Abstract
This is a first time report on the successful cultivation of the red alga Sarconema filiforme, employing floating rafts along the south-east coast of India, with an associated study of carrageenan content. Experiments were carried out on seasonality of growth rate and biomass of S. filiforme for 30 months, from August 2010 to January 2013. Harvest was done at the end of 25-day growth. In addition, experiments were also carried out optimizing harvests at the end of different duration of culture period (i.e. 20-, 25- and 30-day culture), and measurements were made at different localities (e.g. Ervadi and Thonithurai). Maximum biomass density (2.28 ± 0.03 kg fresh wt. m−2) and daily growth rates (DGR; 11.63 ± 0.06 %) were found from August to September each year, during the study, and these values were significantly different. Harvesting at the end of 25-day duration of culture period resulted in a maximum biomass (i.e. 4.24 ± 0.95 kg fresh wt. m−2), while 20-day duration of culture period showed higher DGR i.e. 13.20 ± 0.20 % which were significantly different from those plants harvested after 30 days. The range of biomass (i.e. 2.22 to 6.46 kg fresh wt. m−2) and DGR (i.e. 5.0 to 10.91 %) was higher at Ervadi than at Thonithurai site and was significantly different (P < 0.001). Physico-chemical analyses, infra-red and 13C nuclear magnetic resonance (NMR) spectral analyses of extracted carrageenan revealed the presence of hybrid lambda and iota carrageenan. The carrageenan yield from the cultivated material was higher than that of the wild stock of S. filiforme of Indian waters. The results of the study revealed that the peak growth period of S. filiforme was between September and December. Harvesting at the end of the 25-day growth period was more ideal. This study further revealed that production of better strains having hybrid, sulphated galactan, with higher yield and rheological properties the same as the wild stocks, could be possible through cultivation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carrageenan is a hydrocolloid, consisting mainly of the ammonium, calcium, magnesium, potassium and sodium sulphate esters of galactose and 3,6-anhydrogalactose polysaccharides. These hexoses are alternately linked α-1,3 and β-1,4 in the copolymer (Painter 1983; Usov 1998). There are several carrageenans, differing in their chemical structure and rheological/binding properties, and therefore in their applications and uses. However, the major carrageenans of commercial interest are iota (ι), kappa (κ) and lambda (α). Iota carrageenan is an elastic gel, formed with calcium salts, while kappa is a strong, rigid gel formed with potassium salts. Lambda carrageenan does not form a gel (Craigie 1990). Carrageenans are generally used as thickeners, gelling agents, stabilizers and emulsifiers in various food and cosmetic industries. The worldwide market for all carrageenan was estimated to be approximately US$527 Million in 2009 (Bixler and Porse 2011).
The principal commercial sources of carrageenans are the red algae Furcellaria, Chondrus, Gigartina, Iridia, Hypnea, Sarconema, Phyllophora, Ahnfeltia, Gymnogongrus, Kappaphycus and Eucheuma. The structure and composition of carrageenans in various red seaweeds differs, e.g. Hypnea and Kappaphycus produce mainly kappa carrageenan, while Gymongongrus and Eucheuma yield mainly iota carrageenan. Chondrus and Gigartina may produce mainly kappa and iota and mixes sometimes lambda carrageenan (dependent of the dominant generations involved; McHugh 2003).
The genus Sarconema has three species worldwide, namely Sarconema filiforme (Sonder) Kylin, Sarconema scinaioides Boergesen and Sarconema gracilarioides Zhang and Xia. Of these, S. filiforme and S. scinaioides occur throughout the Indo-Pacific tropics and sub-tropics (Womersley 1994; Silva et al. 1996), whilst the distribution of S. gracilarioides is restricted to China (Zhang and Xia 1999). Indian waters have two species viz. S. scinaioides and S. filiforme (Oza and Zaidi 2001; Jha et al. 2009). S. scinaioides is distributed in north-west coast only, while S. filiforme has been recorded from the north-west and south-east coast. S. filiforme grows up to 15 cm long, slender, with cylindrical axis, regularly and sub-dichotomously branched and tapering towards the tip.
Sarconema filiforme from Indian waters (Parekh et al. 1988) and Tanzania (Semesi and Mshigeni 1977) has been reported to contain iota carrageenan. However, S. filiforme from Australia (Chiovitti et al. 1998) has been reported to contain a hybrid or mixture of lambda carrageenan, iota carrageenan and pyruvated lambda carrageenan. Recently, Kumar et al. (2011) analysed the carrageenan of S. filiforme from the north-west coast of India and confirmed the earlier report of Chiovitti et al. (1998).
India annually produces 120 t of agar and 300 t of alginate to meet the domestic needs (Subba Rao and Mantri 2006). Industrial production of kappa carrageenan has only been in operations since 2009, as a direct result of the successful, commercial farming of Kappaphycus alvarezii, along the south-east Indian coast. Annual production of K. alvarezii through cultivation ranges from 1,000 to 1,500 dry t), and kappa carrageenan production ranges from 250 to 300 t (M. Shanmugam personal communication). However, India does not produce other forms of carrageenan (i.e. iota and lambda), mainly due to the scanty availability of carrageenophyte biomass from wild stocks.
The south-east coast of India is an ideal location for seaweed cultivation. Commercial cultivation of K. alvarezii is currently employing about 1,000 seaweed farmers. In addition, cultivation potential has been demonstrated at the pilot-scale, for other commercial seaweeds, such as Gracilaria edulis (S. Gmelin) P. Silva (Raju and Thomas 1971; Umamaheswara Rao 1974; Kaliaperumal et al. 2003; Ganesan et al. 2011), Gelidiella acerosa, (Forsskål) J. Feldmann & G. Hamel (Patel et al. 1979, 1986; Subbaramaiah and Banumathi 1992; Ganesan et al. 2009, 2011) and Hypnea musciformis (Wulfen) Lamouroux (Rama Rao and Subbaramaiah 1986; Ganesan et al. 2006). The present study aimed to develop the practical and applicable cultivation methods for S. filiforme, optimize the harvest period, cultivate at different locations and analyse the carrageenan yield and quality from the cultivated samples in order to augment the resources of other carrageenophyte in India.
Materials and methods
Cultivation
Field cultivation experiments on S. filiforme were carried out at Thonithurai (09° 16.92′ N–079° 11.40′ E) in the Gulf of Mannar, on the south-east coast of India. The intertidal and shallow sub-tidal regions have a muddy bottom substratum, covered with a dense seagrass meadow (Thalassia sp.). Adjacent to this, there is a sandy coast where pebbles and small coral stones make up the beach. Gracilaria, Hypnea and Padina are the dominant algae attached to these pebbles. The intertidal region is exposed during lowest of low tide and submerged during high tide; the tidal range is 0.7 m.
S. filiforme was cultivated using floating rafts made of bamboo poles (1.5 × 1.5 m size). Young and healthy plants of S. filiforme better suited for cultivation were selected from a wild population on the natural substrata of Krusadai island (09° 14.928′ N; 079° 13.245′ E), Gulf of Mannar coast. Seedlings were cut into small size of having an average of 0.8 g fresh wt. ± 0.25 and inserted between the twists of the polypropylene rope (3-mm diameter) at 5-cm intervals. Each polypropylene rope had 25 seedlings with a total fresh weight of approx. 20 g rope−1 (25 × 0.8 g fresh wt). A raft with 20 ropes had an initial seedling mass of 400 g fresh wt. raft−1 which was equivalent to 160 g fresh wt. m−2. The lower side of the raft, below the seeded ropes, was fully covered with fish net (8 mm mesh) to prevent access by the grazing fish and minimize loss of any experimental material which might detach from the lines (Fig. 1a, b). The raft was anchored securely to stones (25 kg approx.). Harvest was made at the end of 25 days of culture period. All plants grown on the rope were fully harvested, and the ropes were freshly re-seeded with new seedlings from the cultivated material after every harvest.
Biomass and daily growth rate assessments
The biomass of harvested plants was determined after thorough washing in seawater. All attached epiphytes (Lyngbya, Jania and Chaetomorpha are the major epiphytes) and fauna (dominated by gastropods, polychaetes, bivalves and crabs) were removed manually. Excess water was drained by keeping the biomass on a Palmira mat (3 mm thick) over wooden stand for 5 min, and fresh weights were measured. S. filiforme grew well and covered fully the length of rope and distance between the ropes (Fig. 1b). Therefore, biomass (Y) harvested from 20 ropes was expressed as mean kilogram fresh wt. per square meter considering the size of the raft. Biomass was determined using the modified formula of Doty (1986) that included the initial weight of the seedlings:
where W f is the final fresh weight, W i is the initial fresh weight and m 2 is the area covered.
Daily growth rate (DGR (%)) was calculated using the formula of Dawes et al. (1993) as follows:
where W f is the final fresh weight after t days of culture period and W o is the initial fresh weight.
Biomass and daily growth rate values are given with mean ± standard error of the mean (SEM).
Seasonal variation in biomass and growth rate
Seasonal variations in biomass yield and growth rate were studied for 3 years from August 2010 to January 2013. Twenty-five rafts were seeded, and periodical harvests were made at the end of 25 days of culture period.
Harvest at different duration of culture period
In order to understand the best duration of culture period to get optimum biomass yield and DGR, harvests were made at the end of 20-, 25- and 30-day culture period. Three rafts were employed for each culture period. Two harvests were done for each culture period.
Growth at different stations
Three rafts were placed at the same time in the intertidal regions of the Thonithurai coast and the Ervadi coast (9° 12.49′ N, 78° 43.59′ E) in the Gulf of Mannar. The distance between the two stations was 70 km. The bottom substrata of Ervadi consisted of flat, coralline reefs with small patches of sand beds. The flat reef was always submerged at low tide and the tidal range between 0.3 and 1.0 m (lowest low tide and highest high tide). The flat reef supports rich seaweed diversity, and Turbinaria, Sargassum and Gelidiella are the dominant algae. In both the stations, rafts were harvested at the end of the 25-day culture period. Three subsequent harvests were done in both the stations. This experiment was conducted in 2011.
Environmental parameters
Seawater temperature, salinity and dissolved oxygen were recorded every week at the cultivation sites. Seawater temperature and salinity were recorded at 7.00 a.m. and followed the same time throughout the cultivation period. Temperature was measured using a standard thermometer and salinity with a hand refractometer (Atago, Japan). Dissolved oxygen was estimated immediately using the Winklers (1888) method. Seawater samples (in triplicate) were collected monthly, and nutrient analysis was done at the laboratory. Dissolved inorganic phosphate (PO4-P) and nitrate (NO3-N) were determined using a UV-visible spectrophotometer (Hitachi, model 2001, Hitachi, Japan), following the standard method (Strickland and Parsons 1972).
Statistical analyses
Analysis of variance (ANOVA; P = 0.05) was used to determine significant differences in average values of DGR and biomass yield. The Tukey’s HSD test was used to separate means, whenever the F values were significant in the ANOVA. Pearson’s correlation analysis was used to understand the relations between environmental parameters and biomass, DGR, carrageenan yield and its chemical properties. The Student’s t test was performed to examine any differences in biomass and DGR between the two stations examined. The statistical analyses were performed by using the software SYSTAT version 7.
Carrageenan
Extraction of sulphated polysaccharide
S. filiforme plants were harvested from the cultivation rafts during August–September 2010, October–November 2010, November–December 2010 and December 2010–January 2011. The harvested materials were washed three times with sea water, and epiphytes, (Jania, Lyngbya and Chaetomorpha), epifauna (gastropods, polychaetes, bivalves and crabs) and silt were removed and then air dried. Dried biomass (50 g) was soaked in demineralized water for 1 h, followed by extraction with 0.05 M NaHCO3 (1:30 w/v) at 110 °C for 2 h in an autoclave (Craigie and Leigh 1978). The cooked seaweed was homogenized and centrifuged, and the sulphated galactan (SG-FA) was isolated by precipitation of the supernatant with isopropyl alcohol (1:2 v/v).
Chemical analysis
Chemical analysis was done for carrageenan extracted from samples collected during August–September 2010, October–November 2010, November–December 2010 and December 2010–January 2011. The total carbohydrate content was determined by the method of Dubois et al. (1956). Protein content was calculated from the percentage nitrogen (%N) determined by the Kjeldahl method on a KEL PLUS-KES 201 Digestion unit, attached to a KEL PLUS-CLASSIC DX Distillation unit (M/s PELICAN Equipments, India), using the conversion factor 6.25 (Marks et al. 1985). Sulphate contents were estimated on a PerkinElmer inductively coupled plasma optical emission spectrophotometer (ICP-OES) Optima 2000DV following the method of Wolnik, (1998). All analyses were undertaken in triplicate, and the mean values are presented.
FT-IR, 1 H and 13 C NMR
FT-IR, 1 H and 13 C NMR of sulphated galactans were done for samples collected during August–September 2010, October–November 2010, November–December 2010 and December 2010–January 2011. FTIR spectra of sulphated galactans were recorded on a PerkinElmer Spectrum GX (FT-IR System, USA), using the KBr disk method (Pereira et al. 2003). Sulphated galactans were dissolved in D2O (20 mg mL−1) and freeze-dried twice to replace all exchangeable protons with deuterium. The 1H NMR (500 MHz) of sulphated galactans was acquired in D2O after isotopic exchange at 70 °C on a Bruker Avance-II 500 spectrometer. The water peak served as a reference (HOD, 4.69 μL L−1). 13C-NMR spectra at 125 MHz of SG-SF in D2O (50 mg mL−1) solution were recorded on a Bruker Avance-II 500 spectrometer, operating at 70 °C. Chemical shifts were measured relative to internal dimethyl sulphoxide (DMSO) (ca. 39.43 μL L−1). The numbers of acquisitions were 6,000–6,500.
Results
Seasonal variations in biomass and growth rate
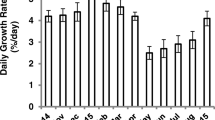
Between 2010 and 2013, a total of 17 harvests were made, 3 harvests during the first year (2010), 6 harvests in the second year (2011), and 7 harvests in the third year (2012); there was 1 harvest in 2013. In the first year, the maximum harvested biomass was recorded as 1.96 ± 0.48 kg fresh wt. m −2, during December to January; the minimum 1.11 ± 0.40 kg fresh wt. m−2 occurred during October to November. No significant differences were observed (P > 0.05) between the biomass harvested as only three harvests have been done. In the second year, a maximum biomass 2.22 ± 0.80 kg fresh wt. m−2 was harvested during September to October; the minimum was in July, i.e. 0.56 ± 0.30 kg fresh wt. m−2. Significant differences were observed (P < 0.001) between the maximum and minimum biomass values. In the third year, the harvested biomass peaked at 2.28 ± 0.03 kg fresh wt. m−2 during August to September, and the lowest was 0.92 ± 0.04 kg fresh wt. m−2 during May to June (Fig. 2a); significant differences were observed between the harvested biomass (P < 0.001).
Maximum daily growth rate (7.49 ± 0.43 %) was observed from November to December during the first year. No significant differences (P > 0.05) were observed between harvests. In the second year, the DGR value peaked from May to June (6.70 ± 1.74 %) harvests, and significant differences were observed in DGR (P < 0.001). In the third year, the DGR was at a maximum (11.63 ± 0.06 %) during August to September harvest (Fig. 2b). Significant differences were observed between DGR of 3 years (P < 0.001).
Harvest at the end of 25-day duration of culture period resulted in the absolute maximum biomass yields (4.24 ± 0.95 kg fresh wt. m−2), while harvest at the end of 30-day duration of culture period yielded (Fig. 3) the lowest biomass (1.92 ± 0.50 kg fresh wt. m−2). However, no significant differences were observed between different durations of culture period (P > 0.05). Harvest at the end of 20-day duration of culture period showed higher daily growth rate (i.e. 13.20 ± 0.20 %) which was closely followed by the 25-day culture period (i.e. 11.73 ± 0.40 %; Fig. 3). The lowest DGR (6.60 ± 0.80 %) was observed at 30-day culture period. Significant differences were observed in the DGR reported amongst the three different duration of culture periods investigated in this study (P < 0.05).
Biomass harvested at Ervadi ranged from 2.22 to 6.46 kg fresh wt. m−2, and significant differences (P < 0.01) were observed in biomass between the three harvests. Biomass harvested at Thonithurai ranged from 1.66 to 2.20 kg fresh wt. m−2, and no significant differences (P > 0.05) were observed in biomass between three harvests (Fig. 4). DGR of S. filiforme at Ervadi ranged from 5.0 to 10.91 %, and significant differences (P < 0.001) were observed between minimum and maximum DGR. DGR values of S. filiforme at Thonithurai ranged from 5.49 to 6.0 %, and no significant differences (P > 0.05) were observed between DGR (Fig. 4).
Monthly variations in environmental parameters for 29 months (August 2010 to December 2012) at Thonithurai cultivation site are shown in Fig. 5a, b. Seawater temperatures ranged from 25 °C (December 2012) to 31.5 °C (May 2011). Salinity values varied from 28.2 ‰ in January 2011 to 38 ‰ in April 2012. Dissolved oxygen content ranged from 1.21 mg L−1 in February 2012 to 5.13 mg L−1 in December 2011. Phosphate content varied from 0.51 μM (September 2010) to 7.2 μM (July 2011). Nitrate content varied from 0.1 μM (August 2011) to 3.2 μM (December 2010).
Monthly variations in environmental parameters for 12 months (January to December 2011) at Ervadi cultivation site are given in Fig. 6a, b. Seawater temperature ranged from 26 °C (January 2011) to 31.5 °C (September 2011). Salinity ranged from 30 ‰ in January 2011 to 37 ‰ in September 2011. Dissolved oxygen content ranged from 2.50 mg L−1 in June 2011 to 5.62 mg L−1 in February 2011. Phosphate content varied from 0.23 μM (January 2011) to 7.8 μM (July 2011). Nitrate content varied from 0.20 μM (October and December 2011) to 0.96 μM (February 2011). ANOVA showed no significant variations in environmental parameters between two cultivation sites (Thonithurai and Ervadi).
Carrageenan
The yield, total sugar, sulphate, protein and pyruvate contents of sulphated galactan of S. filiforme harvested during August–September 2010, October–November 2010, November–December 2010 and December–January 2011 are shown in Table 1. The yield of sulphated galactan ranged between 32 ± 1 and 40 ± 1 % (Table 1). The highest (40 % ± 1) and lowest (32 ± 1 %) yields of sulphated galactan were obtained in August 2010 and October 2010, respectively (Table 1). The sulphate contents of carrageenan were in the range of 22 ± 1 to 23 ± 1 %, and no significant differences were observed between sulphate content (P > 0.05). The apparent viscosities of sulphated galactan from S. filiforme were 18 to 21 cP (in 1 % SG solution in 1 % KCl), 28 to 31 cP (in 1 % SG solution in 1 % CaCl2) and 87 to 98 cP (in 1 % SG solution in 1 % 1:1 KCl & CaCl2).
FT-IR spectra
A strong IR bands at around 1,258 cm−1 were seen in all four polysaccharide samples of S. filiforme harvested during August–September 2010, October–November 2010, November–December 2010 and December–January 2011 (Fig. 7a–d). The appearance of the strong IR bands at around 1,258 cm−1 in all the four polysaccharide samples indicated the presence of sulphated ester (Fig. 7a–d). Bands were also seen in 932, 808 and 848 cm−1. Other characteristic bands were observed at 1,637, 1,158, 1,075 and 1,033 cm−1. The presence of 3-linked galactose residues bearing pyruvate acetal substitution was confirmed by the appearance of the band at 904 cm−1.
13C and 1H NMR spectra
The 13C NMR chemical shifts of extracted polysaccharide are shown in Fig. 8. Anomeric carbon resonance pairs at 102.7 and 92.7, 103 and 92.7, and 103 and 95.4 μL L−1 were assigned to the diads to pyruvated α, α and ι carrageenans respectively (Fig. 8).
The 1H NMR spectrum of polysaccharide (AS-IV-226-04) exhibited the anomeric proton signals (Fig. 9) in the range of 4.56 to 4.73 μL L−1 (assigned to 1H of β-d-galactose residue of α, ι and pyruvated α-carrageenans respectively), at 5.38 μL L−1 (1H of α-d-anhydrogalactose residue of ι carrageenan) and at 5.19 μL L−1 (1H of α-d-anhydrogalactose residue of α carrageenan).
Discussion
A clear seasonal trend was observed in growth patterns. Every year, growth started in August, peaked from September to December and declined from February to July. ANOVA showed significant variations (P < 0.001) in biomass yield during the 3-year period. South-west monsoon brought strong waves during July to September, whilst the north-east monsoon provided good rainfall during October to December. These two monsoon periods favoured the growth of S. filiforme. Dissolved oxygen (3.20–5.13 mg L−1) and nitrate content (0.1–3.12 μM) were higher during the two monsoon periods, and dissolved oxygen and nitrate showed a strong, positive correlation with the biomass (r = 0.960; r = 0.947, respectively). Earlier studies (Craigie 1990; Brown 1995) also reported that nitrate increased the growth of the carrageenophytes Chondrus crispus and Soliera chordalis. The observed seasonal maxima of biomass values during September to December are similar to seasonality in biomass of cultivated H. musciformis (Rama Rao and Subbaramaiah 1986; Ganesan et al. 2006), Gracilaria edulis (Ganesan et al. 2011) and Gelidiella acerosa (Ganesan et al. 2009) along this coast.
Biomass on the cultivated lines was lowest during May to July when both salinity and phosphate values were at their peak (38 ‰ and 7.2 μM respectively). Salinity (r = 0.557) and phosphate (r = 0.669) showed a strong negative correlation with biomass. Similar negative correlations between salinity and biomass were reported for H. musciformis (Ganesan et al. 2006) cultivated on the same coast.
The observed maximum DGR value was the same as that of H. musciformis (11.2 % Ganesan et al. 2006) but much higher than other economically important seaweeds cultivated in the same area, e.g. K. alvarezii (9.99 % Eswaran et al. 2002), Gracilaria edulis (5 % Subbaramaiah and Thomas 1990; 5.3 % Ganesan et al. 2011) and Gelidiella acerosa (1.5 % Subbaramaiah and Banumathi 1992; 1.31 % Ganesan et al. 2009 and 2.63 % Ganesan et al. 2011).
Consistencies in biomass yield and DGR were observed for 3 years of cultivation period. This is because young and actively growing apical portions of thallus of S. filiforme were selected from wild stock for initial seeding. In subsequent cultures, actively growing apical portions of the thalli were selected from harvested crop for seeding. However, other economic seaweeds such as Gracilaria chilensis in Chile (Buschmann et al. 2001), Gracilaria edulis from Lakshadweep (Kaladharan et al. 1996), Gelidiella acerosa (Rama Rao and Subbaramaiah 1977) and H. musciformis (Ganesan et al. 2006) from Indian waters all showed higher crop yields in the first 1 or 2 years and sharp decline in crop yield in subsequent harvests. It has been reported by these authors that repeated harvest and ageing of the thallus are the main reason for decline in crop yield in subsequent harvests.
Harvest at the end of 25-day duration of culture period was more ideal. The lowest biomass and DGR were obtained when harvest took place at the end of 30-day culture period. Cultivation beyond 25 days affected the growth rate due to accumulation of more epiphytic and epifaunal species on the alga and the plant lost its vigour. Harvest at the end of 25-day culture period yielded higher biomass and DGR in the other carrageenophyte H. musciformis (Ganesan et al. 2006). Both algae have fast growth rate (>11 %) and susceptible to epiphytes and epifaunal infestation because of their thin, slender and highly branched thallus structure.
Among the two stations of cultivation, biomass and DGR were higher in Ervadi than in Thonithurai. The t test showed significant difference in biomass (P < 0.001) and DGR (P < 0.001) between the two stations. Correlation analyses revealed that among the various environmental parameters, dissolved oxygen showed positive correlation with biomass (r = 0.696) at Thonithurai, while nitrate had strong positive correlation with biomass (r = 0.795) and DGR (r = 0.914) at Ervadi. Higher nitrate content that resulted in higher biomass was also observed for Gracilaria edulis at Ervadi (Ganesan et al. 2011). Therefore, it is clear that nitrate is an important factor determining the growth of algae in intertidal and subtidal regions.
Environmental parameters were not significantly different between two stations. Nevertheless, significantly higher biomass and DGR were obtained at Ervadi cultivation site. This confirmed the results of our earlier experiment on Gracilaria edulis (Ganesan et al. 2011) which showed higher growth at Ervadi. Clear water, high nitrate content, moderate wave action and rich algal diversity in intertidal and subtidal regions altogether favoured the growth of S. filiforme at Ervadi.
Salinity, seawater temperature and nutrients level showed significant seasonal variations (P < 0.01). Lowest temperature and salinity values were recorded during December and January respectively. These months fall on autumn when temperature is always low along the south-east Indian coast. Nutrient (phosphate, nitrate and nitrite) levels are high during July to December due to the influence of south-west monsoon (July to September) and north-east monsoon (October to December).
Seasonal trends of environmental parameters are similar in both the cultivation sites. Two cultivation sites located in Gulf of Mannar, south-east coast which is influenced by the south-west monsoon during July to September and north-east monsoon during October to December. Wind blowing directions and tidal amplitude are similar in both stations. Therefore, the same seasonal trends of environmental factors were observed.
The results on carrageenan from S. filiforme revealed seasonal variations in the yields of sulphated galactan and dependent on the cultivation time. Among the various environmental parameters recorded at cultivation site, phosphate content of seawater showed a strong negative correlation (r = −0.995) on carrageenan content. Kumar et al. 2011 have reported 31 % yield of carrageenan from S. filiforme harvested from wild stocks of west coast of India. This study indicates that the cultivated S. filiforme produced higher yield of sulphated galactan compared to S. filiforme harvested from wild stocks of west coast of India (Kumar et al. 2011). Chiovitti et al. 1998 have also reported 25 to 35 % carrageenan yields from S. filiforme harvested from Australia. Cultivated S. filiforme possessed a slightly lower sulphate content, as compared to naturally growing S. filiforme (25 % Kumar et al. 2011). The sulphate content of S. filiforme was in between the sulphate value of lambda-carrageenan (17 to 18 %) and iota-carrageenan (35 to 36 %).
Polysaccharide samples (1 % w/v) of S. filiforme did not form a gel with salts (1 % w/v, CaCl2, KCl) solutions while iota-carrageenan forms elastic gels with CaCl2 (1 % w/v) solution. These two characters of carrageenan indicate that S. filiforme produced hybrid galactan. In addition, viscosities of sulphated galactan from S. filiforme were higher i.e. 28 to 31 cP 18 in 1 % SG solution in 1 % CaCl2 than in 1 % SG solution in 1 % KCl (18 to 21 cP). This greater viscosity of sulphated galactans of S. filiforme in the presence of Ca+2 ions, as compared to K+ ions, confirmed the earlier report by Kumar et al. (2011). Varying viscosity of sulphated galactan in the presence of salts makes it potentially useful for various application (De Vries 2002; van de Velde 2008; Guibet et al. 2008; Jouanneau et al. 2010). The bands at 932, 808 and 848 cm−1 FT-IR spectra confirmed the presence of 3,6-anhydrogalactose, axial sulphate ester at O-2 of 4-linked 3,6-anhydrogalactose and sulphate ester at O-4 of 3-linked galactose residues, respectively (Chiovitti et al. 1997; Pereira et al. 2003). Bands at 1,637, 1,158, 1,075 and 1,033 cm−1 were due to -O-H bending, C-O-C stretching of 6-O-methylated Gal, C-O stretching of secondary and primary alcohols of a pyranose ring, respectively. Chiovitti et al. (1997) reported that the band at 904 cm−1 showed the presence of 3-linked galactose residues bearing pyruvate acetal substitution. The band at 848 cm−1 (4-sulphate) was weaker than 808 cm−1 (2-sulphate) suggesting that polysaccharides were not composed merely of iota-carrageenan. Taken together all characters of carrageenan from S. filiforme like the range of sulphate content (22 to 23 %) that lies between lambda (17 to ‘18 %) and iota (35 to 36 %), and no gel-forming capacity with salt (1 % w/v, CaCl2, KCl) solutions and weaker bands at 848 cm−1 than 808 cm−1, we conclude that these polysaccharides were a hybrid of lambda- and iota-carrageenans, having pyruvated acetal substitution (Chiovitti et al. 1997; Pereira et al. 2003; Yermak et al. 2006; Matsuhiro and Rivas 1993).
13C NMR carbon resonance at 59.7 μL L−1 was assigned to the methylated C-6 of the 3-linked galactose (Usov et al. 1980). The 13C NMR chemical shifts of extracted polysaccharide were comparable to those of carrageenans reported earlier (Kumar et al. 2011). Additional 13C NMR resonances at 176.3, 102.0 (shoulder) and 26.2 μL L−1 indicated the presence of carboxyl, acetal and methyl carbons of the pyruvate unit, respectively. Villanueva et al. (2009) gave the 13C NMR of standard ι-carrageenan.
In 1H NMR spectra, the resonance at 3.31 μL L−1 (singlet) was assigned to the methyl protons of 6-O-methyl Gal and the one at 1.33 μL L−1 (singlet) to methyl hydrogens of pyruvated α-carrageenan (Falshaw et al. 2003; Chiovitti et al. 2004). Three weak signals at 67.7, 67.4 and 66.2 μL L−1 were assigned to C-4, C-5 and C-6 of the 3-linked pyruvated galactose unit.
In conclusion, the present study revealed that S. filiforme can be cultivated along the Gulf of Mannar, south-east coast of India for 9 months in a year from August to April. The peak growth period of the alga is from September to December. May to July is the lean period and very little growth of alga was observed. Harvest at the end of 25-day duration of culture period gave higher biomass with high DGR. The observed differences in growth of S. filiforme between different stations raised the scope for expanding its cultivation in other areas along this coast.
The yield and physical properties of the polysachharide associated with cultivated S. filiforme, harvested during different seasons, and did not show any variation. The sulphated galactan of the cultivated S. filiforme was assigned as a hybrid polysaccharide composed of lambda, pyruvated lambda, methylated lambda and iota carrageenans. This result confirmed the earlier report that this alga has a hybrid carrageenan. Cultivated S. filiforme gave higher polysaccharide yield than wild S. filiforme from the west coast of India as earlier reported. However, the physical-chemical properties of the extracted gel were similar. Recently, the industrial utilization of hybrid carrageenans, especially in the food and cosmetic industries has increased tremendously. This study further revealed that production of better strains having hybrid, sulphated galactan, with more yield and identical quality of natural sources, could be done through cultivation.
References
Bixler JH, Porse H (2011) A decade change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–325
Brown MT (1995) Interactions between environmental variables on growth rate and carrageenan content of Solieria chordalis (Solieriaceae, Rhodophyta) in culture. J Appl Phycol 7:427–432
Buschmann AH, Correa JA, Westermeier R, Gonzalez MC, Norambuena R (2001) Re algal farming in Chile—a review. Aquaculture 194:203–220
Chiovitti A, Bacic A, Craik DJ, Munro SLA, Kraft GT, Liao ML (1997) Cell wall polysaccharides from Australian red algae of the family Solieriaceae (Gigartinales, Rhodophyta): novel highly pyruvated carrageenans from the genus Callophycus. Carbohydr Res 299:229–243
Chiovitti A, Bacic A, Craik DJ, Kraft GT, Liao ML, Falshaw R, Furneaux RH (1998) A pyruvated carrageenan from Australian specimens of the red alga Sarconema filiforme. Carbohydr Res 310:77–83
Chiovitti A, Bacic A, Craik DJ, Kraft GT, Liao ML (2004) A nearly idealized 6’-O-methylated ι-carrageenan from the Australian red alga Claviclonium ovatum (Acrotylaceae, Gigartinales). Carbohydr Res 339:1459–1466
Craigie J (1990) Irish moss cultivation: some reflections. In: Yarish C, Penniman CA, van Patten P (eds) Economically important marine plants of the Atlantic. Their Biology and Cultivation. Connecticut Sea Grant College Program, Groton, pp 37–52
Craigie JS, Leigh C (1978) Carrageenans and agars. In: Hellebust JA, Craigie JS (eds) Handbook of phycological methods: physiological and biochemical methods. Cambridge University Press, Cambridge, pp 109–131
Dawes CJ, Lluisma AO, Trono GC (1993) Clonal propagation of Eucheuma denticulatum and Kappaphycus alvarezii for Philippines farms. Hydrobiologia 260/261:1379–1383
De Vries J (2002) Interaction of carrageenan with other ingredients in dairy dessert gels. In: Williams PA, Phillips GO (eds) Gums and stabilisers for food industry. The Royal Society of Chemistry, Cambridge, pp 201–210
Doty MS (1986) Estimating returns from producing Gracilaria and Eucheuma on line farms. Monogr Biol 4:45–62
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 8:350–366
Eswaran K, Ghosh PK, Mairh OP (2002) Experimental field cultivation of Kappaphycus (Doty) Doty. ex. P. Silva at Mandapam region. Seaweed Res Utiln 24:67–72
Falshaw R, Furneaux RH, Wong H (2003) Analysis of pyruvated β-carrageenan by 2D NMR spectroscopy and reductive partial hydrolysis. Carbohydr Res 338:1403–1414
Ganesan M, Thiruppathi S, Jha B (2006) Mariculture of Hypnea musciformis (Wulfen) Lamouroux in south east coast of India. Aquaculture 256:201–211
Ganesan M, Thiruppathi S, Eswaran K, Jha B (2009) Cultivation of Gelidiella acerosa in open sea on the south eastern coast of India. Mar Ecol: Prog Ser 382:9–57
Ganesan M, Sahu N, Eswaran K (2011) Raft culture of Gracilaria edulis in open sea along the south eastern coast of India. Aquaculture 321:145–151
Guibet M, Boulenguer P, Mazoyer J, Kervarec N, Antonopoulos A, Lafosse M, Helbert W (2008) Composition and distribution of carrabiose moieties in hybrid κ-/ι-carrageenans using carrageenases. Biomacromolecules 9:408–415
Jha B, Reddy CRK, Thakur MC, Rao UM (2009) Seaweeds of India: the diversity and distribution of seaweeds of the Gujarat coast. Springer, Dordrecht
Jouanneau D, Guibet M, Boulenguer P, Mazoyer J, Smietana M, Helbert W (2010) New insights into the structure of hybrid κ-/μ-carrageenan and its alkaline conversion. Food Hydrocolloids 24:452–461
Kaladharan P, Vijayakumaran K, Chennubhotla VSK (1996) Optimization of certain physical parameters for the mariculture of Gracilaria edulis (Gmelin) Silva in Minicoy lagoon (Luccadive Archipelago). Aquaculture 139:265–270
Kaliaperumal N, Kalimuthu S, Ramalingam JR (2003) Pilot scale field cultivation of the agarophyte Gracilaria edulis (Gmelin) Silva at Vadakadu (Rameswaram). Seaweed Res Utiln 25:213–219
Kumar S, Mehta GK, Prasad K, Meena R, Siddhanta AK (2011) Chemical investigation of carrageenan from the red alga Sarconema filiforme (Gigartinales, Rhodophyta) of Indian Waters. Nat Prod Commun 6:1327–1332
Marks DL, Buchsbaum R, Swain T (1985) Measurement of total protein in plant samples in the presence of tannins. Anal Biol 1147:136–143
Matsuhiro B, Rivas P (1993) Second-derivative Fourier transform infrared spectra of seaweed galactans. J Appl Phycol 5:45–51
McHugh DJ (2003) A guide to the seaweed industry, FAO Fisheries Technical Report
Oza RM, Zaidi SH (2001) A revised checklist of Indian marine algae. CSMCRI, Bhavnagar
Painter TJ (1983) Algal polysaccharides. In: Aspinall GO (ed) The polysaccharides. Academic Press, New York, pp pp.195–285
Parekh RG, Doshi YA, Rao AV, Chauhan VD (1988) Polysaccharides from Sarconema filiforme of Indian marine alga. Phytochemistry 27:1–3
Patel JB, Gopal BV, Nagulan VR, Subbaramaiah K, Thomas PC (1979) Experimental field cultivation of Gelidiella acerosa at Ervadi in India. In: Proc. int. symp. marine algae of the Indian Ocean region. Bhavnagar, India: CSMCRI; 1979. p. 24-25
Patel JB, Gopal BV, Nagulan VR, Subbaramaiah K, Thomas PC (1986) Experimental field cultivation of Gelidiella acerosa at Ervadi in India. Proc Symp Coastal Aquac 4:1342–1345
Pereira L, Sousa A, Coelho H, Amado AM, Ribeiro-Claro PJA (2003) Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol Eng 20:223–228
Raju PV, Thomas PC (1971) Experimental field cultivation of Gracilaria edulis (Gmel.) Silva. Bot Mar 14:71–75
Rama Rao K, Subbaramaiah K (1977) Regeneration and regrowth of Gelidiella acerosa Forssk.) Feldmann et Hamel at Kilakkarai southeastern shores of India. Indian J Mar Sci 6:175–177
Rama Rao K, Subbaramaiah K (1986) A technique for the field cultivation of Hypnea musciformis. Proc Symp Coastal Aquac 4:1190–1192
Semesi AK, Mshigeni KE (1977) Studies on yield and IR-spectra of Phycocolloids from Porteria hornemannii (Lyngbye) and Sarconema filiforme (Sonder) Kylin from Tanzania. Bot Mar 20:271–275
Silva PC, Bassom PW, Moe RL (1996) Catalogue of the benthic marine algae of Indian Ocean. California: University of California Publication Series 79
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Bull Fish Res Bd Canada 67:1–311
Subba Rao PV, Mantri VA (2006) Indian seaweed resources and sustainable utilization: scenario at the dawn of a new century. Curr Sci 91:164–174
Subbaramaiah K, Banumathi R (1992) A preliminary note on the raft cultivation of Gelidiella acerosa. Phykos 31:1–5
Subbaramaiah K, Thomas PC (1990) Raft cultivation of Gracilaria edulis (Gmel.). Silva Proc Indian Acad Sci 100:123–127
Umamaheswara Rao M (1974) On the cultivation of Gracilaria edulis in the near shore areas around Mandapam. Curr Sci 43:660–661
Usov AI (1998) Structural analysis of red seaweed galactans of agar and carrageenan groups. Food Hydrocolloids 12:301–308
Usov AI, Yarotsky SV, Shashkov AS (1980) 13C-NMR spectroscopy of red algal galactans. Biopolymer 19:977–990
Van de Velde F (2008) Structure and function of hybrid carrageenans. Food Hydrocolloids 22:727–734
Villanueva RD, Montaño MNE, Romero JB (2009) Iota-carrageenan from a newly farmed, rare variety of eucheumoid seaweed—“endong”. J Appl Phycol 21:27–30
Winkler LW (1888) Die Bestimmung des im Wasser gelösten Sauerstoffes. Ber Dtsch Chem Ges 21(2):2843–2855
Wolnik KA (1998) Inductively coupled plasma-emission spectrometry. Methods Enzymol 158:190–205
Womersley HBS (1994) The marine benthic flora of southern Australia. Rhodophyta. Part IIIA. Flora Australia Supplementary Series Number 1, Australian Biological Resources Study, Canberra
Yermak IM, Barabanova AO, Glazunov VP, Isakov VV, Hwan KY, Soon SK, Titlyanova TV, Solov’eva TF (2006) Carrageenans from cystocarpic and sterile plants of Chondrus pinnulatus (Gigartinaceae, Rhodophyta) collected from the Russian Pacific coast. J Appl Phycol 18:361–368
Zhang J, Xia B (1999) Sarconema gracilarioides in: Xia B (ed) Flora Algorum Marinerum Koeltz Scientific Books, Koenigstein, Germany pp. 1–201
Acknowledgements
Our sincere thanks to Dr. Alan Critchley for improving the style of the manuscript and refining the English. The funding support received from the Council of Scientific & Industrial Research (NWP 018) is gratefully acknowledged. The author AKS thank CSIR, New Delhi for awarding Emeritus Scientist Scheme.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ganesan, M., Meena, R., Siddhanta, A.K. et al. Culture of the red alga Sarconema filiforme in open waters and hybrid carrageenan from the cultivated seaweeds. J Appl Phycol 27, 1549–1559 (2015). https://doi.org/10.1007/s10811-014-0442-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0442-z