction to tomato root diffusate compared with distilled water (DW). Moreover, J2 pre-exposed to An lost the ability to differentiate repellent and attractant solutions on agar plates, unlike J2 pre-exposed to Em or DW. A 24-h pre-exposure to An reduced the infectivity of M. chitwoodi and M. hapla, whereas pre-exposure to Em enhanced the infectivity of M. chitwoodi. In a glasshouse pot experiment, treatments with Em reduced M. hapla multiplication on tomato. For M. chitwoodi, no effect on the number of nematodes per gram root was seen. The root biomass significantly reduced for untreated plants infested with M. chitwoodi compared to Em- and An-treated plants. The results indicate that these seaweed products adversely affect hatching and sensory perception in in vitro assays, but assumptions about in vivo effects may be unwise as dilutions of the products when applied as soil drenches may compromise activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant-parasitic nematodes are responsible for an estimated US$157 billion in agricultural damage globally on an annual basis (ScienceDaily 2008). They constitute an exceptionally complex crop pest to control. The root-knot nematodes (Meloidogyne spp.) are among the three most economically damaging genera of plant-parasitic nematodes on horticultural and field crops (Jones et al. 2013). They are distributed worldwide and are obligate parasites of the roots of thousands of plant species, including lower and higher herbaceous and woody plants (Moens et al. 2009). Meloidogyne incognita, Meloidogyne javanica, Meloidogyne arenaria and Meloidogyne hapla are the most important species worldwide, responsible for 95 % of the root-knot nematode infestations of cultivated land (Sasser and Carter 1982). Meloidogyne chitwoodi and Mycobacterium fallax as well as Meloidogyne enterolobii have recently gained importance as quarantine organisms or newly emerging and extremely pathogenic species. Damage caused by root-knot nematodes results in poor growth, loss of quality and yield, as well as reduced resistance to other stresses (e.g. biotic, abiotic and other diseases) and heavy infestations of root-knot nematodes can lead to total crop loss (Wesemael et al. 2011).
The management and control of root-knot nematodes have historically been achieved with the use of crop rotation, plant resistance and nematicides. However, due to the wide host range of root-knot nematodes and highly specialized production processes in vegetable production, rotational options are limited. The use of chemical nematicides has reduced due to increasing concern about pesticide residues and adverse impacts on the environment (Nyczepir and Thomas 2009), and alternative control methods are needed. Biological control of plant-parasitic nematodes is promising, but commercial use is still limited. Control of Meloidogyne spp. has been achieved with nematophagous fungi and bacteria (Stirling 2014). Also, arbuscular mycorrhizal fungi, endophytic fungi that grow within plant tissues without causing disease, can play a protective role against plant-parasitic nematodes (Hol and Cook 2005).
The discovery of inhibitory substances biosynthesized by seaweeds dates back to early 1917 (Harder and Oppermann 1953). Pratt et al. (1944) were the first to report antibiotic activities of algae. Evidence that seaweeds contain cytotoxic (Rocha et al. 2007), antibacterial (Tuney et al. 2006), antifungal (Aliya and Shamaeel 1999; Tang et al. 2002), antiviral (Garg et al. 1992; Serkedjieva 2004) and larvicidal components (Manilal et al. 2009) has been reported. The secondary metabolites synthesized by seaweeds demonstrate a broad spectrum of bioactivity varying from neurologically active in humans to algicidal, nematicidal, insecticidal and ichthyotoxicity in lower forms of animals (Smith 2004). Seaweed products have also been shown to increase seed germination, plant nutrients uptake, frost resistance and resistance to pathogenic fungi (Boot 1964). Tarjan (1977) demonstrated that extracts of Ascophyllum nodosum can cause a significant reduction in the number of nematodes and an increase in plant weight when applied to citrus seedlings infected with Radopholus similis, compared with water-treated control seedlings. Commercial extracts of A. nodosum when applied to the soil effectively controlled Belonolaimus longicaudatus on centipede grass after 1-month application (Morgan and Tarjan 1980). A reduction in root galling by M. incognita of 82 to 87 % was observed on tomato after application of a commercial bio-product containing A. nodosum (Radwan et al. 2011, 2012). Featonby-Smith and van Staden (1983) reported a significant reduction of M. incognita infection of tomato plants with the use of commercially available seaweed concentrate prepared from Ecklonia maxima. Suppression of reproduction of Pratylenchus zeae in vitro on an excised root of Zea mays has also been reported by De Waele et al. (1988) using the same commercial product.
The present study focused on experiments to compare the effects of commercially available products of A. nodosum and E. maxima on hatching, infectivity and host attraction of M. chitwoodi and M. hapla, and on the growth of tomato plants in glasshouse conditions.
Materials and methods
Nematode culture
Pure cultures of M. hapla and M. chitwoodi were maintained on tomato, Solanum lycopersicum cv. Moneymaker grown in small transparent plastic tubes (120 × 20 × 15 mm) in sterilized (100 °C, 18 h) sandy peat soil under controlled (14-h daylight, 22 ± 6 °C) glasshouse conditions. Freshly hatched (<24 h) second-stage juveniles (J2) of M. hapla and M. chitwoodi used throughout the experiments were extracted 8 weeks after inoculation from infected roots by the Baermann funnel technique (Baermann 1917). When required, egg masses were carefully hand-picked from the tomato root system under a binocular stereomicroscope with the aid of a pair of forceps following a gentle cleaning of the roots under slow-running tap water.
Glasshouse experiments
The effects of the seaweed products from E. maxima (Em) and A. nodosum (An) on the infectivity of the Meloidogyne species on tomato plants were examined under controlled glasshouse conditions (14-h light, 22 ± 6 °C). Seeds of S. lycopersicum cv. Moneymaker were germinated on seedling trays. Seedlings were transplanted at the four-leaf stage into 1.7-L pots containing organic soil (Peat, 20 % OM, pH 5.0–6.5, NPK 12-14-24, Saniflor, Belgium). The experimental setup consisted of six treatments: M. chitwoodi, M. chitwoodi + An, M. chitwoodi + Em, M. hapla, M. hapla + An and M. hapla + Em. Each treatment consisted of five replicates, and pots were arranged in a fully randomized design.
The two seaweed concentrates used were Kelpak (Kelp Products (Pty) Ltd, Simon’s Town, South Africa) and OSMO® (OSMO® International NV, Diksmuide, Belgium). Kelpak (Em) is prepared by a cell-burst process from the brown alga E. maxima. OSMO® liquid fertilizer (An) is an aqueous alkaline extract produced from the brown marine alga A. nodosum. Dilutions for the solutions used were 10 mL and 5 mL L−1 for Em and An, respectively. These concentrations are recommended by the manufacturers for application to plants as a soil drench for growth enhancement. One hundred milliliters of the solutions were applied to the seedlings at transplanting and thereafter every 5 days for the first 20 days. This was followed by 200 mL at the same time interval with in-between application of tap water following the increased moisture requirement by the plants as they grew.
Nematode inoculations were done 2 weeks after transplanting of the seedlings by boring small holes around the active growing root region into which nematodes were inoculated with the aid of a micro pipette; approximately 4000 J2 per pot were used for each treatment.
Plants were harvested 2 months after inoculation. The biomass of the shoot and root was determined as well as the length of the above-ground plant parts. Nematodes were extracted from the roots of inoculated plants with the Hendrickx zonal centrifuge (Hendrickx 1995). Roots were carefully washed and macerated for 1 min before centrifugation. The number of juveniles, eggs, females and males were counted with the use of a binocular stereomicroscope. Data was log10 transformed to normalize residuals and statistically analysed using analysis of variance (one-way ANOVA). Significant differences were reported using least significant difference (LSD) test (P < 0.05).
Hatching assays
To examine the effect of An or Em on hatching of J2, egg masses of M. chitwoodi and M. hapla were immersed in 100, 50, 25 or 10 % concentrations of each seaweed product. Young egg masses of both species were carefully removed from heavily infected tomato roots. Five egg masses were placed on a 48-μm sieve that retained the egg masses while hatched J2 could easily move through the sieves. The sieves containing the egg masses were inserted into small plastic tubes each containing 4 mL of the test solutions while distilled water (DW) was used as control. The tubes were capped while tiny holes were perforated on the caps to allow for ventilation and incubated at 20 ± 1 °C. The solutions were refreshed at a 4-day interval, and the number of hatched J2 were counted and recorded. The hatching of J2 was monitored until the number of hatched J2 at each count was fewer than five for individual replicates. To check for reversible effects after treatment, all the sieves with the egg masses were transferred into distilled water and monitored for 8 days and the number of J2 that hatched were counted. The remaining un-embryonated eggs or unhatched juveniles were counted at the end of the experiment after the egg masses had been carefully transferred into counting dishes, covered with 3 mL of 10 % sodium hypochlorite and homogenized to dissolve the gelatinous matrix and liberate the eggs. Each treatment for the hatching assay was replicated four times. The experiment was repeated for M. chitwoodi following the same concentrations of An and Em but with ten replicates of a single egg mass.
Hatching data obtained for both M. chitwoodi and M. hapla were fitted to the logistic model y = c/(1 + exp(−b × (time − m))), where y is the cumulative percentage hatch. The model is described by three parameters: the time at which 50 % hatch is reached (m), the hatching rate (b) and the final hatching percentage (c) (Oude Voshaar 1994). These parameters were calculated for all the replicates of the treatments separately and subjected to one-way ANOVA. Observations were reported as significant or non-significant using the LSD test (P < 0.05). As hatching parameters data did not fulfil the assumptions for ANOVA, data was subjected to analysis of variance using the non-parametric Kruskal-Wallis and Mann-Whitney U tests for significant differences among the treatments (P < 0.05).
Infectivity of J2 after pre-exposure to the seaweed products
Freshly hatched J2 of M. chitwoodi and M. hapla were pre-exposed to either 10 mL L−1 An, 5 mL L−1 Em or DW (control) for 6 or 24 h prior to inoculation on tomato cv. Moneymaker seedlings (four-leaf stage) grown in small transparent plastic tubes (120 × 20 × 15 mm) in sterilized (100 °C, 18 h) sandy soil. Each treatment had approximately 200 J2, and the plants were kept in a growth chamber under controlled temperature (20 ± 1 °C), humidity (70 %) and light (14 h), with daily application of water using a jet spray to maintain the moisture content of the soil. The concentrations used in this experiment were the recommended rate by the product suppliers (10 and 5 mL L−1 for An and Em, respectively), and each treatment was replicated five times. The number of nematodes that penetrated the roots was determined 10 days after inoculation. The tubes were soaked in water, and the soil was carefully washed from the roots. Nematodes inside the roots were stained with fuchsin acid as described by Byrd et al. (1983) and counted under a binocular stereomicroscope. Data were analysed by one-way ANOVA and results reported as significant or non-significant using the LSD test (P < 0.05).
Orientation of J2 after pre-exposure to the seaweed products
The effect of the seaweed products on the orientation of M. chitwoodi and M. hapla juveniles towards tomato roots was examined in vitro. Pluronic F-127 gel (NF Prill Poloxamer 407, BASF) was prepared according to Wang et al. (2009). Seeds of tomato were germinated by placing them on moist filter paper in the dark for 1 week. Petri dishes (5.5 cm diameter) were half-filled with the pluronic gel, one seedling of tomato was placed on the centre of each plate, and the gel was allowed to solidify at room temperature. Six hours after the seedlings were placed in the gel, approximately 200 freshly hatched J2 of each Meloidogyne species, which had been pre-exposed to the test products (DW, An or Em) for 6 or 24 h as previously described, were inoculated onto the gel at the edge of the Petri dish. The setup (Fig. 1a) was incubated for a further 20 h in the dark at room temperature before the number of nematodes within the 0.5 cm area of the root was determined. Each treatment was replicated five times. Data were analysed statistically by one-way ANOVA and results reported as significant or non-significant using the LSD test (P < 0.05).
Illustrations of attraction assay with a pluronic gel and b water-agar to assess the orientation of Meloidogyne chitwoodi and M. hapla second-stage juveniles (J2) after exposure to distilled water, Ascophyllum nodosum or Ecklonia maxima. Quadrants in b delimit the positions of the nematodes on the neutral zone or in the zones with test solutions (water, repellent or attractant)
In a second experiment, the orientation of J2 of M. chitwoodi towards a known attractant (tomato root diffusate (TRD)) and repellent (acetic acid) was examined after pre-exposure to Em and An for 24 h. Petri dishes (5.5-cm diameter) filled with 4 mL water-agar (1 %) were divided in four quadrants. Two opposite quadrants were considered neutral quadrants as no test solution was added. For the other two opposite quadrants, the attractant, repellent or DW (control) were added with a disc (0.5 cm diameter) of filter paper saturated with the solutions. After 2 h, to allow the solutions to be absorbed by the water-agar and a gradient to be formed, 15 J2 pre-exposed to Em, An or DW were placed in the centre of the Petri dish (Fig. 1b). After incubation for 2 h in darkness at 21 ± 1 °C, the movement of the J2 in the water-agar was observed by counting their numbers in the different quadrants. Each treatment was replicated five times, and data were analysed with the G test for goodness of fit (Sokal and Rohlf 1995).
Results
Glasshouse experiments
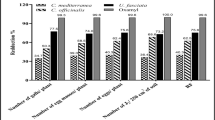
The treatment of Meloidogyne-infected tomato plants with An or Em reduced root damage by the nematodes when compared with untreated plants that were infected with M. chitwoodi only (F = 6.59, P = 0.005) (Fig. 2). However, the shoot biomass was similar between treatments (F = 0.07, P = 0.93) as was shoot length (F = 0.12, P = 0.89). The different treatments had an effect on the number of nematodes per gram root (F = 6.45, P < 0.05). Generally, the population of M. hapla per gram of root was higher when compared with M. chitwoodi (Fig. 3). Root galls caused by M. chitwoodi were not easily visible with the naked eye, unlike those caused by M. hapla which were large and distinct. For M. chitwoodi, application of Em or An gave a lower number of nematodes per gram root than the control but this was non-significant. In contrast, for M. hapla, a significant lower number of nematodes per gram root were found after application of Em compared to the control and the An treatment.
Means ± SE of root (■) or shoot (□) biomass of tomato plants for the glasshouse pot experiment following infestation with either Meloidogyne chitwoodi (Mc) or M. hapla (Mh) and treatments with different seaweed extracts from Ascophyllum nodosum (An) or Ecklonia maxima (Em). Significant differences (P < 0.05) are marked with different letters according to LSD test
Means ± SE of nematodes per gram of tomato roots in the glasshouse pot experiment after infestation with either Meloidogyne chitwoodi (Mc) or M. hapla (Mh) and treatments with different seaweed extracts from Ascophyllum nodosum (An) or Ecklonia maxima (Em). Significant differences (P < 0.05) are marked with different letters according to LSD test
Hatching assays
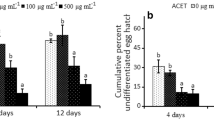
Exposure of the egg masses of M. hapla and M. chitwoodi to the seaweed products had no significant effect (P < 0.05) on the hatching process (Fig. 4). For both species, there was no difference between treatments in the final hatching percentage, rate of hatching as well as the time at which 50 % hatch was attained (Table 1). On average, the final hatching percentage of M. chitwoodi was higher than that of M. hapla. Subsequent transfer of the egg masses from the respective treatments with An or Em into DW after eight successive counts at 4-day intervals did not induce additional hatch for both Meloidogyne species (Tables 2 and 3).
Cumulative hatching curves for Meloidogyne chitwoodi (a) and M. hapla (b) in different concentrations of Ascophyllum nodosum (An) or Ecklonia maxima (Em) with distilled water (DW) as control based on the means ± SE of the test parameters: the time at which 50 % hatch was attained (m), the hatching rate (b) and the final hatching percentage (c)
In the repeated experiment with M. chitwoodi, there was a significant reduction in the percentage of hatched J2 when egg masses were exposed to 100 and 50 % An (Fig. 5). Hatching of M. chitwoodi in 100 % An (10 mL L−1) was delayed for 8 days and commenced slowly afterwards, with a significantly lower percentage hatch as compared to DW control. There was no significant effect on hatching in 10 and 25 % concentrations of An and in all treatments with Em.
Cumulative hatching curves for Meloidogyne chitwoodi in the repeated experiment with different concentrations of Ascophyllum nodosum (An) or Ecklonia maxima (Em) with distilled water (DW) as control based on the means ± SE of the test parameters: the time at which 50 % hatch is reached (m), the hatching rate (b) and the final hatching percentage (c)
Infectivity of J2 after pre-exposure to the seaweed products
When pre-exposed to the two seaweed products and DW for 6 h, there were no significant differences in the number of J2 of M. chitwoodi that infected the plants (Fig. 6a). After a 24-h pre-exposure to the two seaweed solutions, there was a significant reduction (P < 0.05) in the number of J2 of M. chitwoodi that infected the plant compared with infectivity in the distilled water control treatment (Fig. 6a). However, the reduction achieved with pre-treatment with An was much greater than with Em.
Infectivity of hatched J2 of Meloidogyne chitwoodi (a) and M. hapla (b) after 6-h (■) and 24-h (□) pre-exposures to Ascophyllum nodosum (An) or Ecklonia maxima (Em) with distilled water (DW) as control. Bars show means ± SE of the number of J2 that infected the roots. Significant differences are marked with different letters, LSD test (P < 0.05)
A 6-h pre-exposure of the J2 of M. hapla did not show any significant difference between the treatment with An and DW, but the treatment with Em demonstrated an unexpected increase in the number of J2 that infected the plants (Fig. 6b). After a 24-h pre-exposure to An, there was a significant reduction (P < 0.05) of the number of M. hapla J2 that infected the tomato plant roots in relation to Em treatment (Fig. 6b).
Orientation of J2 after pre-exposure to the seaweed products
In vitro bioassay with pluronic gel
The number of J2 found within the 0.5 cm area of the root after a 6 h pre-exposure of the J2 of M. chitwoodi did not differ significantly between the treatments (Fig. 7a). When exposed to the test solutions for 24 h prior to inoculation, there was a significant increase (P < 0.05) in the number of J2 of M. chitwoodi that could be seen within the 0.5-cm area of the root for the treatments with Em (Fig. 7a). A similar situation was observed for M. hapla, but unlike M. chitwoodi, there was an increase in the number of J2 of M. hapla within the 0.5-cm area after a 6 h pre-exposure time (Fig. 7b). Treatments with DW control and An both resulted in low numbers of J2 that could reach the 0.5 cm area around the root for M. hapla. The number was much lower for J2 pre-exposed for 24 h to An than those pre-exposed to DW, although the difference was non-significant (Fig. 7b).
Attraction of hatched J2 of Meloidogyne chitwoodi (a) and M. hapla (b) after 6-h (■) and 24-h (□) pre-exposures to the Ascophyllum nodosum (An) or Ecklonia maxima (Em) with distilled water (DW) as control. Bars show means ± SE of the number of J2 that could be seen within the 0.5-cm area around the root. Significant differences are marked with different letters, LSD test (P < 0.05)
In vitro bioassay on water-agar plates
For all the treatments, the number of J2 moving around the Petri dish was significantly higher than the number of nematodes that remained located at the inoculation point in the Petri dish (G T > 11.1, P < 0.05). The orientation towards water, attractant or repellent solutions was determined based on the J2 that moved out of the centre of the plate. For the control plates, there was no significant difference in the number of juveniles that moved to the water quadrants or to the neutral quadrants (G T < 11.1, P > 0.05) (Fig. 8a). However, in plates with the attractant, only the nematodes that were pre-exposed to DW represented a significantly higher migration towards the root diffusate gradient (G T > 11.1, P < 0.05). A similar number of nematodes pre-exposed to An moved to the attractant and neutral quadrants while for those J2 pre-exposed to Em, the migration was significantly higher towards the neutral quadrants (G T > 11.1, P < 0.05) (Fig. 8b). The orientation in agar plates with a repellent solution revealed that a significantly higher number of J2 pre-exposed to DW and Em moved towards the neutral quadrants, whereas nematodes pre-exposed to An moved either to the acetic acid or the neutral quadrant of the plates (G T < 11.1, P > 0.05) (Fig. 8c).
Orientation of Meloidogyne chitwoodi pre-exposed to Ascophyllum nodosum (An) or Ecklonia maxima (Em) with distilled water (DW) as control on agar plates, with test solutions water (a), tomato root diffusate as the attractant solution (b) and acetic acid as repellent solution (c). Data were calculated based on the number of J2 that moved away from the inoculation place. Bars show means ± SE of the number of J2 recorded at neutral (□) and test solution (■) quadrants. Asterisks indicate significant differences (P < 0.05) for the pair-wise comparison between each test solution
Discussion
A wide range of beneficial effects of seaweed extract applications on plants, such as improved crop performance and yield, early seed germination and establishment, enhanced post-harvest shelf-life of perishable products, and elevated resistance to biotic and abiotic stress have been well documented (Beckett and van Staden 1989; Hankins and Hockey 1990; Blunden 1991; Norrie and Keathley 2006). Genard et al. (1991) attributed yield enhancement effects due to improved chlorophyll content in leaves of various crop plants treated with seaweed to the betaines present in the extract. Observations from the glasshouse pot test in the present study are a confirmation of previous findings by Featonby-Smith and van Staden (1983) that seaweed concentrates improve the growth of nematode-infected tomato plants. In the present study, there were no statistical differences in the length and fresh weight of the above-ground plant parts but visible differences between treatments in the aerial plant parts were observed.
Although there have been a number of reports demonstrating significant reductions of the level of root-knot nematodes attack on plants treated with seaweed extract from An and Em (Featonby-Smith and van Staden 1983; Crouch and van Staden 1993; Whapham et al. 1994; Wu et al. 1997; Radwan et al. 2011, 2012), the experiments have mostly been done on tropical root-knot nematode species. In the present study, tomato roots were challenged with the temperate root-knot nematodes M. chitwoodi and M. hapla. No significant difference between treatments in the level of attack on the root of the tomato plants by M. chitwoodi upon application as a soil drench of the two seaweed products used was seen. However, treatments with Em and An gave a lower number of M. chitwoodi per gram of root compared to the control. This difference can be allocated to the higher root biomass after application of Em and An. For M. hapla, a significant lower number of nematodes per gram root were found in the treatment with Em. There was no difference in root biomass between treatments for M. hapla, so other factors must have been influencing this result. The number of M. hapla that infected the tomato plants was consistently higher than M. chitwoodi for all treatments with the two seaweed products and the DW control. The reason for this is unclear, although differences in biology, for example temperature preferences for activity, may have been influential.
In many species of plant-parasitic nematodes, the hatching behaviour is considered an essential component of the life cycle for optimizing the chances of successful infection by synchronization with host availability (Perry 2002). Meloidogyne chitwoodi and M. hapla have a remarkable difference in this aspect of their biology. It has been shown (Santo and O’Bannon 1981) that M. chitwoodi is able to reproduce at lower soil temperatures than M. hapla. However, Inserra et al. (1983) had demonstrated that temperatures of approximately 15, 20 and 25 °C give similar effects on the hatch of both species. The maximum-percentage hatch obtained during the present study was higher for M. chitwoodi (98 %) than for M. hapla (85 %). The high-percentage hatch obtained in DW confirms that Meloidogyne hatch spontaneously in water under favourable environmental conditions (Perry 1997). Inserra et al. (1983) showed that root diffusates of tomato, potato and wheat did not increase hatch of M. chitwoodi and M. hapla. However, recent findings by Perry and Wesemael (2008) show exceptions, suggesting that responses to root exudates may be more important than previously realized. This is supported by a more recent study by Oka and Mizukubo (2009) who demonstrated an increased number of J2 of M. incognita that hatched in hydroponic culture media in which tomato and okra had been grown as compared with those in water or fresh culture medium. In our study, reduction in hatching has been clearly demonstrated by the 100 and 50 % concentrations of An compared with the DW control treatment in the repeated experiment with M. chitwoodi. This, together with the amount of reduction in the number of J2 that infected the tomato plant root after a 24-h pre-exposure to An, confirms previous reports (Whapham et al. 1994; Wu et al. 1997, 1998; Radwan et al. 2011, 2012) that extracts from this seaweed product reduce infection of tomato plants by J2 of Meloidogyne spp. However, in the first experiment, no differences in hatching were seen between treatments. As experiments were separated in time, the condition of the egg masses might have been different. Wesemael et al. (2006) showed that for M. chitwoodi, hatching behaviour is influenced by the condition of the host plant. Preliminary experiment had shown that the seaweed extract has no toxic effect on the J2 upon exposure to the product for a longer period of time and this confirms an earlier report by Wu (1996). In addition, the fact that hatched J2 after being in the product for 4 days were still actively moving is an additional support. Whapham et al. (1994) showed that J2 of M. javanica hatched directly into seaweed extract from An had a greatly reduced level of attack on tomato plant roots. The role of extract of the seaweed products on hatching and infectivity therefore can be considered as an indirect effect. A variety of enzymes, including lipase, proteinase and chitinase, are involved in the hatching process of nematodes (Perry 1997). The extract of An consists of a variety of compounds, and some of these compounds could possibly have interacted with and interrupted activities of the enzymes that are correlated with the hatching percentage; these enzymes are capable of increasing the flexibility of the egg shell (Perry et al. 1992), which is a characteristic for hatching of these Meloidogyne spp.
Migration by root-knot nematodes through the soil can be limited or stopped by adverse conditions of moisture, porosity, oxygen availability, toxins and temperature (Curtis et al. 2009). Sandy soils are well known as a good medium for root-knot nematodes, and low compaction of the soil appears to favour the movement of these nematodes (Eo et al. 2007). In the bioassay with pluronic gel on Petri dishes, the behaviour of both Meloidogyne spp. are in some cases consistent with their infectivity after prior exposure to the seaweeds products. Meloidogyne chitwoodi appeared to be more attracted to the roots on both the gel and in sandy soil after a 24-h pre-exposure to Em, even twofold more than the DW control in the attraction test. The same holds for M. hapla, but unlike M. chitwoodi, M. hapla was more attracted to the root after a 6-h pre-exposure to Em. This could either be due to the fact that the J2 became adapted to the conditions in the solution after the said time period or that this seaweed product has some chemical properties that enhance sensory perceptions of the roots by the nematodes after these time periods. Perry (2005) provided a useful generalized framework to visualize attractants by classifying them as long-distance, short-distance and local attractants. Root-knot nematodes are said to be attracted to the root area by long-distance attractants. Short-distance attractants attract the nematodes to the roots themselves while local attractants are responsible for orientation to the preferred invasion site by endoparasitic nematodes. Early in vitro experiments demonstrated the attraction of nematodes to the roots as well as within sand to the zones where the roots had been growing (Prot 1980). Wang et al. (2009) using in vitro assays with pluronic gel demonstrated that M. javanica and M. incognita moved to the roots much more rapidly than M. hapla. However, it could be that the two tropical root-knot nematodes were more favoured by the temperature of the gel than the northern root-knot nematode. Pluronic gel becomes semi-solid at room temperature while at temperatures below 20 °C, it is in the liquid state when concentrated at 20–30 %. They also found aggregations of J2 when the nematodes were in contact with root tips, indicating that a signal from the root is involved in the attraction. In the present study, a similar situation was observed in treatment with Em. Wang et al. (2009) suggested that lower oxygen or a volatile attractant is involved in this aggregation behaviour. Perhaps, the absence of these type of signals might be one of the reasons for the results obtained in agar plates, where M. chitwoodi treated with Em and An were not clearly attracted by tomato root diffusate and moved towards the neutral quadrants. Although the importance of root diffusates on the orientation of endoparasitic nematodes to the host roots is well known (Perry 1997), it could be seen through the result of this study that other factors directly related to the presence of the host by itself can strengthen the signal for the nematode orientation and migration to the host. In addition, it was unclear if the diffusion of the attracting components of TRD in the agar was somehow restricted by the agar medium itself. It is possible that changing the attractant from TRD to a tomato seedling or increasing the time for the agar to absorb the test solutions would result in a better nematode chemoreception. Juveniles exposed to An and Em could sense the presence of a repellent in the agar. The fact that nematodes treated with DW and Em migrated significantly more to the neutral quadrants confirms that sensory perception was not disrupted by the exposure to the seaweed extracts. The responses to this type of allelochemicals are mediated by organs such as amphids, which are situated laterally on either sides of the mouth and are the primary chemosensory organs of nematodes (Riga et al. 1995).
Seaweed extracts from A. nodosum used as a soil drench in the glasshouse pot test to improve the growth of Meloidogyne-infected tomato as well as reduction in hatch and inhibition of infectivity of J2 in vitro have been clearly demonstrated. However, the level of control achieved with these applications alone may be insufficient under normal agricultural conditions, and thus, such treatments would have to be incorporated into an integrated control programme. This would include other nematode control measures, for example, the addition of organic supplements to the soil (Radwan et al. 2011) and the sustainable use of nematicides.
Growth enhancers are mostly developed without assessment of possible effects on pests and diseases. Only when marketed, products will be tested for these aspects to create an added value. Both companies and science would benefit if crop protection properties would be included from the beginning. Further research and development of biological control methods must be given high priority, and people in general and farmers in particular should be educated about the risk associated with the handling and use of chemical nematicides. The general public is advised to demand farm products where chemical nematicides application is minimal or absent. All these will lead to a general enlightenment about the benefits of bio-based products and integrated approaches thereby compelling policy makers to take decisions that would reduce the use of chemical nematicides and increase the use of a green substitute.
References
Aliya R, Shamaeel M (1999) Phytochemical evaluation of four coenocytic green seaweeds from the coast of Karachi. Pak J Mar Biol 5:65–76
Baermann G (1917) Eine einfache Methode zur Auffindung von Anklyostomum (Nematoden) Larven in Erdproben. Geneesk Tijdschr Ned-Indie 57:131–137
Beckett RP, van Staden J (1989) The effect of seaweed concentrate on the growth and yield of potassium stressed wheat. Plant Soil 116:29–36
Blunden G (1991) Agricultural uses of seaweeds and seaweed extracts. In: Guiry MD, Blunden G (eds) Seaweed resources in Europe: uses and potential. Wiley, Chichester, pp 65–81
Boot CO (1964) Seaweed has possibilities apart from its fertilizer use. Grower 62:442–443
Byrd DW, Kirkpatrick T Jr, Barker KR (1983) An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol 15:142–143
Crouch IJ, van Staden J (1993) Effect of seaweed concentrate from Ecklonia maxima (Osbeck) Papenfuss on Meloidogyne incognita infestation on tomato. J Appl Phycol 5:37–43
Curtis RHC, Robinson AF, Perry RN (2009) Hatch and host location. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CABI, Wallingford, pp 139–162
De Waele D, Mc Donald AH, De Waele E (1988) Influence of seaweed concentrate on the reproduction of Pratylenchus zeae (Nematoda) on maize. Nematol 34:71–77
Eo J, Nakamoto T, Otobe K, Mizukubo T (2007) The role of pore size on the migration of Meloidogyne incognita juveniles under different tillage systems. Nematol 9:751–758
Featonby-Smith BC, van Staden J (1983) The effect of seaweed concentrates on the growth of tomato plants in nematode-infested soil. Sci Hort 20:137–146
Garg HS, Sharma T, Bhakuni DS, Pramanik BN, Bose AK (1992) An antiviral sphingosine derivative from green alga Ulva fasciata. Tetrahedron Lett 33:1641–1644
Genard H, Le Saos J, Billard JP, Tremolieres A, Boucaud J (1991) Effect of salinity on lipid composition, glycine betaine content and photosynthetic activity in chloroplasts of Suaeda maritima. Plant Physiol Biochem 29:421–427
Hankins SD, Hockey HP (1990) The effect of a liquid seaweed extract from Ascophyllum nodosum (Fucales, Phaeophyta) on the two-spotted red spider mite Tetranychus urticae. Hydrobiologia 204:555–559
Harder R, Oppermann A (1953) Über Antibiotische Stoffe bei den Grünalgen Stichoccus bacillaris and Protosiphon botryoides. Arch Mikrobiol 19:398–401
Hendrickx G (1995) An automated apparatus for extracting free-living nematode stages from soil (Abstr.). Nematologica 41:308
Hol GWH, Cook R (2005) An overview of arbuscular mycorrhizal fungi-nematode interactions. Basic Appl Ecol 6:489–503
Inserra RN, Griffin GD, Sisson DV (1983) Effects of temperature and root leachates on embryonic development and hatching of Meloidogyne chitwoodi and M. hapla. J Nematol 15:123–127
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WML, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Path 14:946–961
Manilal A, Sugathan S, George SK, Joseph S, Chippu S, Ramakrishnan G, Mamkoottathil VNP (2009) Biopotentials of seaweeds collected from southwest coast of India. J Mar Sci Tech 17:67–73
Moens M, Perry RN, Starr JL (2009) Meloidogyne species—a diverse group of novel and important plant parasites. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CABI, Wallingford, pp 1–17
Morgan KT, Tarjan AC (1980) Management of sting nematode on centipede grass with kelp extracts. Proc Fla State Hort Soc 93:97–99
Norrie J, Keathley JP (2006) Benefits of Ascophyllum nodosum marine-plant extract applications to ‘Thompson seedless’ grape production. Acta Hort 727:243–247
Nyczepir AP, Thomas SH (2009) Current and future management strategies in intensive crop production systems. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CABI, Wallingford, pp 412–443
Oka Y, Mizukubo T (2009) Tomato culture filtrate stimulates hatching and activity of Meloidogyne incognita juveniles. Nematol 11:51–61
Oude Voshaar JH (1994) Statistiek voor onderzoekers. Wageningen Academic Publishers, Wageningen
Perry RN (1997) Plant signals in nematode hatching and attraction. In: Fenoll C, Grundler MW, Ohl SA (eds) Molecular aspects of plant-nematode interactions. Kluwer Academic Publishers, Dortrecht, pp 38–50
Perry RN (2002) Hatching. In: Lee DL (ed) The biology of nematodes. Taylor and Francis, London, pp 147–169
Perry RN (2005) An evaluation of types of attractants enabling plant-parasitic nematodes to locate plant roots. Russ J Nematol 13:83–88
Perry RN, Wesemael WML (2008) Host plant effects on hatching of root-knot nematodes. Russ J Nematol 16:1–5
Perry RN, Knox DP, Beane J (1992) Enzymes released during hatching of Globodera rostochiensis and Meloidogyne incognita. Fund Appl Nematol 15:283–28
Pratt R, Daniel TC, Gunnison JB, Kumler WD, Oneto JF, Strait LA, Spoehr HA, Hardin GJ, Milner HW, Smith JHC, Strain HH (1944) Chlorellin: an antibacterial substance from Chlorella. Science 99:351–352
Prot JC (1980) Migration of plant-parasitic nematodes towards plant roots. Rev Nématol 3:305–318
Radwan MA, Abu-Elamayem MM, Farrag SAA, Ahmed NS (2011) Integrated management of Meloidogyne incognita infecting tomato using bio-agents mixed with either oxamyl or organic amendments. Nematol Medit 39:151–156
Radwan MA, Farrag SAA, Abu-Elamayem MM, Ahmed NS (2012) Biological control of the root-knot nematode, Meloidogyne incognita on tomato using bioproducts of microbial origin. Appl Soil Ecol 56:58–62
Riga E, Perry RN, Barrett J, Johnston MRL (1995) Investigation of the chemosensory function of amphids of Syngamus trachea using electrophysiological techniques. Parasitology 111:347–35
Rocha FD, Soares AR, Houghton PJ, Pereira RC, Kaplan MAC, Teixeira VL (2007) Potential cytotoxic activity of some Brazilian seaweeds on human melanoma cells. Phytoth Res 21:170–175
Santo GS, O’Bannon JH (1981) Effect of soil temperature on the pathogenicity and reproduction of Meloidogyne chitwoodi and M. hapla on Russet Burbank potato. J Nematol 13:483–4862
Sasser JN, Carter CC (1982) Overview of the international Meloidogyne project—rationale, goals, implementation and progress to date. Proc. 3rd Res. Plan. Conf. on root-knot nematodes Meloidogyne spp. Panama pp:1–7
ScienceDaily (2008) Seaweed products. accessed in 2009
Serkedjieva J (2004) Antiviral activity of the red marine algae Ceramium rubrum. Phytoth Res 18:480–483
Smith AJ (2004) Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol 16:245–262
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. WH Freeman and Co., New York
Stirling GR (2014) Biological control of plant-parasitic nematodes. CABI, Wallingford
Tang HF, Yi YH, Yao XS, Xu QZ, Zhang SY, Lin HW (2002) Bioactive steroids from the brown algae Sargassum carpophyllum. J Asian Nat Prod Res 4:95–105
Tarjan AC (1977) Kelp derivatives for nematode-infected citrus trees. J Nematol 9:28
Tuney I, CadircI BH, Unal D, Sukatar A (2006) Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey). Turkish J Biol 30:171–175
Wang C, Lower S, Williamson VM (2009) Application of pluronic gel to the study of root-knot nematode behaviour. Nematol 11:453–464
Wesemael WML, Perry RN, Moens M (2006) The influence of root diffusate and host age on hatching or the root-knot nematodes, Meloidogyne chitwoodi and M. fallax. Nematol 8:895–902
Wesemael WML, Viaene N, Moens M (2011) Root-knot nematodes (Meloidogyne spp.) in Europe. Nematol 13:3–16
Whapham C, Jenkins T, Blunden G, Hankins D (1994) The role of seaweed extracts, Ascophyllum nodosum, in the reduction in fecundity of Meloidogyne javanica. Fund Appl Nematol 17:181–183
Wu Y (1996) Biologically active compounds in seaweed extracts. Ph.D. Dissertation, University of Portsmouth
Wu Y, Jenkins T, Blunden G, Whapham C, Hankins D (1997) The role of betaines in alkaline extracts of Ascophyllum nodosum in the reduction of Meloidogyne javanica and M. incognita infestations of tomato plants. Fund Appl Nematol 20:99–102
Wu Y, Jenkins T, Blunden G, Mende VN, Hankins SD (1998) Suppression of fecundity of the root-knot nematode, Meloidogyne javanica, in monoxenic cultures of Arabidopsis thaliana treated with an alkaline extract of Ascophyllum nodosum. J Appl Phycol 1:91–94
Acknowledgments
The authors would like to thank Riaan Lourens (Kelpak) and OSMO company for providing the products used. The second author, Yirina Valdes received financial support from the Flemish Interuniversity Council (VLIR) at Ghent University. This study was supported by the Belgian Plant Protection Service (FAVV).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ngala, B.M., Valdes, Y., dos Santos, G. et al. Seaweed-based products from Ecklonia maxima and Ascophyllum nodosum as control agents for the root-knot nematodes Meloidogyne chitwoodi and Meloidogyne hapla on tomato plants. J Appl Phycol 28, 2073–2082 (2016). https://doi.org/10.1007/s10811-015-0684-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0684-4