Abstract
Emergence of extended antibiotic resistance among bacterial pathogens often leads to the failure of existing antibiotics to treat bacterial infections; therefore, there is an urgent need to look for novel alternative treatment measures. The aim of this study was to evaluate the anti-quorum sensing (QS) potential of Synechococcus sp., to prevent the onset of bacterial infections as an alternate to antibiotics. A total of 110 marine cyanobacterial strains were screened for their anti-QS activity against biomarker strain Chromobacterium violaceum (ATCC 12472) and aquatic bacterial pathogens Vibrio harveyi (MTCC 3438) and Vibrio vulnificus (MTCC 1145). Of the 110 strains tested, the extract of unicellular algae Synechococcus sp. (Q-25) exhibited the efficient reduction in the production of violacein pigment of C. violaceum to the level of 82 %, bioluminescence of V. harveyi to 91 % and protease in V. vulnificus to 63 %. In V. harveyi and V. vulnificus, it exhibited a significant reduction of 71 and 84 % in biofilm formation and 66 and 68 % in EPS production, respectively, without any antibacterial activity. Confocal laser scanning microscopic and light microscopic analyses further confirmed that the Q-25 extract effectively prevented initial attachment as well as disrupting the architecture of mature biofilm, when compared to their untreated controls. In addition, the characterization of active principle by gas chromatography–mass spectrometry analysis confirmed the presence of stable bioactive compound hexadecanoic acid in the extract. Hence, this study clearly revealed the antibiofilm and QS inhibitory potential of the cyanobacterium, Synechococcus sp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is one of the fastest growing food producing sectors worldwide (Leung and Bates 2012). Despite such progressive growth, the emerging microbial diseases hamper the growth of aquaculture and cause severe economic losses worldwide. One of the prime threats to the intensive aquaculture industry is infectious diseases caused by Vibrio species. The most significant bacterial infectious agents in marine aquaculture includes Vibrio harveyi, Vibrio parahaemolyticus, Vibrio alginolyticus, Vibrio anguillarum, Vibrio vulnificus, Vibrio salmonicida and Aeromonas hydrophila (Austin and Zhang 2006; Ye et al. 2008; Qi et al. 2009). V. harveyi, a luminous marine bacterium, is the most important etiological agent for mass mortalities in black tiger shrimp (Penaeus monodon) larval rearing systems. V. parahaemolyticus is a halophilic bacterium distributed in temperate water and causes early mortality syndrome in Penaeid shrimp and hepatopancreatitis in humans via consumption of contaminated uncooked seafood (Matsumoto et al. 2000). It is also responsible for the most common vibrio-associated seafood-borne gastroenteritis. Likewise, V. vulnificus is also well documented to cause gastrointestinal infections in humans through the consumption of uncooked seafood and can also cause wound or soft tissue infections in marine organisms (Bross et al. 2007).
Consequently, aquaculture has experienced relatively severe disease problems owing to lack of control on microbiota in culture systems. Habitually, a wide range of common antibiotics are being used either as a prophylactic measure or to treat established infections. This kind of routine and indiscriminate usage of antibiotics to prevent bacterial infections in aquaculture leads to the development of resistance among bacterial pathogens and makes the existing antibiotics ineffective, which necessitates the finding of suitable alternative strategies to control such problems (Defoirdt et al. 2011). The necessary factors responsible for the resistance development in bacterial pathogens are biofilm formation and virulence factor production, which come under the control of quorum sensing (QS) systems (Hentzer and Givskov 2003).
In vibrios, QS is the phenomenon of coordinated gene expression regulated by three different types of autoinducers. The LuxM/N QS system utilizes acylated homoserine lactones (AHLs) as signal molecules, whereas CqsA/S system utilizes (S)-3-hydroxytridecan-4-one (“Cholera autoinducer 1”, CAI-1), and in the third QS system, the signal molecules are collectively referred as autoinducer-2 (AI-2) (Milton 2006). These signal molecules are known to coordinate the production of virulence factors and biofilm formation in response to the cell density of the surrounding bacterial population (You et al. 2007; Yildiz and Visick 2009). The biofilm forming potential of Vibrio spp. is responsible for their survival, virulence and stress resistance (Packiavathy et al. 2013). Such biofilms are the preferable lifestyle for bacteria as they enhance growth and survival by affording the free entry to nutrients and avoid the entry of antibiotics. Hence, interfering with such QS mechanisms by targeting these signal molecules and to inhibit their virulence enzyme production and biofilm formation will pave the way to combat bacterial infections especially vibriosis in the aquaculture industry.
In recent years, there is a growing interest in the discovery of novel anti-quorum sensing (anti-QS) compounds from natural sources to interrupt or hinder the QS signalling pathway in pathogenic bacteria. Fascinatingly, various anti-QS compounds have already been identified from a wide range of natural resources, including medicinal plants (Nostro et al. 2007), edible vegetables and fruits (Musthafa et al. 2010), marine sponges and coral associated bacteria (Thenmozhi et al. 2009; Nithya et al. 2011; Annapoorani et al. 2012). These anti-QS compounds are believed to provide complementary medicine for emerging bacterial infections by inhibiting the QS system. Similarly, marine cyanobacteria have been reported as one of the promising candidates for bioactive compounds. Marine cyanobacteria have gained much attention due to their wide range of potential biological applications such as anticancer, antibacterial, antifungal, antiviral and protease inhibitor activities. They are a group of Gram-negative photoautotrophic prokaryotes, capable of performing oxygenic photosynthesis (Singh et al. 2011; Uzair et al. 2011; Amrei et al. 2014). It has also been well demonstrated that metabolites from cyanobacteria can interfere with autoinducer-dependent QS system in pathogenic bacteria (Clark et al. 2008; Dobretsov et al. 2010, 2011). However, reports on marine cyanobacteria for anti-QS activity are very limited. Thus, it would be valuable to screen marine cyanobacterial communities for their ability to produce anti-QS compounds. This will provide an alternate to antibiotic use to battle emerging aquatic bacterial infections. Hence, in the present study, we investigated the anti-QS potential of the marine cyanobacterium Synechococcus sp. (Q-25) extract against aquatic bacterial pathogens.
Materials and methods
The marine cyanobacterial strains used in this study were obtained from the Cyanobacteria Culture Collection Centre, Department of Microbiology, Bharathidasan University, Tiruchirappalli, India. The samples were aseptically transferred to sterile marine nutrient (MN+) medium (Shao et al. 2013) and maintained in the same medium for further studies. Experimental cultures were incubated at 25 ± 2 °C, under a 14/10-h light/dark cycle, with illumination of 20 μmol photons m−2 s−1 under cool white fluorescent lamps (Philips, India) (Shao et al. 2013). The cultures were gently shaken by manually on alternate days to prevent attachment.
Cells were pelleted by centrifugation at 10,000 rpm for 15 min, and the culture supernatant was filtered through a 0.22-μm nitrocellulose membrane filter (Merck, India). The cell-free culture supernatant (CFCS) was extracted with an equal volume of chloroform/ethyl acetate (1:1) solvent system. The solvent extract was evaporated and dried under reduced pressure at room temperature to yield crude extract. The obtained crude extract was weighed and dissolved in MilliQ water (Millipore, USA) (Nithyanand and Pandian 2009). The stocks were stored in 4 °C and used for further experimental studies.
Bacterial strains, growth media and culture conditions
The bacterial strains Chromobacterium violaceum (ATCC 12472), Vibrio harveyi (MTCC 3438), Vibrio parahaemolyticus (ATCC 17802), Vibrio alginolyticus (ATCC 17749), Vibrio vulnificus (MTCC 1145) and Aeromonas hydrophila (ATCC 7966) were used as the test pathogens. C. violaceum and CV026 (ATCC 31532) were used as marker strains to test the anti-QS activity. C. violaceum and CV026 were cultured in Luria-Bertani (LB) broth (pH 7 ± 0.2) at 37 and 30 °C, respectively, and all the other test bacterial pathogens were cultivated in marine LB (mLB) broth (pH 7.5 ± 0.2) at 30 °C. For experimental purpose, all the test bacterial pathogens were subcultured in mLB until it reaches the OD of 0.4 at 600 nm.
Qualitative and quantitative analysis of violacein inhibition
The preliminary violacein inhibition assay was performed according to the method of Choo et al. (2006). A total of 110 marine cyanobacterial strains were subjected to qualitative analysis to find their anti-QS potential against C. violaceum. It synthesizes a violet-coloured pigment known as violacein by responding to its QS-mediated signal molecule N-hexanoyl-L-homoserine lactone (HHL) produced by its autoinducer synthase CviI. This HHL binds to its receptor CviR, and this complex triggers the expression of violacein production (Choo et al. 2006). Overnight culture (1 %) of C. violaceum (adjusted to 0.4 OD at 600 nm) was added into the wells of sterile micro-titre plates (MTPs) containing 1 mL of LB broth and incubated in the presence and absence of varying concentrations of test extracts. The MTPs were incubated at 30 °C for 16 h and observed for the reduction in violacein pigment production.
Further, all the positive extracts were subjected to the quantitative analysis with CV026 in a 24-well MTPs. CV026 is a violacein-negative, double mini-Tn5 mutant of C. violaceum (ATCC 31532), deficient in the autoinducer synthase CviI but known to respond the exogenous supply of autoinducer molecules (Choo et al. 2006). Hence, in order to induce the violacein production in CV026, the autoinducer molecules HHL (Sigma-Aldrich, Switzerland) was added at a working concentration of 5 μM to 0-h culture of CV026 and incubated at 30 °C for 16 h. One-millilitre culture from each well was centrifuged at 10,000 rpm for 10 min to pelletize the insoluble violacein along with bacterial cells. The culture supernatant was discarded, and the cell pellet was resuspended in 1 mL of dimethyl sulfoxide (DMSO) for the extraction of violacein. The suspension was vortexed vigorously for 30 s and centrifuged at 10,000 rpm for 10 min to remove the cells, and the extracted violacein was quantified spectrophotometrically (Hitachi, U-2800, Japan) at 585 nm. All these cyanobacterial strains with potential anti-QS activity were further considered for their efficiency to inhibit biofilm formation and virulence factor production against target bacterial pathogens.
Bioluminescence inhibition assay
The bioluminescence inhibition assay was done by following the method of Teasdale et al. (2009) with little modifications. V. harveyi cells at the aforementioned cell density were inoculated into mLB and cultivated with 0, 50, 100, 150 and 200 μg mL−1 of Q-25 extract. The tubes were incubated at 30 °C for 12 h. After incubation, the intensity of bioluminescence was measured in terms of relative light units (RLU) using luminometer (Turner Biosystem Inc., USA). The percentage inhibition of bioluminescence was calculated based on the obtained RLU.
Anti-virulent assay
Total proteolytic assay
Proteolytic activity of CFCS of V. harveyi (MTCC 3438) and V. vulnificus (MTCC 1145) cultivated (0.4 OD at 600 nm) in mLB broth (pH 7.5 ± 0.2) at 30 °C with 0, 50, 100, 150 and 200 μg mL−1 of Q-25 extract was determined by azocasein assay by following the method of Kessler et al. (1993). Briefly, 75 μL of Q-25 extract treated and untreated CFCS were added to 125 μL of 0.3 % azocasein (Sigma, USA) in 0.05 M Tris–HCl and 0.5 mM CaCl2 (pH 7.5) and incubated at 37 °C for 15 min. The reaction was stopped by the addition of trichloroacetic acid (l0 %, 0.5 mL) followed by centrifugation, and the absorbance of clear supernatant was measured at 400 nm in a UV-visible spectrophotometer.
Gelatinase assay
Gelatinase activity was semi-quantitatively tested according to the method of Austin et al. (2005) using a plate with nutrient agar supplemented with 0.4 % gelatin. Ten microlitres of CFCS from both Q-25 extract treated (50–200 μg mL−1) and untreated cultures of V. harveyi and V. vulnificus were added to the seperate wells with 10 mm in diameter. After incubation for 2–4 days at the optimal temperature, the medium was flooded with 10 % HCl-15 % HgCl2 solution. A clear zone of inhibition around the growth of the bacteria indicates the gelatinase inhibition activity.
Effect of Q-25 extract on biofilm development of V. harveyi and V. vulnificus
Biofilm biomass quantification by crystal violet staining assay
The inhibition of biofilm biomass was assessed through crystal violet (CV) assay in MTP by following the method of Nithya and Pandian (2010). Briefly, 1 % of V. harveyi (MTCC 3438) and V. vulnificus (MTCC 1145) cultivated in mLB broth (pH 7.5 ± 0.2) at 30 °C with 0, 50, 100, 150 and 200 μg mL−1 of Q-25 extract (OD adjusted to 0.4 at 600 nm) for 16 h. After incubation, the plates were washed thrice with sterile-deionized water to remove loosely attached cells. The washed MTP wells were air-dried and stained with 1 mL of 0.4 % CV solution (w/v) for 15 min. Stained wells were washed thrice with deionized water to remove excess stain. The CV in the stained cells was solubilized with 1 mL of 20 % glacial acetic acid, and the absorbance was measured at 570 nm using UV-visible spectrophotometer. Percentage inhibition of biofilm biomass was calculated by following the formula [control OD-test OD / control OD] × 100.
Light microscopic observation of biofilm development on cover glasses
The test bacterial strains (OD adjusted to 0.4 at 600 nm) were incubated with cover glasses (1 cm2) in the wells of MTP containing 1 mL of mLB medium at 30 °C for 24 h in the presence (50–200 μg mL−1) and absence of Q-25 extract. After incubation, the biofilms on the cover glasses were stained as mentioned above and observed under light microscope at a magnification of × 400 (Nikon Eclipse Ti 100, Japan) (Bakkiyaraj and Pandian 2010).
Confocal laser scanning microscopic (CLSM) analysis
The effect of bacterial extract on biofilm formation was studied on cover glasses. The biofilms of V. harveyi and V. vulnificus were developed on cover glasses as described above and stained with 0.1 % acridine orange solution (w/v) for 3 min. The biofilms were monitored under a CLSM (Model LSM 710) (Carl Zeiss, Germany). The 488-nm argon laser and a 500- to 640-nm band pass emission filters were used to excite and detect the stained cells (Gowrishankar et al. 2012).
Disintegration of mature biofilm
To screen the destroyer of mature biofilm, the preformed mature biofilm on the cover glasses of V. harveyi and V. vulnificus was incubated with Q-25 extract at the concentration of 200 μg mL−1 for 5 h at 30 °C. After incubation, the biofilms on the cover glasses were stained and washed twice with distilled water and air-dried. The stained cover glasses were observed under both light microscopy and CLSM (You et al. 2007).
Exopolysaccharide (EPS) inhibition assay
EPS inhibition assay was carried out by following the method of Nithya et al. (2011) with small modifications. Briefly, cover glasses immersed in the cultures of Q-25 extract treated (50–200 μg mL−1) and untreated bacterial pathogens in 24-well MTP were incubated for 16 h. After incubation, the cover glasses were removed and washed with 0.9 % NaCl. The cell suspensions in 0.9 % NaCl (0.5 mL) were incubated in test tubes with an equal volume of 5 % phenol (0.5 mL) to which 5 volumes of concentrated H2SO4 were added. The mixture was incubated for 1 h in the dark and centrifuged at 10,000 rpm for 10 min, followed by the absorbance that was measured at 490 nm.
Motility inhibitory assay
Swimming and swarming assay
In swimming assay, 3-μL overnight culture of the aquatic bacterial pathogens (0.4 OD at 600 nm) was point inoculated at the centre of the swimming agar medium consisting of 1 % tryptone, 0.5 % NaCl and 0.3 % agar supplemented with 100 μg mL−1 of test extract. For swarming assay, 5 μL (0.4 OD at 600 nm) overnight culture of test pathogens was inoculated at the centre of the swarming agar medium consisting of 1 % peptone, 0.5 % NaCl, 0.5 % agar and 0.5 % of filter-sterilized D-glucose. The plates were incubated at 30 °C for 16 h and observed for reduction in swimming and swarming migration zones.
Antibacterial activity by well diffusion agar assay
In order to determine whether the Q-25 extracts have any antibacterial efficacy against V. harveyi and V. vulnificus, well diffusion agar assay was performed using Muller-Hinton agar (MHA) (Himedia, India). Overnight cultures of V. harveyi and V. vulnificus were subcultured in mLB until a turbidity of 0.5 McFarland (1 × 108 CFU mL−1) was reached. Using a sterile cotton swab, the cultures were uniformly spread over the surface of the agar plates, and to absorb the excess moisture, plates were kept undisrupted for 10 min. In the swabbed plate, wells were punched with a diameter of 10 mm and loaded with extract (at the concentrations of 50–200 μg mL−1). The MHA plates were then incubated at 37 °C, and the plates were observed for zone of clearance after 24 h (Bakkiyaraj and Pandian 2010).
Confirmation of non-enzymatic nature of active principle
In order to determine the nature of the active principle in Q-25, the CFCS of Q-25 was subjected to enzymatic (proteinase K) treatment. One-millilitre CFCS of test marine cyanobacteria was treated with proteinase K (Sigma-Aldrich, USA) at the concentration of 1 mg mL−1. The proteinase K-treated samples were incubated at 55 and 70 °C for 1 h and 20 min, respectively. After incubation, samples were tested for anti-QS activity as described above with C. violaceum.
Purification and characterization of active compound by gas chromatography–mass spectrometry (GC-MS) analysis
The Q-25 crude extract showing anti-QS activity was partially purified through thin layer chromatography (TLC) plates (Merck, Germany) by using the solvent system of chloroform/ethyl acetate/methanol (6:3:1). Further, the partially purified fractions were loaded into the silica gel packed column (20 cm length and 2 cm in diameter) and eluted with chloroform/ethyl acetate (50:50). The fractions were collected and checked for activity by bioassay.
The fractions showing anti-QS activity were characterized by gas chromatograph (GC-2010) interfaced with a quadrupole mass spectrometer (QP-2010) (Shimadzu Corporation, Japan) analyzer in order to determine its chemical constituents using Rtx-PCB capillary column (60 m × 0.25 mm i.d., 0.25-mm film thickness, RESTEK, Bellefonte, PA). Helium with a purity of 99.99 % was used as the carrier gas at a flow rate of 1 mL min−1. One millilitre of extract was injected in split mode using an autosampler. The injector port, interface and ion source temperatures were set at 250, 270 and 230 °C, respectively. GC temperature was programmed as follows: 50 °C (1 min), 10 °C min−1 ramp to 320 °C (10 min hold). The mass spectrometer was operated in electron ionization (EI) mode at 70 eV and at an emission current of 60 mA. Full scan data were obtained in a mass range of m/z 50–500.
Data collection and statistical analysis
All experiments were performed in triplicate, and the data obtained were analyzed by one-way ANOVA, with a P value of 0.05 being significant, using Student’s t test.
Results
Qualitative and quantitative assay for violacein inhibition
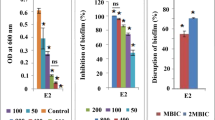
In qualitative screening for anti-QS potential, among the 110 samples tested, the chloroform/ethyl acetate (1:1) extract of Q-25 was showed consistent as well as maximum inhibition of violacein production in C. violaceum without any antibacterial activity (Table 1, Supplementary Fig. 1). Hence, Q-25 extract alone was used for further experiments. Similarly, in CV026 mutant, a gradual decrease in the production of violacein pigment was quantitatively observed when treated with the increasing concentrations of Q-25 extract (50–200 μg mL−1). A maximum of 39, 65, 73 and 82 % of inhibition in violacein production was observed at the concentrations of 50, 100, 150 and 200 μg mL−1, respectively (Fig. 1).
Effect of Q-25 extract on bacterial growth and violacein production. Quantification of violacein production in CV026 treated with Q-25 extract. Data are represented as percentage of violacein inhibition. Mean values of triplicate independent experiments and SE are shown. Single asterisk indicates significant at P ≤ 0.025 and triple asterisk indicates significant at P ≤ 0.005 of treated compared to control
Bioluminescence inhibition assay
Bioluminescence production is one of the important factors in V. harveyi, which is positively regulated by QS system. Interestingly, Q-25 extract exhibited a dose-dependent inhibition of bioluminescence, and a maximum range of 17, 35, 60 and 91 % inhibition was attained with the aforementioned concentrations (Fig. 2).
Effect of Q-25 extract on bioluminescence production. At 50–200 μg mL−1 concentration in V. harveyi. The results were shown as percentage of inhibition. Mean values of triplicate independent experiments and SE are shown. Single asterisk indicates significant at P ≤ 0.025 and triple asterisk indicates significant at P ≤ 0.005 of treated compared to control
Effect of Q-25 extract on total proteolytic activity
In protease assay, the extract of Q-25 at 50 and 100 μg mL−1 concentrations inhibited protease production of V. vulnificus to the level of 45 and 63 %, respectively (Fig. 3). There was no observed in V. harveyi treated with Q-25 extract.
Effect of Q-25 extract on protease enzyme production. Quantification of protease inhibition in V. vulnificus at 50 and 100 μg mL−1 concentrations represented as percentage of inhibition. Mean values of triplicate independent experiments and SE are shown. Single asterisk indicates significant at P ≤ 0.025 of treated compared to control, respectively
Gelatinase inhibition
In order to show that the reduction in gelatinase production was due to the anti-QS activity of Q-25 extract, gelatinase inhibition was determined semi-quantitatively. As expected, gelatinase production was inhibited by the test extract at the concentration of 100 μg mL−1 in comparison to the untreated samples (Supplementary Fig. 2). Significant reduction in zone around the wells was observed in both V. harveyi and V. vulnificus treated with Q-25.
Assessment of biofilm biomass
QS mechanism is well recognized to regulate the biofilm formation of aquatic bacterial pathogens which plays an important role in both initiation and maturation of biofilms. In the present study, the anti-biofilm effect of Q-25 extract was evaluated by biofilm biomass inhibition assay. The obtained results show a concentration-dependent inhibition (50–200 μg mL−1) in biofilms of test bacterial pathogens, with a significant level of inhibition up to 25, 37, 53 and 71 %, respectively, in V. harveyi, and 33, 47, 62 and 84 %, respectively, in V. vulnificus treated with Q-25 extract (Fig. 4a).
Biofilm inhibition by Q-25 extract at 50–200 μg mL−1. a Biofilm formation of V. harveyi and V. vulnificus, as quantified by crystal violet staining and measuring absorbance at 570 nm. b EPS inhibition of V. harveyi and V. vulnificus at 50–200 μg mL−1 and measured OD at 490 nm. a, b Represented as percentage of inhibition. Mean values of triplicate independent experiments and SE are shown. Mean single asterisk indicates significant at P ≤ 0.025, double asterisk indicates significant at P ≤ 0.01 and triple asterisk indicates significant at P ≤ 0.005 of treated compared to control
EPS inhibition by Q-25 extract
In addition to biofilm inhibition, EPS were significantly inhibited by Q-25 extract in a dose-dependent manner (50–200 μg mL−1). Q-25 efficiently inhibited the EPS production in V. harveyi to the range of 27, 39, 46 and 66 %, respectively, whereas, in V. vulnificus, it was up to 24, 51, 54 and 68 %, respectively (Fig. 4b).
Light microscopic and CLSM analyses of biofilm inhibition on cover glasses
Based on the results obtained from biofilm inhibition assay, the inhibition of biofilm formed by V. harveyi and V. vulnificus formed on cover glasses in the presence and absence of Q-25 extract were visually confirmed by light microscopic (Fig. 5a) and CLSM analyses (Fig. 5b). Results of the image analysis (Fig. 5a) clearly indicate that the biofilm formation on cover glasses was reduced efficiently by Q-25 extract. Similarly, the preformed biofilms of V. harveyi and V. vulnificus treated with Q-25 extract also effectively disintegrated when compared with control at the concentration of 200 μg mL−1 (Fig. 6a). Interestingly, the results of CLSM analysis also strengthened the anti-biofilm activity of Q-25 extract (Fig. 6b).
Light microscopic analysis and CLSM analysis. a Light microscopic images showing the effect of Q-25 extract on bacterial biofilms (a, c control biofilms of V. harveyi and V. vulnificus, respectively, b, d Q-25 extract treated at 200 μg mL−1 of V. harveyi and V. vulnificus). b CLSM analysis: three-dimensional (3D) images of control and treated biofilms
Swimming and swarming motility
The flagellar-mediated motility plays an important role in QS-mediated biofilm formation in several aquatic bacterial pathogens. Hence, an effort was made to examine the anti-QS potential of Q-25 extract against the swimming and swarming motility of V. harveyi and V. vulnificus. The results clearly indicated the anti-swimming and anti-swarming activities of Q-25 in V. harveyi and V. vulnificus at 200 μg mL−1 concentration (Supplementary Fig. 3a, b). In the present study, inhibition of swimming and swarming motility by Q-25 has further strengthened its QS-mediated anti-biofilm activity.
Antibacterial activity of Q-25 extract
The effect of Q-25 extract on growth of V. harveyi and V. vulnificus was assessed by well diffusion agar assay. In which, there was no zone of inhibition observed around the loaded wells (Supplementary Fig. 4a, b) as there was no bactericidal or bacteriostatic activity observed with 50–200 μg mL−1 concentrations of Q-25 extract. This was further confirmed by spectrophotometric analysis at OD 600 nm.
Non-enzymatic nature of the active compound
In order to determine the nature of the anti-QS compound present in Q-25 extract, whether enzymatic or non-enzymatic, the CFCS was subjected to enzymatic treatment and heat treatment. It was observed that the supernatant of Synechococcus sp., Q-25 retained its original level of violacein inhibition in C. violaceum even after heat treatment at 70 °C for 10 min and proteinase K (1 mg mL−1) treatment for 1 h and 20 min, confirming the non-enzymatic nature of the anti-QS compound present in Synechococcus sp., Q-25 extract.
Compounds identified by GC-MS
In GC-MS analysis, a total of 10 different compounds were identified from the active fraction. The target mass ions (m/z) and retention time of all identified compounds are shown in Fig. 7 and Table 2. Interestingly, this extract comprises the known potent anti-QS agent hexadecanoic acid (palmitic acid).
Discussion
Vibriosis is one of the major aquatic bacterial diseases that causes major economic losses in shellfish and finfish aquaculture industry as a result of mass mortalities in aquatic organisms. More than 14 Vibrio spp. are known to cause infections in aquatic animals (Adams and Boopathy 2013). The social and economic impacts of the pandemics caused by these pathogens have been profound in many countries wherein shrimp farming constitutes a significant industry. In fact, the massive usage of antibiotics to control infections in aquaculture has resulted in the development of resistant strains, which have rendered antibiotic treatment futile. Therefore, it is necessary to develop an alternative control method for large-scale yield from aquaculture (Defoirdt et al. 2007). In aquatic bacterial pathogens, the production of virulence factors such as caseinase, gelatinase, chitinase, siderophore, motility and also biofilm formation are under the control of AHL-mediated QS mechanism (Milton 2006). Hence, inhibition of such QS mechanism in vibriosis causing bacterial pathogens could eventually result to reduce the pathogenicity on aquatic species. It is worth noting that several studies have already reported that the CFCS of coral and sponge associated bacterial organisms, and the extracts from fruits, spices and medicinal plants efficiently inhibited AHL-mediated virulence factors and biofilm formation of human bacterial pathogens including Serratia marcescens, Pseudomonas aeruginosa and Staphylococcus aureus, as well as the aquatic bacterial pathogens such as V. harveyi, V. vulnificus and V. alginolyticus (Brackman et al. 2008; Dobretsov et al. 2010, 2011; Vikram et al. 2011). In the present investigation, we demonstrated the anti-QS potential of an extracellular non-protein-based compound obtained from marine cyanobacterium Synechococcus sp., (Q-25) extract to inhibit QS-dependent violacein production in C. violaceum (ATCC 12472), virulence factors and biofilm formation of V. harveyi (MTCC 3438) and V. vulnificus (MTCC 1145). Among the different marine cyanobacteria strains tested, the extract of Q-25 alone showed anti-QS potential by strongly inhibiting violacein production in C. violaceum, and phenotypic factors of V. harveyi and V. vulnificus. In quantitative analysis, the Q-25 extract inhibited violacein production of CV026 by up to 82 % (Fig. 1). The results support the findings of Zhu and Sun (2008), who have reported the inhibition of QS-regulated behaviours of violacein by Tremella fuciformis fruiting bodies extract. Although some species of Synechococcus and other cyanobacterial strains are known to produce bioactive compounds with anti-bacterial, anti-cancer and anti-inflammatory activity (Dobretsov et al. 2011), the application of a Synechococcus sp. extract as an anti-QS and anti-biofilm agent remains unexplored.
It is well recognized that bioluminescence is one of the important QS-dependent factors in V. harveyi. Therefore, the results of anti-bioluminescence activity was evidence for the presence of anti-QS activity in Q-25 against V. harveyi without any growth inhibitory activity (Fig. 2). The results are similar to those of Manefield et al. (2000), who studied the effect of the AHL antagonist furanone from Delisea pulchra on bioluminescence and toxin production against a virulent strain of V. harveyi. The bioluminescence inhibitory potential of Q-25 extract is also comparable with the activity of curcumin (Packiavathy et al. 2013). The pathogenicity of bacterial pathogens is mainly correlated with the QS-mediated secretion of extracellular virulence factors including caseinase, protease and gelatinase enzymes, which helps the bacteria to resist the host immune response. In the present study, the enzymatic assay indicated that protease and gelatinase were positively regulated by the QS and the test extract Q-25 inhibited these enzymes in a concentration-dependent manner (Fig. 3). Consistent with the results of protease and gelatinase obtained in this study, a positive regulation of proteases has been reported previously in the V. harveyi metalloprotease gene (vhp) and also in other vibrios such as V. alginolyticus (Natrah et al. 2011).
It is well known that QS triggers the pathogenicity of aquatic bacterial pathogens through biofilm formation, which enables the bacteria to develop resistance against antibiotics (Yildiz and Visick 2009; Guzman et al. 2004). It has also been reported that in vivo formed biofilms are responsible for enhanced infectivity of V. cholerae shed in human stools (Faruque et al. 2006). Similarly, the biofilms shed from the marine animals are more harmful, especially in the aquaculture environment. They can contaminate the whole farm and enhance the prevalence of vibriosis. Therefore, the anti-QS potential of Q-25 in reducing the biofilm formation of V. harveyi and V. vulnificus was evaluated. The Q-25 extract effectively inhibited biofilm formation and significantly reduced the number of microcolonies in the biofilm community (Fig. 4a). These results indicate that the anti-biofilm potential of Q-25 was mainly due to inhibiting the development of biofilms, as well as disrupting the mature biofilm architecture.
Another important step in biofilm formation is the development and maintenance of architecture. In the current study, the characteristics of biofilm architecture visualized by light microscopy and CLSM showed the inhibition of dynamic complexity of vibrio biofilm and also confirmed the architecture of the biofilm was looser with a reduced number of microcolonies compared to the untreated controls. Similarly, Vikram et al. (2010) also found that limonoids such as obacunone and nomilin from grapefruit supressed biofilm formation of V. harveyi in non-growth inhibitory fashion and plausibly in a QS-dependent manner.
Exopolymeric substances (EPS) are an essential factor for the development of biofilm architecture in bacterial pathogens (Watnick and Kolter 1999). Overproduction of EPS can lead to alterations in biofilm architecture that correlate with an increased resistance of the cells to osmotic and oxidative stresses as well as killing by biocides such as chlorine (Wai et al. 1998). The importance of QS-dependent EPS production in biofilm development and its maturation is well reported. Moreover, increased biofilm formation often correlates with increased EPS production. In this study, the extract of Synechococcus sp. (Q-25) effectively interrupted EPS production (Fig. 4b). Further, it is envisaged that the reduction in EPS production may possibly decrease the resistance of the sessile cells to antibiotics (Nithya and Pandian 2010).
In addition, another significant phase in the process of biofilm development is the biofilm maturation. QS mechanism which is also known to play an important role in biofilm maturation (Davey and O’Toole 2000). Bacterial cells in mature biofilm can be up to 1000-fold more resistant to antibiotic treatment than their counterpart growing planktonically (Brooun et al. 2000). Thus, it is also important to destroy the mature biofilm in the environment to avoid the subsequent bacterial infections. The Synechococcus sp. (Q-25) extract was able to destroy preformed biofilms of V. harveyi and V. vulnificus (Fig. 6a and b).. Similarly, curcumin from turmeric and supernatants of marine actinomycetes (A66) have been reported to inhibit mature biofilms of Vibrio spp. (You et al. 2007; Packiavathy et al. 2013). Since QS-mediated swimming and swarming motility plays an important role in biofilm formation, any interruption in such motility could possibly inhibit biofilm formation. Here, the motility assay revealed a significant inhibition in flagellar-mediated swimming and swarming migration of V. harveyi and V. vulnificus treated with extract when compared with control. Similarly, Niu and Gilbert (2004) found that cinnamaldehyde reduced biofilm formation of E. coli by interfering with its swimming motility.
It is also well understood that the good QSI compound should not possess any antibacterial activity. In view of this fact, the attained results with well diffusion agar assay revealed the nil antibacterial activity of Q-25 against the target pathogens. Hence, it is envisaged that the reduced phenomena of biofilm behaviour and virulence of the target pathogens might possibly due to the QS interference activity of test extract.
Pertaining the nature of anti-QS compounds, AHL analogue compounds and AHL-degrading enzymes have been reported to interfere with AHL-mediated QS systems. The anti-QS compounds present in Synechococcus sp., extract did not lose activity upon proteinase K treatment suggesting that the constituents present in the extract are not assimilated as AHL-degrading enzymes, but possibly may be an analogue compound similar to previous studies of anti-QS compounds from Bacillus sp. which have been found to be AHL analogues, rather than AHL-degrading enzymes, and which inhibit QS and biofilm formation in P. aeruginosa PAO1. The GC-MS analysis showed the presence of a known fatty acid and bioactive compound hexadecanoic acid (Fig. 7, Table 2). Several reports have described the anti-QS property of hexadecanoic acid against various bacterial pathogens including V. harveyi, P. aeruginosa and Bacillus pumilus, but the mode of action is unknown. However, it was medium and long chain fatty acids from ground beef have been shown to inhibit AI-2 based signalling in V. harveyi (Soni et al. 2008). Similarly, there is another report of antibiofilm activity of glycolipid (glucose and palmitic acid) from marine bacterium against biofouling bacterial pathogens (Dusane et al. 2012). In one of our earlier reports, we have evaluated the anti-QS property of hexadecanoic acid through molecular docking approach using bioinformatics tools. The results clearly indicated that the anti-QS property of the hexadecanoic acid was mainly due to antagonostic binding to the AHL receptor protein (Musthafa 2011).
In conclusion, to the best of our knowledge, this is the first study showing the anti-QS potential of the marine cyanobacterium Synechococcus sp. against aquatic bacterial pathogens such as V. harveyi and V. vulnificus. The results of this study provide information on the anti-QS potential of Synechococcus sp. against emerging aquatic bacterial infections. Further research is needed to understand the complete mode of action and potential applications of hexadecanoic acid to prevent bacterial diseases among cultivable organisms in aquaculture.
References
Adams D, Boopathy R (2013) Use of formic acid to control vibriosis in shrimp aquaculture. Biologia 68:1017–1021
Amrei HD, Nasernejad B, Ranjbar R, Rastegar S (2014) Spectral shifting of UV-A wavelengths to blue light for enhancing growth rate of cyanobacteria. J Appl Phycol 26:1493–1500
Annapoorani A, Jabbar AKKA, Musthafa SKS, Pandian SK, Ravi AV (2012) Inhibition of quorum sensing mediated virulence factors production in urinary pathogen Serratia marcescens PS1 by marine sponges. Indian J Microbiol 52:160–166
Austin B, Austin D, Southerland R, Thompson F, Swings J (2005) Pathogenicity of vibrios to rainbow trout (Oncothynchus mykiss, Walbaum) and Artemia nauplii. Environ Microbiol 7:1488–1495
Austin B, Zhang X-H (2006) Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol 43:119–124
Bakkiyaraj D, Pandian STK (2010) In vitro and in vivo antibiofilm activity of a coral associated actinomycete against drug resistant Staphylococcus aureus biofilms. Biofouling 26:711–717
Brackman G, Defoirdt T, Miyamoto C, Bossier P, Calenbergh SV, Nelis H, Coenye T (2008) Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol 8:149
Brooun A, Liu S, Lewis K (2000) A dose–response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 44:640–646
Bross MH, Soch K, Morales R, Mitchell RB (2007) Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician 76:539–544
Choo JH, Rukayadi Y, Hwang JK (2006) Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol 42:637–641
Clark BR, Engene N, Teasdale ME, Rowley DC, Matainaho T, Valeriote FA, Gerwick WH (2008) Natural products chemistry and taxonomy of the marine cyanobacterium Blennothrix cantharidosmum. J Nat Prod 71:1530–1537
Davey ME, O’Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867
Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P (2007) Alternatives to antibiotics to control bacterial infections: luminescent vibriosis in aquaculture as an example. Trends Biotechnol 25:472–479
Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14:251–258
Dobretsov S, Teplitski M, Alagely A, Gunasekera SP, Paul VJ (2010) Malyngolide from the cyanobacterium Lyngbya majuscula interferes with quorum sensing circuitry. Environ Microbiol Rep 2:739–744
Dobretsov S, Abed RMM, Maskari SMSA, Sabahi JNA, Victor R (2011) Cyanobacterial mats from hot springs produce antimicrobial compounds and quorum-sensing inhibitors under natural conditions. J Appl Phycol 23:983–993
Dusane DH, Pawar VS, Nancharaiah YV, Venugopalan VP, Kumar AR, Zinjarde SS (2012) Antibiofilm potential of a glycolipid biosurfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 27:645–654
Faruque SM, Biswas K, Udden SMN, Ahmad QS, Sack DA, Nair GB, Mekalanos JJ (2006) Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc Natl Acad Sci 103:6350–6355
Gowrishankar S, Mosioma ND, Pandian SK (2012) Coral-associated bacteria as a promising antibiofilm agent against methicillin-resistant and-susceptible Staphylococcus aureus biofilms. Evid Based Complement Alternat Med 2012:862374
Guzman GA, Ruiz HM, Ascencio F (2004) A review of extracellular virulence product of Vibrio species important in disease of cultivated shrimp. Aquacult Res 35:1395–1404
Hentzer M, Givskov M (2003) Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest 112:1300–1307
Kessler E, Safrin M, Olson JC, Ohman DE (1993) Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem 268:7503–7508
Leung TLF, Bates AE (2012) More rapid and severe disease outbreaks for aquaculture at the tropics: Implications for food security. J Appl Ecol 50:215–222
Manefield M, Harris L, Rice SA, de Nys R, Kjelleberg S (2000) Inhibition of luminescence and virulence in the black tiger prawn (Penaeus monodon) pathogen Vibrio harveyi by intercellular signal antagonists. Appl Environ Microbiol 66:2079–2084
Matsumoto C, Okuda J, Ishibashi M, Iwanaga M, Garg P, Rammamurthy T, Wong HC, Depaola A, Kim YB, Albert MJ, Nishibuchi M (2000) Pandemic spread of an O3:K6 clone of Vibrio parahaemolyticus and emergence of related strains evidenced by arbitrarily primed PCR and toxRS sequence analyses. J Clin Microbiol 38:578–585
Milton DL (2006) Quorum sensing in vibrios: complexity for diversification. Int J Med Microbiol 296:61–71
Musthafa KS, Ravi AV, Annapoorani A, Packiavathy IASV, Pandian SK (2010) Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of the N-Acyl-Homoserine Lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy 56:333–339
Musthafa KS (2011) Anti-quorum sensing and antimicrobial potential of marine sediment bacteria. Ph.D Thesis, Alagappa University, India 230 pp
Natrah FMI, Ruwandeepika HAD, Pawar S, Karunasagar I, Sorgeloos P, Bossier P, Defoirdt T (2011) Regulation of virulence factors by quorum sensing in Vibrio harveyi. Vet Microbiol 154:124–129
Nithya C, Pandian SK (2010) The in vitro antibiofilm activity of selected marine bacterial culture supernatants against Vibrio spp. Arch Microbiol 192:843–854
Nithya C, Devi MG, Pandian SK (2011) A novel compound from the marine bacterium Bacillus pumilus S6-15 inhibits biofilm formation in Gram-positive and Gram-negative species. Biofouling 27:519–528
Nithyanand P, Pandian SK (2009) Phylogenetic characterization of culturable bacterial diversity associated with the mucous and tissue of the coral Acropora digitifera from the Gulf of Mannar. FEMS Microbial Ecol 69:384–394
Niu C, Gilbert ES (2004) Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl Environ Microbiol 70:6951–6956
Nostro A, Roccaro AS, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, Cioni PL, Procopio F, Blanco AR (2007) Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol 56:519–523
Packiavathy IASV, Sasikumar P, Pandian SK, Ravi AV (2013) Prevention of quorum-sensing-mediated biofilm development and virulence factors production in Vibrio spp. by curcumin. Appl Microbiol Biotechnol 97:10177–10187
Qi Z, Zhang XH, Boon N, Bossier P (2009) Probiotics in aquaculture of China - current state, problems and prospect. Aquaculture 290:15–21
Shao J, Peng L, Luo S, Yu G, Gu J, Lin S, Li R (2013) First report on the allelopathic effect of Tychonema bourrellyi (Cyanobacteria) against Microcystis aeruginosa (Cyanobacteria). J Appl Phycol 25:1567–1573
Singh RK, Tiwari SP, Rai AK, Mohapatra TM (2011) Cyanobacteria: an emerging source for drug discovery. J Antibiot 64:401–412
Soni KA, Jesudhasan P, Cepeda M, Widmer K, Jayaprakasha GK, Patil BS, Hume ME, Pillai SD (2008) Identification of ground-beef derived fatty acid inhibitors of AI-2 based- cell signalling. J Food Prot 71:134–138
Teasdale ME, Liu J, Wallace J, Akhlaghi F, Rowley DC (2009) Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in Gram-negative bacteria. Appl Environ Microbiol 75:567–572
Thenmozhi R, Nithyanand P, Rathna J, Pandian SK (2009) Antibiofilm activity of coral-associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol Med Microbiol 57:284–294
Uzair B, Mahmood Z, Tabassum S (2011) Antiviral activity of natural products extracted from marine organisms. BioImpacts 1:203–211
Vikram A, Jesudhasan PR, Jayaprakasha GK, Pillai BS, Patil BS (2010) Grapefruit bioactive limonoids modulate E. coli O157:H7 TTSS and biofilm. Int J Food Microbiol 140:109–116
Vikram A, Jesudhasan PR, Jayaprakasha GK, Pillai SD, Patil BS (2011) Citrus limonoids interfere with Vibrio harveyi cell-cell signalling and biofilm formation by modulating the response regulator LuxO. Microbiol 157:99–110
Wai SN, Mizunoe Y, Takade A, Kawabata SI, Yoshida SI (1998) Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl Microbiol Biotechnol 64:3648–3655
Watnick PI, Kolter R (1999) Steps in the development of a Vibrio cholerae EI Tor biofilm. Mol Microbiol 34:586–595
Ye J, Ma Y, Liu Q, Zhao DL, Wang QY, Zhang YX (2008) Regulation of Vibrio alginolyticus virulence by the LuxS quorum-sensing system. J Fish Dis 31:161–169
Yildiz FH, Visick KL (2009) Vibrio biofilms: so much the same yet so different. Trends Microbiol 17:109–118
You JL, Xue XL, Cao LX, Lu X, Wang J, Zhang LX, Zhou SN (2007) Inhibition of Vibrio biofilm formation by a marine actinomycete strain A66. Appl Microbiol Biotechnol 76:1137–1144
Zhu H, Sun SJ (2008) Inhibition of bacterial quorum sensing-regulated behaviors by Tremella fuciformis extract. Curr Microbiol 57:418–422
Acknowledgments
The authors gratefully acknowledge the Department of Biotechnology (DBT), the Government of India, (Grant No. BT/PR4815/AAQ/3/587/2012) for the financial support in the form of DBT major research project. The technical assistance extended by Mr. K. Vimal Kumar, Department of Environmental Biotechnology, Bharathidasan University, in GC-MS analysis is thankfully acknowledged. Professor N. Thajuddin thanks King Saud University for the Visiting Professorship and also acknowledges the Deanship of Scientific Research, College of Science Research Center, King Saud University, Kingdom of Saudi Arabia. The authors also thankfully acknowledge the computational and bioinformatics facility provided by the Alagappa University Bioinformatics Infrastructure Facility (funded by DBT, GOI; Grant No. BT/BI/25/001/2006).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Qualitative analysis of Q-25 extract at 50–200 μg mL−1 concentrations in inhibiting the AHL dependent violacein production in C. violaceum (ATCC 12472). (JPEG 217 kb)
Supplementary Fig. 2
Gelatinase inhibition of V. harveyi and V. vulnificus (a-Control, b-100 μg mL−1 treated), (JPEG 373 kb)
Supplementary Fig. 3
Effect of Q-25 extract on swimming and swarming motility. A. Swimming motility; a, c-control of V. harveyi and V. vulnificus, b, d-treated at 200 μg mL−1. B. Swarming motility; a, c-control and b, d-treated at 200 μg mL−1 of Q-25 extract. (JPEG 1209 kb)
Supplementary Fig. 4
Well diffusion agar assay. A. V. harveyi, B. V. vulnificus, a-control, b, c, d and e- Q-25 extract treated at 50, 100, 150 and 200 μg mL−1 concentrations, respectively. (JPEG 457 kb)
Rights and permissions
About this article
Cite this article
Santhakumari, S., Kannappan, A., Pandian, S.K. et al. Inhibitory effect of marine cyanobacterial extract on biofilm formation and virulence factor production of bacterial pathogens causing vibriosis in aquaculture. J Appl Phycol 28, 313–324 (2016). https://doi.org/10.1007/s10811-015-0554-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0554-0