Abstract
Extracts of four heterocystous cyanobacteria isolated from wheat field soils (Triticum aestivum L.) were used as a promoter of growth and essential oil production in Mentha piperita L., which is mostly used for medicinal purposes. An axenic monoalgal culture was prepared using nitrate-free BG-11 medium. Pot experiments were performed by spraying algal extracts on the soil of treated plants on the first day of planting and every 20 days thereafter. Growth of plants was evaluated by measuring growth parameters such as plant height, root length, dry and fresh weight of plant, as well as leaf number, leaf area, and ramification after 100 days planting. In addition to growth factors, the quantity of essential oil of plants was also assessed. Statistical analysis showed that there were significant differences in the studied parameters compared to the control. The addition of some cyanobacterial inocula had a positive effect on the plant growth parameters and essential oil content. HPLC analysis of the cyanobacterial extracts detected some auxins, which may be the possible factor contributing to the positive growth responses and enhanced essential oil content of the treated plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mentha piperita L., commonly known as peppermint, is a hybrid created by cross-breeding of Mentha aquatica and Mentha spicata. It is native to Europe and has been commonly cultivated and naturalized in several countries. The chemical components identified in the mint species, mainly, menthone, menthol, pulegone, and carvone (Kizil et al. 2010; Santoro et al. 2011), are among the most popular and widely used essential oils. The metabolites of the herbal plants such as peppermint have economic impact due to their wide applications in food, cosmetic, and pharmaceutical industries (Torres Salazar et al. 2011; Neeraj et al. 2013).
The chemical composition and yield of these essential oils can be affected by several exogenous factors. Therefore, due to their wide application and beneficial effects, the study of exogenous factors, particularly the composition of soil (Mihajilov-Krstev et al. 2010), are of great significance.
Cyanobacteria or blue-green algae (BGA) constitute important components of the soil microflora that directly or indirectly increase soil productivity (Vaishampayan et al. 2001; Mishra and Pabbi 2004). Earlier studies indicate that cyanobacteria play a major role in increased nitrogen content of the surface soil, growth-promoting substances, and essential microelements known to be necessary for plant growth and ion uptake (Misra and Kaushik 1989a, b; Stirk et al. 2002; Karthikeyan et al. 2007; Obana et al. 2007; Maqubela et al. 2008). Moreover, cyanobacteria have also been shown to enhance the production of secondary metabolites in plants (Saker et al. 2000, Shanab 2001), among which essential oils are of particular importance.
Although numerous studies have shown the effects of cyanobacteria as a biofertilizer source on the growth of rice and many other crop plants, not much attention has been paid to the impact of these microorganisms on medicinal plants. The aim of this study was to investigate the effect of some monospecific cyanobacterial cultures, applied as an inoculum, on growth and essential oil content of peppermint. In addition, the presence of three major auxins, indole 3-acetic acid (IAA), indole 3- propionic acid (IPA), and indole 3- butyric acid (IBA) was evaluated, and chemical content of algae extracts was determined.
Materials and methods
Isolation of cyanobacteria species
For isolation of cyanobacterial species employed in this study, soil samples were collected from several western fields of Iran (Kermanshah Province) under cultivation of Triticum aestivum L. (common wheat). Over two consecutive summers of 2012 and 2013, and according to the methodology of Rangaswamy (1966), the soil samples were collected, and several cyanobacterial species were isolated from them.
The sieved soils from different sites were transferred to sterile Petri dishes containing sterile liquid nitrate free BG-11 medium (Stanier et al. 1971). The Petri dishes were incubated at 25 ± 2 °C for 2 weeks of artificial light illumination (74 μmol photons m−2 s−1) with a 12/12 h light/dark cycle. After colonization, isolates were transferred to agar plates for purification. For taxonomic determinations, the semi-permanent slides of colonies were prepared and morphometric study was performed by light microscopy and based on Desikachary (1959), Prescott (1970), Wehr et al. (2002) and John et al. (2002). For molecular determination of the isolates, 16S rRNA gene sequencing was performed.
Seedling growth test
To select efficient strains, a seedling growth test was performed. For this purpose, air-dried seeds of T. aestivum were soaked in separate algal extracts (5.0 g fresh algal material in 500 mL of distilled water; experimental) for 24 h. For controls, seeds were soaked in distilled water for 24 h. Twenty experimental and control-treated seeds of each were spread on thick filter papers in a Petri dish soaked with 5.0 mL of a specific cyanobacterial extract or water, respectively. Each experiment was performed with four replicates. The plates were incubated in a culture chamber at 25 °C with illumination cycle of light–dark (12/12 h). Efficiency of seedling growth was evaluated by calculating the percent seed germinations and measurement of root and leaf lengths after 10 days of incubation.
Preparation of cyanobacterial extract
Based on the morphological, molecular, as well as seedling growth tests described above, the following four species of heterocystous cyanobacteria were chosen for this study: Anabaena vaginicola ISB42, Nostoc calcicola ISB43, Trichormus ellipsosporus ISB44, and Cylindrospermum michailovskoense ISB45. These species were cultured in nitrate-free BG-11 solid medium at 25 ± 2 °C exposed to artificial illumination (74 μmol photons m−2 s−1) with a 12/12 h light–dark cycle. The cultures were harvested after 4 weeks of incubation and cyanobacterial extracts were prepared by grinding in a blender; 1 % cyanobacterial extract contains 5.0 g of fresh cyanobacteria material ground in 500 mL distilled water.
Pot culture
Rhizomes of M. piperita were obtained from Medicinal Plants and Drugs Research Institute, Shahid Beheshti University, Tehran, Iran. Healthy rhizomes (20 for each treatment) were grown in 3-L pots (14 cm diameter), containing 60 % peat, 25 % sand, and 15 % normal soil, for 100 days. In place of fertilizer, M. piperita planted pots were sprayed with individual 1 % cyanobacterial extracts, and for controls, the similarly planted pots were sprayed with water alone. The cyanobacterial extracts were applied on the first day of planting and every 20 days thereafter. The pots were arranged in a completely randomized order in an experimental greenhouse. The parameters employed to assay for plant growth rates are as follows: plant height, root length, dry and fresh weights, leaf number, leaf area, and ramification. In addition to growth rates, the quantity of the essential oils extracted from these plants was analyzed.
Extraction and quantification of essential oil
The essential oils of air-dried leaf samples (100 g) were isolated by hydro-distillation for 3 h (until no more essential oil was recovered), using a Clevenger-type apparatus and according to the method recommended in Anon (1993). Subsequently, the distilled essential oils were dried over anhydrous sodium sulfate and refrigerated at 4 °C before weighing. The yield of extraction for each sample was calculated based on the fraction of the weight of the dried oils and total dry weight of the plant used (w/w %).
Preparation of auxin standards and cyanobacterial extracts
Identification and quantification of the endogenous auxins was performed according to the procedure of Hashtroudi et al. (2013). The standard solutions of the studied auxins, namely, IAA, IPA, and IBA were prepared by dissolving 1 mg of each standard in methanol–water 80:20, and successive dilution was made to obtain the required concentration of 5, 10, 25, 50, 100, and 250 ng mL−1 for calibration curves. The freeze dried algal biomass was extracted with methanol–water 80:20 by ultrasonication in a Sonorex Digitec DT103H ultrasonic bath (Bandelin, Germany) with an ultrasonic frequency of 35 kHz and an effective ultrasound power of 140 W for 30 min at 20 °C. The suspensions were centrifuged at 6728×g for 10 min, and the supernatants were filtered through 0.45-μm PTFE filters and concentrated to the final volumes of 500–1000 μL under a mild stream of pure nitrogen.
HPLC analysis
An Agilent 1200 series high-performance liquid chromatography (HPLC) system with a quaternary pump and a degasser, equipped with a G1315D diode array detector and a G1321A fluorescence detector and a Eurosphere RP-column (100–5 C18 column, 250 × 4.6 mm Knauer, Germany) was used for the chromatographic separations and quantification of the studied auxins. The Agilent LC Chemstation software was employed for instrument control, data acquisition, and processing. The mobile phase system consisted of solvent A (methanol) and solvent B (water) containing 0.3 % AcOH. The elution was started with an isocratic step of 60 % A for 5 min and continued with a linear gradient to 100 % A within 15 min. The flow rate was 1 mL min−1, and the column temperature was maintained at 25 °C. UV detection was at 225 nm, and excitation and emission wavelengths were 280 and 360 nm, respectively.
The injections of standards and extracts were both manually done through a 20-μL loop and the analytes were identified with comparing the retention times and UV spectra of the sample peaks with the standard mixture. Each experiment was repeated three times and run in triplicate. Recoveries were also calculated by adding known amounts of auxin standards to the cyanobacteria and extracting the auxins with the same procedure as the studied samples. Calibration curves of the peak areas versus six concentrations of the three studied auxins were used to quantify the auxins. Linearity of the calibration curve was assessed by coefficient of determination (R 2). High purity standards were used for high accuracy and precision was expressed as relative standard deviation (RSD) for each of replicate concentrations (n = 3).
Evaluation of chemical contents of cyanobacterial extracts
The chemical content of cyanobacterial biomass homogenates (cyanobacterial extracts) was determined by Arian Fan Azma Institute, Tehran, Iran. The chemical analyses included the total nitrogen and inorganic nitrogen (NO2 −, NO3 −, and NH4 +), phosphate, bicarbonate, and cation contents (Na+, K+, Mg++, Ca++).
Statistical analysis
One-way ANOVA statistical analysis was performed employing SPSS software version 16 (Package for the Social Sciences, SPSS Inc., Chicago IL, USA). Means were separated using the Tukey HSD test at P < 0.05.
Results
Cyanobacterial extracts as inoculants for Triticum aestivum L.
In total, seven heterocystous morphospecies from four genera were isolated from several sites (Table 1) and tested in a T. aestivum seedling growth test. There was a significant growth difference between treated and the control seedlings. Among the strains tested, as compared with controls, A. vaginicola ISB42, C. michailovskoense ISB45, T. ellipsosporus ISB44, and N. calcicola ISB43 significantly enhanced the length of both roots and leaves of the seedlings (Table 1). Thus, for this study, these four species were selected as inocula for the M. piperita pot trial. Among the taxa, seedlings treated with A. vaginicola ISB42 and C. michailovskoense ISB45 exhibited the most significant differences, and seedlings treated with N. calcicola ISB43 showed the least as compared with the controls. Among the positive effects observed in seedling growth parameters, the differences in root length are the most prominent. Interestingly, despite these positive effects, the cyanobacterial extracts did not affect other growth parameters such as germination rates (Table 1).
For further characterization, the morphology of each cyanobacterial strain was examined by light microscopy showing their filamentous trichomes with distinctive heterocysts (Fig. 1).
Selected cyanobacterial extracts as inoculum for Mentha piperita L.
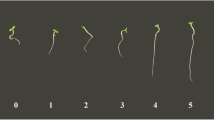
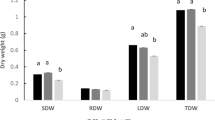
Compared with the controls, treatment of M. piperita with the cyanobacterial extracts induced differential changes in morphology of the treated plants. These differences were obvious in the density and length of roots, stems, leaves, and the plant ramifications. Fresh and dry weight of roots treated with cyanobacterial extracts were also significantly higher than control plants irrigated with water alone (P < 0.05; Fig. 2, Table 2). These differences are more pronounced in peppermint plants irrigated with the extracts of A. vaginicola ISB42 and C. michailovskoence ISB45 than, although significant, the plants irrigated with N. calcicola ISB43 extract. On the other hand, the extract from T. ellipsosporus ISB44, unlike its positive effect on T. aestivum growth, did not induce significant changes in the growth of the peppermint plant.
Comparison between control and treated plants in growth parameters. Treatment conditions: 1 % algal extracts (5.0 g fresh algal material in 500 mL of distilled water) were sprayed on soil of treated pots. 0 Plant irrigated by distilled water (control plant). 1 Plant treated by Anabaena vaginicola ISB42. 2 Plant treated by Cylindrospermum michailovskoense ISB45. 3 Plant treated by Nostoc calcicola ISB43. 4 Plant treated by Trichormus ellipsosporus ISB44 (bar = 5 cm)
In addition, as compared with control peppermint plants, the content of essential oils in plants treated with the extracts of A. vaginicola ISB42, C. michailovskoence ISB45 and N. calcicola ISB43 were significantly increased (Table 2). As expected, there was no significant difference in the essential oil content of the peppermint plants treated with the extract of T. ellipsosporus ISB44 and that of the control plants.
Determination of plant growth stimulating factors in cyanobacterial extracts
The presence of the growth promoting hormones (auxins) in the microcyanobacterial biomass was quantified. The HPLC chromatograms of three studied auxin standards and four microcyanobacterial samples under the optimized HPLC conditions are shown in Figs. 3 and 4, respectively. The retention times for each auxin were 4.26, 5.24, and 7.36 min for IAA, IPA, and IBA, respectively. To examine the reproducibility of the retention times, the standards were injected at 100 ng mL−1, and each injection was done five times (n = 5). The RSDs of the retention times for all analytes were in the range of 0.45–0.85 %. There was a linear correlation between concentration and the peak area for the three auxins in the range of calibration concentrations; typically R 2 values were in the range of 0.997–0.998. The auxin recoveries were 84–86 %. The equation of calibration curves, R 2, RSDs, and recoveries are summarized in Table 3.
HPLC chromatograms of the ultrasonicated samples for 30 min with fluorescence detector. a HPLC chromatograms of the ultrasonicated sample of Cylindrospermum michailovskoense ISB45 for 30 min. b HPLC chromatograms of the ultrasonicated sample of Nostoc calcicola ISB43 for 30 min. c HPLC chromatograms of the ultrasonicated sample of Trichormus ellipsosporus ISB44 for 30 min. d HPLC chromatograms of the ultrasonicated sample of Anabaena vaginicola ISB42 for 30 min
Two endogenous auxins, i.e., IAA and IPA, were present in three cyanobactrial biomasses but not in the biomass of T. ellipsosporus ISB44. The concentration of IAA in C. michailovskoense ISB44 was higher than that in the other species, whereas IPA concentration was the highest in Anabaena (Table 4).
Total content of nitrogen, ammonium, and nitrate were higher in the extract of A. vaginicola ISB42 than that in the other extracts (Table 5). Compared with T. ellipsosporus ISB44, the extracts of N. calcicola ISB43, C. michailovskoense ISB45, and A. vaginicola ISB42 had higher total contents of nitrogen, nitrate, nitrite, ammonium, and bicarbonate and K+.
Discussion
In this study, as environmentally safe fertilizers, we have investigated the potential effects of widespread and naturally occurring cyanobacteria on the peppermint plant. There are few reports regarding cyanobacteria as biofertilizers for medicinal plants, although these plants are an important economic bioresource. Some studies have revealed that the cyanobacterial content of soil can positively affect several growth parameters of these herbaceous plants. For example, initial work by Riahi et al. (2013) demonstrated the positive effect of cyanobacteria on vegetative and reproductive growth of the medicinal plants Matricaria chamomilla L. (chamomile), Satureja hortensis L. (garden savory), and Mentha aquatica L. (water mint). Similarly, the results of the present study showed that cyanobacteria can significantly increase several growth parameters of the treated plants, from the root to the stem and leaf growth parameters. The amount of essential oil was also increased in all treated plants. This increase was significant in most treatments.
Although the inoculation of M. piperita with cyanobacterial culture showed significant differences in growth factors, the effect was not the same for all parts of the treated plants (Fig. 2). For example, as seen in Fig. 2, compared with controls, within the same cyanobacterial treatment, M. piperita exhibited significant differences in leaf numbers, while the differences, although significant, in the dry weight of roots, stems, or leaves were not as prominent. Likewise, as compared with controls, there is a variable enhancement of vegetative growth among the different cyanobacterial-treated plants. These differences are significant and include increases in root, stem, and leaf dry weights as well as sizes.
Our data suggest a direct correlation between the improvement in growth parameters of the treated peppermint plants and the nutrient and auxin concentrations of cyanobacterial inocula. In general, as compared with controls, peppermint plants treated with A. vaginicola ISB42 and C. michailovskoense ISB45 demonstrated the most significant differences in their growth parameters and total essential oil contents, which paralleled the rich chemical content as well as the high levels of auxins in the corresponding cyanobacterial extracts. Conversely, the low levels of nutrients and auxins in extract of T. ellipsospours ISB44 were associated with the low growth rates and content of the essential oils in the peppermint plants treated with the same cyanobacterial extract.
Recent studies indicate that several nitrogen-fixing rhizobacteria such as Azotobacters and Azospirilla, purified from soil, stimulated vegetative growth and essential oil content of Mentha plants. Thus, the nitrogen-fixing ability of these bacteria was proposed to be one of the reasons for the growth improvement of the treated plants (Gantar et al. 1995; Irisarri et al. 2001; Nilsson et al. 2002). In our previous work, we have also reported the positive effect of heterocystous cyanobacteria, including Nostoc and Anabaena, on nitrogen content of the surface soil (Shariatmadari et al. 2013). Our results here also revealed the presence of high levels of total nitrogen and ammonium contents in the cyanobacterial extracts, efficiently promoting growth. Therefore, we can draw the conclusion that, one way, these cyanobacteria can enhance the growth of peppermint plant by elevating the readily available nitrogenous compounds. This conclusion is in line with our findings that the highest levels of the total nitrogen, nitrite, nitrate, and ammonium contents in the extracts of A. vaginicola ISB42 and C. michailovskoence ISB45 were associated with the highest improvements in growth parameters of the treated plants.
Besides the nitrogenous content of cyanobacterial suspensions, stimulation of growth in the treated plants can be attributed to other chemical elements in the cyanobacterial extracts. Obana et al. (2007) suggested that the necessary micro-elements for plant growth can be supplied by cyanobacteria. This fact was strongly supported by the analysis of chemical content of cyanobacterial extracts in our study (Table 5). This analysis demonstrated that, in addition to the total nitrogen, nitrite, nitrate, and ammonium contents, elemental cations such as K+ in the extracts of A. vaginicola ISB42 and C. michailovskoence ISB45 are present at highest and in the extract of T. ellipsosporus ISB44 at lowest levels. Furthermore, we have previously reported that high amounts of cations such as K+ and Ca2+, found in the cyanobacterial extracts (genera Anabaena and Nostoc), correlate with enhanced effects on plant growth (Shariatmadari et al. 2013). Because of the necessity of such cations in regulation of metabolic and molecular activities of plant cells, cyanobacterial growth or provision of their extracts in the soil should be considered as natural, free, and productive means to promote growth. Therefore, as in this study, subsequent research work in the field should partly be concentrated on determining the cyanobacterial species, in isolation or in combination, capable of exerting the most effects on plant growth. Thus, the chemical content in the cyanobacterial extracts used in the present study may also influence plant growth.
In addition to the above effects, heterocystous cyanobacteria produce organic compounds, such as phytohormones, that can greatly influence plant growth. For example, the positive effect of rhizobacteria, especially cyanobacteria, on growth parameters and composition of essential oils in M. pipperita and other plants was suggested to be the result of special metabolites, phytohormones (auxins), which are synthesized and released by these microorganisms (Sergeeva et al. 2002; Santoro et al. 2011; Hashtroudi et al. 2013; Thangavelu et al. 2013). These reports are further strengthened by the results of the current study. Specifically, we have shown the presence of IAA and IPA to be prominent in the cyanobactrial biomass employed in our studies (Table 4). The dominant auxin observed in three isolates of cyanobacteria was IAA (in the range of 1935.32–16,751.59 ng g−1 dry weight) followed by lower levels of IPA (in the range of 20.54–503.10 ng g−1 dry weight). Among the studied isolates, C. michailovskoense ISB45 with highest amounts of IAA exerted the most effect and T. ellipsosporus ISB44 with the lowest amounts of this phytohormone exerted the least impact on peppermint growth and essential oil production.
Particular effects of auxins, as phytohormones, are found to be in mediating a number of essential plant growth and developmental processes, such as cell elongation, cell division, as well as induction of root growth and flower development (Kukavica et al. 2007). Previous studies also have shown that low concentrations of IAA can increase some growth parameters such as internodal length and leaf area (Meenakashi and Lingakumar 2011). Phytohormones also have been shown to stimulate root induction and initiation as well as to improve rooting (Simpson 1986). Our findings suggest that improvement of rooting and increase in lateral root production in the treated plants may be the effect of auxins such as IAA. Furthermore, our studies also indicate that the leaf number and area size are other vegetative growth property that can be influenced by these especial secondary metabolites (Table 2).
In conclusion, the cyanobacteria A. vaginicola ISB42, N. calcicola ISB43, and C. michailovskoense ISB45 are able to promote M. piperita growth and production of essential oils. Enhancement of number and size of peppermint leaf are of particular importance, considering the value and application of these structures in food, cosmetic, and pharmaceutical products. Altogether, our findings suggest that the selected cyanobacteria, isolated domestically, can serve as potential biofertilizer candidates to promote robust production of medicinal plants such as peppermint.
References
Anon (1993) British pharmacopoeia. HMSO, London
Desikachary TV (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi
Gantar M, Kerby NW, Rowell P, Obreht Z, Scrimgeour C (1995) Colonization of wheat (Triticum vulgare L.) by N2-fixing cyanobacteria: IV. Dark nitrogenase activity and effects of cyanobacteria on natural 15 N abundance in the plants. New Phytol 129:337–343
Hashtroudi MS, Ghassempour AR, Riahi H, Shariatmadari Z, Khanjir M (2013) Endogenous auxins in plant growth promoting cyanobacteria— Anabaena vaginicola and Nostoc calcicola. J Appl Phycol 25:379–386
Irisarri P, Gonnet S, Monza J (2001) Cyanobacteria in Uruguayan rice fields: diversity, nitrogen fixing ability and tolerance to herbicides and combined nitrogen. J Biotechnol 91:95–103
John DM, Whitton BA, Brook A (2002) The freshwater algal flora of the British Isles: an identification guide to freshwater and terrestrial algae. Cambridge University Press, Cambridge
Karthikeyan N, Prasanna R, Nain L, Kaushik BD (2007) Evaluating the potential of plant growth promoting cyanobacteria as inoculants for wheat. Eur J Soil Biol 43:23–30
Kizil S, Haşimi N, Tolan V, Kilinç E, Yuksel U (2010) Mineral content, essential oil components and biological activity of two Mentha (M. piperita L., M. spicata L.). Turk J Field Crop 15(2):148–153
Kukavica B, Mitrović A, Mojović M, Veljović-Jovanović S (2007) Effect of indole-3-acetic acid on pea root growth, peroxidase profiles and hydroxyl radical formation. Arch Biol Sci Belgrade 59(4):319–326
Maqubela MP, Mnkeni PNS, Malam Issa O, Pardo MT, Acqui LPD (2008) Nostoc cyanobacterial inoculation in South African agricultural soils enhances soil structure, fertility and maize growth. Plant Soil 315:79–92
Meenakashi V, Lingakumar K (2011) The role of IAA and 2,4-D on growth and biochemical constituents in vegetatively propagated Mentha arvensis L. J Biosci Res 2:10–15
Mihajilov-Krstev T, Radnović D, Kitić D, Stojanović-Radić Z, Zlatković B (2010) Antimicrobial activity of Satureja hortensis L. essential oil against pathogenic microbial strains. Arch Biol Sci Belgrade 62(1):159–166
Mishra U, Pabbi S (2004) Cyanobacteria: a potential biofertilizer for rice. Resonance 2004:6–10
Misra S, Kaushik BD (1989a) Growth promoting substances of cyanobacteria I. Vitamins and their influence on rice plant. Proc Indian Natl Sci Acad B55:295–300
Misra S, Kaushik BD (1989b) Growth promoting substances of cyanobacteria II. Detection of amino acids, sugars and auxins. Proc Indian Natl Sci Acad B55:499–504
Neeraj T, Prakash A, Seema Y (2013) Antimicrobial activity and medicinal values of essential oil of Mentha piperita L. Int J Eng Innov Technol 2:214–218
Nilsson M, Bhattacharya J, Rai AN, Bergman B (2002) Colonization of roots of rice (Oryza sativa) by symbiotic Nostoc strains. New Phytol 156:517–525
Obana S, Miyamoto K, Morita S, Ohmori M, Inubushi K (2007) Effect of Nostoc sp. on soil characteristics, plant growth and nutrient uptake. J Appl Phycol 19:641–646
Prescott GW (1970) Algae of the western Great Lakes area. WM.C.Brown, Dubuque, Iowa
Rangaswamy G (1966) Agricultural microbiology. Asia Publishing House, Bombay
Riahi H, Shariatmadari Z, Khanjir M, Azizi A (2013) Effect of Anabaena vaginicola inoculums on growth of pot plants. Acta Hort (ISHS) 1013:507–513
Saker M, Shanab S, Khater M (2000) In vitro studies on Ambrosia maritima. I- Morphogenic responses and algal toxins elicitation. Arab J Biotechnol 3:217–224
Santoro MV, Zygadlo J, Giordano W, Banchio E (2011) Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiol Biochem 49:1177–1182
Sergeeva E, Liaimer A, Bergman B (2002) Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215:229–238
Shanab S (2001) Effect of fresh water cyanobacterial extracts on alkaloid production of the in vitro Solanum elaeagnifolium tissue culture. Arab J Biotechnol 4:129–140
Shariatmadari Z, Riahi H, Seyed Hashtroudi M, Ghassempour AR, Aghashariatmadary Z (2013) Plant growth promoting cyanobacteria and their distribution in terrestrial habitats of Iran. Soil Sci Plant Nutr 59:535–547
Simpson DG (1986) Auxin stimulates lateral root formation of container-grown interior Douglas-fir seedlings. Can J For Res 16:1135–1139
Stanier RY, Kunisawa R, Mandal M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacteriol Rev 35:171–305
Stirk MA, Ördog V, Van Staden J, Jäger K (2002) Cytokinin and auxin-like activity in cyanophyta and microalgae. J Appl Phycol 14:215–221
Thangavelu B, Vadivel B, Selvaraj G, Muthuraman S (2013) Characterization of IAA production by the mangrove cyanobacterium Phormidium sp. MI405019 and its influence on tobacco seed germination and organogenesis. J Plant Growth Regul 32:758–766
Torres Salazar A, Hoheisel J, Youns M, Wink M (2011) Anti-inflammatory and anti-cancer activities of essential oils and their biological constituents. Int J Clin Pharmacol Ther 49:93–95
Vaishampayan A, Sinha RP, Hader DP, Dey T, Gupta AK, Bhan U, Rao AL (2001) Cyanobacterial biofertilizers in rice agriculture. Bot Rev 67:453–516
Wehr JD, Sheath RG, Thorp JH (2002) Freshwater algae of North America: ecology and classification. Aquatic Ecology Press, California
Acknowledgments
The authors wish to thank University of Shahid Beheshti for funding this project. Thanks are also due to Dr. F. Najafi and Dr. Z. Aghashariatmadary for their kind assistance during the research. Special thanks to Dr. M. R. Seghatoleslami for his valuable suggestions and help in editing and reviewing this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shariatmadari, Z., Riahi, H., Abdi, M. et al. Impact of cyanobacterial extracts on the growth and oil content of the medicinal plant Mentha piperita L.. J Appl Phycol 27, 2279–2287 (2015). https://doi.org/10.1007/s10811-014-0512-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0512-2