Abstract
Autistic people often show difficulty with facial expression recognition. However, the degree of difficulty varies widely, which might reflect varying symptom profiles. We examined three domains of autistic traits in the typical population and found that more autistic-like social skills were associated with greater difficulty labelling expressions, and more autistic-like communication was associated with greater difficulty labelling and perceptually discriminating between expressions. There were no associations with autistic-like attention to detail. We also found that labelling, but not perceptual, difficulty was mediated by alexithymia. We found no evidence that labelling or perceptual difficulty was mediated by weakened adaptive coding. Results suggest expression recognition varies between the sub-clinical expressions of autistic symptom domains and reflects both co-occurring alexithymia and perceptual difficulty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (autism) is a neurodevelopmental disorder characterised by restrictive, repetitive patterns of behaviour and interests, difficulties with social interaction, and difficulties with social communication (APA 2013). Given the diagnostic feature of difficulty with social communication and interaction, numerous studies have investigated whether autistic people have difficulty recognising facial expressions, which provide information about others’ emotional states and guide social behaviour (Adams et al. 2006; Ekman et al. 1992). Meta analyses of this literature have concluded that autism is associated with difficulty in facial expression recognition (Lozier et al. 2014; Uljarevic and Hamilton 2013). However, results of individual studies give a more complex and varied picture of expression recognition ability in autism. Findings in different samples are inconsistent, with some showing clear difficulty (e.g. Corbett et al. 2009; Humphreys et al. 2007) and others finding that autistic participants perform as accurately as typical controls (e.g. Castelli 2005; Lacroix et al. 2009).
While some variation between the results of individual studies could be accounted for by different study designs, another important explanation could be that the autistic people in these studies, whose expression recognition as a group was compared with controls, varied in their expression recognition ability. In support of this possibility, recent evidence has shown considerable heterogeneity in expression recognition ability within the autistic population, with some autistic people showing substantial difficulty and others performing no differently to typical controls (Loth et al. 2018). This variation in expression recognition ability might reflect meaningful variation as a function of an individual’s unique symptom profile. Autism is a heterogeneous disorder, with wide variation in strengths and difficulties among autistic individuals such that an individual might report more difficulty, for example, with social communication than with restrictive repetitive behaviours. This heterogeneity is well established, with large scale studies finding that the phenotypic expressions of different features of autism only correlate modestly with each other (Ronald et al. 2005, 2006). This observation has led to suggestions that research should routinely study different symptom profiles separately, because attempts to characterise universal difficulties experienced by autistic people have been unsuccessful (Happé et al. 2006).
The importance of taking symptom profiles into account is supported by the finding that subsets of autistic symptoms appear to have distinct genetic aetiologies (Ronald et al. 2005, 2006). However, within these subsets, autistic symptoms appear to share a common aetiology with sub-clinical autistic traits (Ronald et al. 2006). The broader autism phenotype describes the distribution of autistic traits that correspond to milder levels of autistic symptoms throughout the typical population, with autism spectrum disorder lying at the extreme end of this distribution (Baron-Cohen et al. 2001; Lundström et al. 2012). Individual differences in autistic traits can be measured both in the typical population and in people with autism. In the broader autism phenotype in the typical population, three domains of autistic traits have been identified from several large factor analyses of the Autism Spectrum Quotient (AQ; Austin2005; Baron-Cohen et al. 2001; Hurst et al. 2007; Russell-Smith et al. 2011). The first two domains resemble the diagnostic criterion for Autism Spectrum Disorder of difficulty with social communication and social interaction. Poor communication and mind reading describes difficulties in understanding others’ intentions, nonverbal cues, and the non-literal meaning of speech. Poor social skills describes reduced interest in peers and difficulties in keeping and maintaining relationships, and adapting behaviour to different social contexts. The third domain, attention to details and patterns, describes the detail-oriented processing style common in autistic people. This processing style has been hypothesised to have the same root cause as the second diagnostic criterion of restrictive, repetitive patterns of behaviour (Happé and Frith 2006).
Measuring these different domains of autistic traits in the typical population might increase our understanding of which aspects of the autism phenotype are associated with poorer expression recognition, because it allows sufficiently large samples with large variation in levels of autistic traits to adequately assess different domains. In contrast, in autistic samples, even with clear, meaningful variation in autistic symptom profiles, the extent of this variation will necessarily be restricted because of the range of difficulties required for diagnosis. For example, an individual autistic person might experience much greater difficulty with social skills than with social communication, but every autistic person must have some difficulty with social communication to have met diagnostic criteria. Studying different domains of autistic traits in the typical population has previously clarified the relationship between face processing and autistic traits. For example, poorer recognition of face identity has been associated with autistic traits related to a “Social Interaction” domain (Hoekstra et al. 2008), which comprises items relating to both communication and social skills, but not attention to detail (Davis et al. 2017; Rhodes et al. 2013). Therefore, it is possible that the relationship between autistic traits and expression recognition ability varies in a similar manner.
In addition to determining which autistic individuals are likely to experience expression recognition difficulty, a second important question is which factors underlie this expression recognition difficulty. Examination of individual differences in the broader autism phenotype can provide insight into this question, via the measurement of individual differences in potentially mediating variables. A range of personality, perceptual and behavioural factors have been suggested as explanations for difficulty in expression recognition in autism (for review see Uljarevic and Hamilton 2013). Here we investigate two factors that have been hypothesised to be key contributors to the experience of autistic people and could be expected to underlie expression recognition difficulty. The first, alexithymia, is a personality trait characterised by a limited ability to identify and describe internal emotional experience. According to the ‘alexithymia hypothesis,’ the emotional symptoms of autism, including difficulty in expression recognition, reflect co-occurring alexithymia rather than autism per se, such that they are only experienced by the subset of autistic people (approximately half; Kinnaird et al. 2019) who also experience significant levels of alexithymia (Bird and Cook 2013). Approximately 10% of the typical population (Taylor et al. 1999) also show clinically significant levels of alexithymia. Importantly, higher levels of alexithymia are associated with poorer expression recognition, in both the typical population (for review see Grynberg et al. 2012) and people with autism (Cook et al. 2013; Milosavljevic et al. 2016).
Two previous studies have measured the contribution of alexithymia to expression recognition ability in the context of autistic traits using the Twenty-Item Toronto Alexithymia Scale (TAS-20; Bagby et al. 1994) and the AQ (Baron-Cohen et al. 2001). The first measured both perceptual discrimination and labelling of expressions. The perceptual discrimination task involved determining which of two emotions—surprise vs. fear or disgust vs. anger—was displayed in morphed stimuli with varying percentages of each emotion (Cook et al. 2013). This task was designed to be a measure of the perceptual aspect of expression recognition, and would not necessarily require emotional knowledge to complete, because expressions could be discriminated on the basis of consistent visual differences even without knowledge of what emotion they are conveying. The labelling task was designed to be a measure of the ability to match emotion concepts to perceptual representations, in addition to perceptual ability. Results indicated that in autistic adults (n = 16), as well as in controls (n = 16), neither autistic traits nor alexithymia were associated with poorer perceptual discrimination. Further, autistic traits were not associated with expression labelling accuracy once the effect of alexithymia was accounted for, implying that expression recognition difficulty is not a feature of autism but of co-occurring alexithymia (Cook et al. 2013). In contrast, a second study with typical individuals (N = 389) found that autistic traits predicted facial expression labelling ability independently of alexithymia (Lewis et al. 2016). Although we note that this study used a brief, ten-item version of the AQ (AQ-10; Allison et al. 2012) developed as a screening measure, that might not capture the same individual differences in the broader autism phenotype as the AQ. This study did not measure perceptual discrimination of expressions.
It is important to understand whether alexithymia can fully account for expression recognition difficulty in autism, because taken together with evidence of the role of alexithymia in driving other emotional symptoms of autism (such as difficulty with empathy), this finding has led to arguments that the diagnostic criteria for autism should be revised (Cook et al. 2013). However, neither of the above studies analysed different domains of autistic traits separately, which we will do here. It is reasonable to expect that the presence of alexithymia and any associated impact on expression recognition ability might vary between symptom domains, based on the genetic and phenotypic variation between these domains and the observation that only a subgroup of autistic people show significant levels of alexithymia.
A second factor which has been identified as a potential characteristic of autism leading to a range of difficulties is differences in the nature of perceptual processing. There is increasing interest in perception in autism, with some theories suggesting that perception in autistic people is characterised by differences in reliance on prior perceptual experience (Lawson et al. 2014; Pellicano and Burr 2012; Van de Cruys et al. 2014). One key perceptual process for expression perception, adaptive norm-based coding, relies on the use of prior perceptual experience. Adaptive norm-based coding is a perceptual process where faces are encoded by how they differ from a norm. The norm, which represents the average expression, is constantly recalibrated by visual experience. Adaptive norm-based coding likely facilitates expression recognition by calibrating facial expression perception to suit current demands (Burton et al. 2015). The strength of adaptive coding can be measured by testing the degree to which participants experience perceptual aftereffects, where exposure to a stimulus biases perception of subsequent stimuli away from this initial stimulus. The strength of adaptive norm-based coding of expression varies among typical adults, and stronger adaptive norm-based coding of expression is associated with better expression recognition (Palermo et al. 2018; Rhodes et al. 2015).
Research into whether adaptive norm-based coding of expression is weakened in autistic people has produced mixed results. In one study, children and adolescents with autism showed weaker adaptive norm-based coding of expression than a typically-developing control group (Rhodes et al. 2017). This weakened adaptive norm-based coding showed a numerical association with greater severity scores on the Autism Diagnostic Observation Schedule (Lord et al. 2012) and Social Communication Questionnaire (Lord and Rutter 2003), though these associations were non-significant in the relatively small sample. However, another study reported no reduction in the strength of adaptive coding of expression in adults with autism (Cook et al. 2014). One explanation for these inconsistent results is that the development of adaptive norm-based coding might only be delayed in autism, so that reduced adaptive norm-based coding is observed in children but not adults.
Alternatively, the strength of adaptive coding might vary between autistic symptom domains. In typical men, reduced adaptive coding of face identity is associated with traits relating to poor social skills but not attention to detail (Rhodes et al. 2013). It would therefore be reasonable to expect that weakened adaptive norm-based coding of expression might also only be present in one or both of the social domains (poor communication and mind reading and poor social skills), and that results of the above studies varied because of varying severities of these symptoms in their samples. Finally, given the possibility that alexithymia might drive expression recognition difficulty in the autism phenotype, it is possible that alexithymia is also a driving factor in weakened adaptive coding of expression. Results could therefore have varied based on varying levels of alexithymia in different samples.
In the present study we aimed to identify the domains of autistic traits, as measured by the AQ, that are associated with difficulty in expression recognition in typical individuals. Importantly, we also sought to identify potential sources underlying this difficulty by measuring individual differences in alexithymia, as measured by the TAS-20, and adaptive norm-based coding, as measured by an aftereffect task (Rhodes et al. 2015) and determine the degree to which each could independently account for associations between three domains of autistic traits and expression recognition ability in typical adults. In order to measure both perceptual discrimination and labelling of facial expressions, we used a matching and a labelling task. Performance on these tasks correlates moderately, and this is argued to reflect that perceptual discrimination and labelling of expressions reflect partly overlapping and partly distinct processes (Palermo et al. 2013). We separately measured the mediating effects of alexithymia and adaptive norm-based coding on each task. Depression and anxiety are also often associated with autistic traits (Lundström et al. 2011) and alexithymia (Berthoz et al. 1999; Honkalampi et al. 2000) and may affect performance on expression tasks (Demenescu et al. 2010). We measured these to ensure results were not confounded by these additional factors.
Method
Participants
One hundred and fifty-two participants completed the measures. Most (72%) were students, friends and family members recruited by third-year psychology students enrolled in a research skills class at the University of Western Australia. The remainder were students enrolled in a first-year psychology unit, who participated for course credit. All reported normal or corrected to normal vision. This research was approved by the University of Western Australia Human Research Ethics Office and all participants provided informed consent.
Seven participants were excluded from the final sample because experimenter comments indicated that the participant was not attending to the tasks (n = 2), because computer or experimenter error resulted in lost data on one or more of the measures (n = 3), or because the participant failed to meet minimum performance criteria on the task measuring adaptive norm-based coding, described in more detail in the “Method” section below (n = 2). The final sample consisted of 145 participants (99 female, age range 17–43 years, M = 21.16).
Tasks
Expression Labelling Ability: Labelling Task
Expression labelling ability was measured using the expression labelling task described in Palermo et al. (2018). Participants were presented with a face displaying a fearful, happy, sad, angry, disgusted or surprised expression for 400 ms. Face images could be front-on or a ¾ view. Participants used a mouse to choose which one of 6 emotion labels best represented the expression, within 7 s. Following four practice trials, there were 24 trials for each of the 6 expressions for a total of 144 trials. Scores were calculated as the percentage of expressions labelled correctly overall.
Expression Perceptual Discrimination Ability: Matching Task
Perceptual discrimination was measured using the 100-item expression matching task from Palermo et al. (2013). Participants were presented with three faces for 4500 ms, of which one was a target expression (happy, sad, angry, disgusted, fearful or surprised), and the other two were an expression commonly confused with the target expression (e.g., happy target and surprised distractors). Faces could be front-on or with ¾ view, each face was a different identity but always the same viewpoint and sex for the triad on each trial. There were a total of 100 trials, preceded by three additional practice trials. Participants were required to pick the ‘odd’ target expression within 11.5 s. Scores were calculated as the percentage of correctly identified ‘odd’ target expressions.
Adaptive Norm-Based Coding of Facial Expression
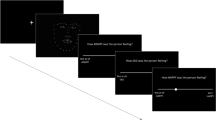
Adaptive norm-based coding is demonstrated by visual aftereffects, where presentation of an initial “adapting” face shifts our perceptual norm towards this face. This shift biases perception of subsequent faces towards the perceptual opposite of the initial face (Rhodes et al. 2015; Skinner and Benton 2010). To create the perceptual opposite of an expression, we can morph a target expression towards an average expression and continue to morph along this trajectory beyond the average expression. This morphing procedure produces an anti-expression that has features that differ from the features of the average expression to the same extent as do those of the target expression, but in the opposite direction—see Fig. 1. Adapting to an anti-expression (e.g. anti-happy) is expected to bias perception towards its perceptual opposite, the target expression (e.g. happy). We used Rhodes et al.’s (2015) expression aftereffect task in which participants adapt to anti-expressions made for four target expressions: fearful, happy, sad, and angry.
Participants first practised discriminating between the four target expressions in two practice phases to familiarise them with the response format. In the first practice phase, participants were required to label two faces displaying each expression presented on screen for an unlimited time for a total of eight trials. For each target expression, one was a weaker, 50% strength expression created by morphing the original face with an average expression; the other was the original 100% strength expression. The second practice phase followed the same format as the first, but faces were presented for 400 ms only. Participants received auditory accuracy feedback during practice. If participants failed to perform at 75% accuracy, this practice phase was repeated up to three times. One participant repeated the second training phase three times but failed to perform at 75% accuracy. However, this participant was not excluded from analyses as they met accuracy requirements throughout the remainder of the task, described below.
Participants then completed 64 adaptation trials. In these trials, participants were first presented with the same adapting anti-expression for 4 × 2 s exposures for a total of 8 s. Following all four exposures of the anti-expression, test faces were presented for 400 ms. Test faces did not differ from the anti-expression in overall luminance by more than 1%, and were 80% the size of the adapting faces to minimize low-level adaptation. To maintain attention to the adapting face, participants were required to indicate whether or not an asterisk appeared between exposures. One participant who performed at chance on this measure was excluded from the final sample, as their performance indicated they may not have attended to the adapting faces. There were 32 trials with the average face as the test face. The expression aftereffect was calculated from these trials as the proportion of trials in which the participant labelled the average test face with the expression that corresponded to the adapting anti-expression, for a maximum aftereffect size of 1. In addition, there were 32 trials where the test face displayed a 100% strength expression that should have been easily identified. These trials were included to maintain motivation. In addition, we could assess accuracy on these “easy” trials to ensure participants were performing the task correctly. One participant who performed at chance on these trials was excluded from the final sample. The mean aftereffect in the full sample was significantly different from chance, M = .58, SD = .14, t(144) = 28.27, p < .001, chance = .25, indicating a significant effect of adaptation in our sample.
Questionnaires
Autistic Traits: The Autism Spectrum Quotient (Baron-Cohen et al. 2001)
The AQ is a widely-used 50-item self report questionnaire designed to measure autistic traits in the typical population. Each item is a descriptive statement and participants rated their agreement with each statement using a 4-point Likert scale from definitely disagree (1) to definitely agree (4).
We measured the three domains of the AQ identified by Austin (2005), Hurst et al. (2007), and Russell-Smith et al. (2011). Given that cultural variation may affect item interpretation (Hurst et al. 2007), we used items for the three domains identified in an Australian sample (Russell-Smith et al. 2011). The domains consist of social skills (social skills; maximum score of 52), communication/mind reading (communication; maximum score of 32) and details/patterns (details and patterns; maximum score of 28). Higher scores on each domain are associated with higher levels of autistic traits in that domain.
We also calculated binary AQ total scores for each participant for the purpose of determining whether any of our participants reached the clinically significant cut-off of 32 identified by Baron-Cohen et al. (2001). Binary scores were calculated by assigning a score of 0 to an item if a participant responded with definitely disagree or slightly disagree, and a score of 1 if the participant responded with definitely agree or slightly agree. The total binary score was calculated as the sum of these scores. Of the final sample, two participants (1.4%) scored at or above the clinically significant cut-off of 32, which is broadly consistent with the rate in the general population reported in the original development of the AQ (2%; Baron-Cohen et al. 2001). These participants were included in the analyses.
Alexithymia: The 20-Item Toronto Alexithymia Scale (Bagby et al. 1994)
The TAS-20 is a 20-item self report scale designed to measure levels of alexithymia using a total score derived from the sum of three scales: difficulty identifying feelings, difficulty describing feelings, and externally oriented thinking1.Footnote 1 Participants rated their agreement with each item on a 5-point Likert scale from strongly disagree (1) to strongly agree (5). The maximum possible score was 100. A total score of 61 or above is associated with clinically significant alexithymia (Bagby et al. 1994). Sixteen participants, 11% of the total sample, met the criteria for alexithymia. This is consistent with the estimated prevalence of alexithymia in the typical population (10%; Taylor et al. 1999) and these participants were included in the final sample.
Depression and Anxiety: The 21-Item Depression Anxiety Stress Scales (DASS-21; Lovibond and Lovibond 1995)
The DASS-21 is a 21-item self-report questionnaire that includes seven items each for three subscales measuring levels of depression, anxiety and stress over the past week. Participants rated their agreement with statements on a 4-point scale from “did not apply to me at all” (0) to “applied to me very much, or most of the time” (3). The maximum possible score on each subscale was 21.
Procedure
All participants completed all tasks in the following order: expression aftereffect task, expression matching task, expression labelling task (and an identity aftereffect task unrelated to the present study that will not be discussed), followed by the questionnaires in the order: AQ, DASS-21 and TAS-20. A single testing session took approximately one hour and fifteen minutes. The expression aftereffect, matching and labelling tasks were completed using Superlab 4 (Cedrus Corporation 2008) on one of two iMacs running OS X 10.5.8 or 10.7.5. Questionnaires were completed online through Qualtrics (2005, Provo, UT, USA; copyright 2019).
Results
Distributions
The data were screened for univariate outliers using the outlier labelling rule described by Hoaglin and Iglewicz (1987). In this rule, the interquartile range is multiplied by 2.2 and added to the third quartile and taken from the first quartile to define a range outside of which data points are considered outliers. Three univariate outliers were identified on the expression labelling task, three on the DASS depression scale and one on the DASS anxiety scale. These outliers were winsorized by replacing them with values 1% higher or lower than the next acceptable value (Tabachnick and Fidell 2007). Skew and kurtosis for each variable indicated all were sufficiently normal for parametric analyses—see Table 1 (skew within |2.0| and kurtosis within |7.0|; West et al. 1995). Minimum and maximum values and standard deviations for each measure indicated the data were free from floor and ceiling effects and showed sufficient range for individual differences analysis (see Table 1). Scores on each of our measures were similar to other reports of these measures in typical samples (Hurst et al. 2007; Palermo et al. 2013, 2018; Parker et al. 2005).
Reliability
Internal consistency reliability for all measures calculated using Cronbach’s alpha is reported in Table 1. Most measures had good to acceptable internal consistency reliability according to George and Mallery’s (2003) guidelines. However, reliability for the AQ domains were not optimal, ranging from good to poor, although modest reliability is consistent with other reports (e.g. Austin2005; Hurst et al. 2007; Russell-Smith et al. 2011).
Sex Differences
Although previous research has reported men scoring more highly on the AQ and TAS-20 than women (Baron-Cohen et al. 2001; Levant et al. 2009; Rhodes et al. 2013), we did not find sex differences on the TAS-20, or the AQ, with the exception of AQ Social Skills (see Table 1). However, women performed significantly more accurately than men on the expression labelling and matching tasks (see independent samples t-tests in Table 1). Therefore, to statistically remove variance related to sex, unstandardized residuals were calculated by regressing scores on AQ Social Skills and the matching and labelling tasks onto sex and used instead of raw scores for these three variables in subsequent analyses.
Effects of Anxiety and Depression
Correlations between the DASS-21 anxiety and depression scales and each of our measures are reported in Table 2. As in previous research (Berthoz et al. 1999; Honkalampi et al. 2000; Lundström et al. 2011), autistic traits and alexithymia were both associated with higher levels of depression and anxiety, though the association between AQ Communication and anxiety, and between AQ Details and Patterns and depression were non-significant. DASS-21 depression and anxiety scales were not significantly associated with either of the expression tasks, or the aftereffect task, suggesting that performance was not affected by levels of depression and anxiety in our sample.
Correlations
We first report correlations to examine bivariate relationships between our measures (Table 3). As our tasks showed varying internal consistency reliability, we calculated disattenuated correlations to correct for measurement error to ensure different effect sizes did not reflect different levels of measurement error (Schmidt and Hunter 1996). These values are used in subsequent analyses.
We observed a negative correlation between both AQ Total and TAS-20 scores and expression labelling but not expression matching. The latter result is consistent with a previous finding that neither autism, as measured by the AQ Total, nor alexithymia, were associated with a perceptual difficulty in expression processing (Cook et al. 2013). Autistic traits, as measured by the AQ Total, and alexithymia were positively associated, again in line with previous research (Cook et al. 2013). We also replicated previous findings that the strength of expression aftereffects was positively associated with performance on both the expression matching and labelling tasks, indicating that stronger adaptive norm-based coding of expression was associated with better expression ability (Palermo et al. 2018; Rhodes et al. 2015).
Turning to our novel findings, we found that the TAS-20 was positively associated with two of the three AQ domains identified by Russell-Smith et al. (2011), AQ Communication and AQ Social Skills. However, AQ Details and Patterns was negatively associated with alexithymia as measured by the TAS-20, though we note that this relationship was marginal prior to correcting for attenuation, p = .062. We found no evidence of any substantial associations between autistic traits and expression aftereffect size in any of the three trait domains. Finally, we found no evidence of a relationship between expression aftereffect size and levels of alexithymia.
Path Analyses
AQ Total and Expression Labelling Performance
Our first aim was to measure the contributions of alexithymia and adaptive norm-based coding to the relationship between autistic traits and expression labelling performance, using path analysis. Figure 2 shows that the indirect effect of alexithymia (TAS-20) largely accounted for the relationship between AQ Total and expression labelling performance. Alexithymia explained 81% of variance in the relationship between AQ Total and expression labelling performance. There was no significant indirect effect of adaptive norm-based coding, and after accounting for the indirect effect of alexithymia and the non-significant indirect effect of adaptive norm-based coding, the remaining direct effect of AQ Total on expression labelling performance (path c) fell below significance (p = .777).
These results are consistent with a previous finding in autistic people and typical controls that AQ Total scores did not account for any variance in expression labelling ability when accounting for alexithymia (Cook et al. 2013). However, our results differ from another previous study that found that scores on a 10-item version of the AQ predicted expression labelling ability independently of alexithymia in the typical population (Lewis et al. 2016).
Our next aim was to examine whether the relationship between autistic traits and expression labelling and expression matching varied between different domains of autistic traits. We found that AQ Communication predicted poorer performance on both expression labelling and matching, though we note that the effect on the matching task was marginal prior to correcting for attenuation, p = .063. AQ Social Skills predicted poorer performance on the expression labelling but not matching task. Finally, AQ Details and Patterns was not substantially or significantly associated with either expression labelling or matching.
We next determined the extent to which alexithymia and adaptive norm-based coding of expression accounted for the relationships that we found between AQ Communication and AQ Social Skills and expression recognition ability.
AQ Communication and Expression Labelling Performance
Figure 3 shows that the indirect effect of alexithymia (TAS-20) largely accounted for the relationship between AQ Communication and expression labelling performance. Alexithymia explained 59% of variance in the relationship between AQ Communication and expression labelling performance. There was no significant indirect effect of adaptive norm-based coding, and after accounting for the indirect effect of alexithymia and the non-significant indirect effect of adaptive norm-based coding, the remaining direct effect of AQ Communication on expression labelling performance (path c) fell below significance (p = .435).
AQ Communication and Expression Matching Performance
Figure 4 shows that there were no significant indirect effects of alexithymia or adaptive norm-based coding which mediated the negative effect of AQ Communication on expression matching performance. However, once the non-significant indirect effects of alexithymia and adaptive norm-based coding were accounted for, the remaining direct effect (path c) of AQ Communication on expression matching performance fell below significance (p = .099).
AQ Social Skills and Expression Labelling Performance
Figure 5 shows that the indirect effect of alexithymia (TAS-20) fully accounted for the relationship between AQ Social Skills and expression labelling performance. There was no significant indirect effect of adaptive norm-based coding, and after accounting for the indirect effect of alexithymia and the non-significant indirect effect of adaptive norm-based coding, there was no remaining negative direct effect of AQ Social Skills on expression labelling performance (path c).
Discussion
Here we demonstrated for the first time that the relationship between autistic traits and facial expression recognition ability differs between subsets of autistic traits. Expression recognition ability, as measured by both perceptual discrimination and labelling, was primarily related to the subset of autistic traits associated with social communication. The subset of traits relating to social skills were associated with more difficulty labelling, but not perceptually discriminating between, facial expressions. Finally, traits relating to attention to detail were not substantially associated with expression recognition ability. These differences across trait domains might explain inconsistencies in previous research on expression recognition in autism and autistic traits. Our findings indicate that performance may have varied as a function of the distribution of trait or symptom profiles in the samples in previous studies, but this variation may not be apparent when using a single overall measure of autistic traits. Further highlighting the importance of considering different autistic symptom profiles, we also show that alexithymia is not uniformly associated across different subsets of autistic traits. Rather, we found that traits relating to social skills and social communication were associated with higher levels of alexithymia, and in contrast, traits relating to attention to detail were associated with lower levels of alexithymia.
Our results provide further evidence that poorer expression labelling in the autism phenotype reflects a substantial effect of co-occurring alexithymia, supporting the ‘alexithymia hypothesis’ (Bird and Cook 2013; Cook et al. 2013). When considering total AQ scores, our results showed a similar pattern to Cook et al. (2013), with autistic traits showing no association with perceptual discrimination ability, and after accounting for alexithymia, no association with expression labelling. Further, only the subsets of autistic traits that were associated with higher levels of alexithymia were also associated with poorer expression labelling, namely Social Skills and Communication. Consistent with the ‘alexithymia hypothesis,’ alexithymia was the primary driving factor in these associations such that no significant variance in expression labelling was explained by autistic traits when alexithymia was accounted for.
However, our results also show that autistic traits are associated with perceptual difficulty with expressions. In the domain of AQ Communication, autistic traits were associated with poorer perceptual discrimination of expressions, and we found no evidence that this association was driven by levels of alexithymia. Perceptual difficulty could play a causal role in social communication difficulties in the autism phenotype. Some accounts suggest that perceptual differences directly contribute to social difficulties in autism, by impeding the ability to read social cues and by rendering social stimuli overwhelmingly complex, motivating autistic people to avoid attending to social information (Robertson and Baron-Cohen 2017). The present results, which suggest perceptual differences in the autism phenotype contribute to the important social skill of expression recognition, highlight the importance of considering the role of perceptual and sensory differences in autistic symptoms. Sensory sensitivities are experienced by the overwhelming majority of autistic people (Robertson and Baron-Cohen 2017)
However, the source of this perceptual difficulty recognising facial expressions is unclear. One theory suggests that perceptual and sensory differences reflect differences in the use of prior perceptual information, such that autistic people are relatively less biased by prior experience when forming perceptual representations (Pellicano and Burr 2012). This would imply that autistic traits could be associated with reduced adaptive norm-based coding, which uses prior perceptual information to tune perception of facial expressions to current demands (Burton et al. 2015). The present results replicate previous research finding that stronger adaptive norm-based coding is associated with better performance on expression tasks (Palermo et al. 2018; Rhodes et al. 2015). However, we found no evidence that autistic traits in any of the three domains were associated with reduced adaptive norm-based coding of expression. Our results therefore do not support the theory that autistic people show less bias from prior experience when forming perceptual representations, in the case of facial expression. This is consistent with one previous study that found that adaptive coding of expression was intact in autistic adults (Cook et al. 2014). However, other research has found reduced adaptive coding of expression in autistic children (Rhodes et al. 2017), suggesting that the development of adaptive coding of expression could be delayed in people with autism such that autistic children but not adults show weaker adaptive coding than their non-autistic peers.
We therefore consider other driving factors that could explain the relationship between autistic traits in the domain of Communication and poorer perceptual discrimination of expressions in adults. One possible factor might be that people with higher levels of autistic traits in this domain give less attention to the eye area of emotional faces. This has been suggested as a potential causal mechanism for facial expression recognition difficulty in autism (Uljarevic and Hamilton 2013), but results of individual studies are mixed (e.g. Boraston et al. 2007; López et al. 2004). Future research might benefit from measuring attention to the eye area as a function of different symptom domains separately. However, any effect of reduced attention to the eye area might be a function of co-occurring alexithymia. In one study, when autistic people passively viewed social films with emotional content, participants’ level of alexithymia, but not the severity of their autistic symptoms, predicted the ratio of attention to actors’ eyes relative to their mouths (Bird et al. 2011).
Another important factor leading to poorer expression recognition performance in people with higher levels of autistic traits might be reduced experience with facial expressions. The social motivation theory suggests that face processing difficulty in autism is a result of experience with faces being limited by reduced interest (Dawson et al. 2005; Schultz 2005). This theory is particularly relevant to autistic traits relating to communication, because difficulty with social communication could be expected to reduce motivation to seek out non-verbal cues from faces. Reduced attention to the eye area could therefore be both a cause and a consequence of difficulty with social communication. Importantly, autism, but not alexithymia, predicts the amount of attention given to faces, despite alexithymia predicting attention to the eye region (Bird et al. 2011). Further, autistic traits are associated with less experience of social reward from interactions, independently of the effect of alexithymia (Foulkes et al. 2015), implying that any effect of social motivation would be an independent contributor to the association between autistic traits and expression recognition ability, rather than simply reflecting co-occurring alexithymia.
We now consider the implications of the current findings for our understanding of expression recognition in autism. The broader autism phenotype in the typical population appears to be continuous with autism and qualitatively similar in aetiology (Colvert et al. 2015; Lundström 2011; Lundström et al. 2012; Ronald et al. 2006). Thus, we would expect that the present findings would extend into expression recognition ability in the autistic population, when considering different symptom profiles separately. Further, our finding that there was no relationship between autistic traits as measured by AQ total scores and expression recognition when accounting for alexithymia replicates a previous study which included a sample of autistic people (Cook et al. 2013). We would therefore expect that autistic people with most difficulties with social skills and social communication should show the most difficulty in facial expression recognition, with labelling, but not perceptual difficulty reflecting alexithymia. Future research with autistic participants would be needed to confirm this hypothesis, because it is possible that qualitatively different relationships could exist at diagnostic levels of autistic traits (for discussion see Gregory and Plaisted-Grant 2016). We also note that the analyses presented here do not demonstrate causal relationships.
A further potential limitation in the present study and other research in this area is the use of self-report measures to measure autistic traits and alexithymia, given the potential for difficulty with introspection to limit the validity of self-report measures. Future research might benefit from using other methods in addition to self-report. However, we note that self-report scores on the TAS-20 correlate with interviewer scores on the Toronto Structured Interview for Alexithymia in both control and clinical populations (TSIA; Bagby et al. 2006), and the AQ discriminates well between people with and without clinical diagnoses of autism (Hoekstra et al. 2008), suggesting that the TAS-20 and AQ are valid measures of alexithymia and autistic traits despite using self-report.
Finally, consistent with two previous studies (Cook et al. 2013; Fujiwara 2018), we found no evidence that alexithymia is associated with a perceptual difficulty processing facial expressions, with alexithymia predicting poorer performance on the labelling but not perceptual discrimination task. This finding seems plausible given that alexithymia is characterised by a difficulty in attending to and evaluating emotions (Preece et al. 2017). It follows that this difficulty in attending to and evaluating emotions could also produce difficulty recognising others’ emotions, in the absence of a perceptual difficulty with expression recognition. However, studies which have measured expression perception under impoverished viewing conditions (visual degradation or very brief stimulus presentation time), have found that higher alexithymia is associated with poorer perceptual discrimination (e.g. Parker et al. 2005; Prkachin et al. 2009). It is possible, therefore, that alexithymia is associated with relatively subtle difficulty in expression perception that is revealed only under more difficult viewing conditions than those used in the present study.
In conclusion, the present results suggest that the relationship between autistic traits and expression recognition reflects both variation in alexithymia and perceptual difficulty. This perceptual difficulty was independent of alexithymia, suggesting that poorer expression ability in the broader autism phenotype has at least two independent sources. Further, we found no evidence that this perceptual difficulty reflects reduced adaptive norm-based coding of expression, leaving an open question as to the precise mechanism. Finally, our results show that the association between autistic traits and both expression recognition and alexithymia varies meaningfully between trait domains, highlighting the importance of considering these domains separately.
Notes
Here we treat alexithymia as a unitary construct, as in previous research into autistic traits, alexithymia and expression recognition (Cook et al. 2013)
References
Adams, R. B., Ambady, N., Macrae, C. N., & Kleck, R. E. (2006). Emotional expressions forecast approach-avoidance behavior. Motivation and Emotion, 30(2), 177–186. https://doi.org/10.1007/s11031-006-9020-2.
Allison, C., Auyeung, B., & Baron-Cohen, S. (2012). Toward brief “red flags” for autism screening: The short autism spectrum quotient and the short quantitative checklist in 1,000 cases and 3,000 controls. Journal of the American Academy of Child & Adolescent Psychiatry, 51(2), 202–212.e207. https://doi.org/10.1016/j.jaac.2011.11.003.
APA. (2013). Diagnostic and statistical manual of mental disorders (DSM-5 ® ). Philadelphia: American Psychiatric Publishing.
Austin, E. J. (2005). Personality correlates of the broader autism phenotype as assessed by the autism spectrum quotient (AQ). Personality and Individual Differences, 38(2), 451–460. https://doi.org/10.1016/j.paid.2004.04.022.
Bagby, R. M., Parker, J. D. A., & Taylor, G. J. (1994). The twenty-item Toronto Alexithymia Scale—I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. https://doi.org/10.1016/0022-3999(94)90005-1.
Bagby, R. M., Taylor, G. J., Parker, J. D., & Dickens, S. E. (2006). The development of the Toronto Structured Interview for Alexithymia: Item selection, factor structure, reliability and concurrent validity. Psychotherapy and Psychosomatics, 75(1), 25–39. https://doi.org/10.1159/000089224.
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001). The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. https://doi.org/10.1023/A:1005653411471.
Berthoz, S., Consoli, S., Perez-Diaz, F., & Jouvent, R. (1999). Alexithymia and anxiety: Compounded relationships? A psychometric study. European Psychiatry, 14(7), 372–378. https://doi.org/10.1016/S0924-9338(99)00233-3.
Bird, G., & Cook, R. (2013). Mixed emotions: The contribution of alexithymia to the emotional symptoms of autism. Translational Psychiatry, 3(7), e285. https://doi.org/10.1038/tp.2013.61.
Bird, G., Press, C., & Richardson, D. C. (2011). The role of alexithymia in reduced eye-fixation in autism spectrum conditions. Journal of Autism and Developmental Disorders, 41(11), 1556–1564. https://doi.org/10.1007/s10803-011-1183-3.
Boraston, Z., Blakemore, S. J., Chilvers, R., & Skuse, D. (2007). Impaired sadness recognition is linked to social interaction deficit in autism. Neuropsychologia, 45(7), 1501–1510. https://doi.org/10.1016/j.neuropsychologia.2006.11.010.
Burton, N., Jeffery, L., Calder, A. J., & Rhodes, G. (2015). How is facial expression coded? Journal of Vision, 15(1), 1–13. https://doi.org/10.1167/15.1.1.
Castelli, F. (2005). Understanding emotions from standardized facial expressions in autism and normal development. Autism, 9(4), 428–449. https://doi.org/10.1177/1362361305056082.
Cedrus Corporation. (2008). SuperLab 4.0. San Pedro, CA: Cedrus Corporation.
Colvert, E., Tick, B., McEwen, F., Stewart, C., Curran, S. R., Woodhouse, E., et al. (2015). Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry, 72(5), 415–423. https://doi.org/10.1001/jamapsychiatry.2014.3028.
Cook, R., Brewer, R., Shah, P., & Bird, G. (2013). Alexithymia, not autism, predicts poor recognition of emotional facial expressions. Psychological Science, 24(5), 723–732. https://doi.org/10.1177/0956797612463582.
Cook, R., Brewer, R., Shah, P., & Bird, G. (2014). Intact facial adaptation in autistic adults. Autism Research, 7(4), 481–490. https://doi.org/10.1002/aur.1381.
Corbett, B. A., Carmean, V., Ravizza, S., Wendelken, C., Henry, M. L., Carter, C., et al. (2009). A functional and structural study of emotion and face processing in children with autism. Psychiatry Research: Neuroimaging, 173(3), 196–205. https://doi.org/10.1016/j.pscychresns.2008.08.005.
Davis, J., McKone, E., Zirnsak, M., Moore, T., O’Kearney, R., Apthorp, D., et al. (2017). Social and attention-to-detail subclusters of autistic traits differentially predict looking at eyes and face identity recognition ability. British Journal of Psychology, 108(1), 191–219. https://doi.org/10.1111/bjop.12188.
Dawson, G., Webb, S. J., & McPartland, J. (2005). Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology, 27(3), 403–424. https://doi.org/10.1207/s15326942dn2703_6.
Demenescu, L. R., Kortekaas, R., den Boer, J. A., & Aleman, A. (2010). Impaired attribution of emotion to facial expressions in anxiety and major depression. PLoS ONE, 5(12), e15058. https://doi.org/10.1371/journal.pone.0015058.
Ekman, P., Rolls, E. T., Perrett, D. I., & Ellis, H. D. (1992). Facial expressions of emotion: An old controversy and new findings. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 335(1273), 63–69. https://doi.org/10.1098/rstb.1992.0008.
Foulkes, L., Bird, G., Gökçen, E., McCrory, E., & Viding, E. (2015). Common and distinct impacts of autistic traits and alexithymia on social reward. PLoS ONE, 10(4), e0121018. https://doi.org/10.1371/journal.pone.0121018.
Fujiwara, E. (2018). Looking at the eyes interferes with facial emotion recognition in alexithymia. Journal of Abnormal Psychology, 127(6), 571. https://doi.org/10.1037/abn0000361.
George, D., & Mallery, M. (2003). Using SPSS for Windows step by step: A simple guide and reference (4th ed.). Boston: Allyn & Bacon.
Gregory, B., & Plaisted-Grant, K. (2016). The autism-spectrum quotient and visual search: Shallow and deep autistic endophenotypes. Journal of Autism and Developmental Disorders, 46(5), 1503–1512. https://doi.org/10.1007/s10803-013-1951-3.
Grynberg, D., Chang, B., Corneille, O., Maurage, P., Vermeulen, N., Berthoz, S., et al. (2012). Alexithymia and the processing of emotional facial expressions (EFEs): Systematic review, unanswered questions and further perspectives. PLoS ONE, 7(8), e42429. https://doi.org/10.1371/journal.pone.0042429.
Happé, F., & Frith, U. (2006). The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders, 36(1), 5–25. https://doi.org/10.1007/s10803-005-0039-0.
Happé, F., Ronald, A., & Plomin, R. (2006). Time to give up on a single explanation for autism. Nature Neuroscience, 9(10), 1218. https://doi.org/10.1038/nn1770.
Hoaglin, D. C., & Iglewicz, B. (1987). Fine-tuning some resistant rules for outlier labeling. Journal of the American Statistical Association, 82(400), 1147–1149. https://doi.org/10.2307/2289392.
Hoekstra, R. A., Bartels, M., Cath, D. C., & Boomsma, D. I. (2008). Factor structure, reliability and criterion validity of the autism-spectrum quotient (AQ): A study in Dutch population and patient groups. Journal of Autism and Developmental Disorders, 38(8), 1555–1566. https://doi.org/10.1007/s10803-008-0538-x.
Honkalampi, K., Hintikka, J., Tanskanen, A., Lehtonen, J., & Viinamäki, H. (2000). Depression is strongly associated with alexithymia in the general population. Journal of Psychosomatic Research, 48(1), 99–104. https://doi.org/10.1016/S0022-3999(99)00083-5.
Humphreys, K., Minshew, N., Leonard, G. L., & Behrmann, M. (2007). A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia, 45(4), 685–695. https://doi.org/10.1016/j.neuropsychologia.2006.08.003.
Hurst, R. M., Mitchell, J. T., Kimbrel, N. A., Kwapil, T. K., & Nelson-Gray, R. O. (2007). Examination of the reliability and factor structure of the autism spectrum quotient (AQ) in a non-clinical sample. Personality and Individual Differences, 43(7), 1938–1949. https://doi.org/10.1016/j.paid.2007.06.012.
Kinnaird, E., Stewart, C., & Tchanturia, K. (2019). Investigating alexithymia in autism: A systematic review and meta-analysis. European Psychiatry, 55, 80–89. https://doi.org/10.1016/j.eurpsy.2018.09.004.
Lacroix, A., Guidetti, M., Rogé, B., & Reilly, J. (2009). Recognition of emotional and nonemotional facial expressions: A comparison between Williams syndrome and autism. Research in Developmental Disabilities, 30(5), 976–985. https://doi.org/10.1016/j.ridd.2009.02.002.
Lawson, R. P., Rees, G., & Friston, K. J. (2014). An aberrant precision account of autism. Frontiers in Human Neuroscience, 8, 302. https://doi.org/10.3389/fnhum.2014.00302.
Levant, R. F., Hall, R. J., Williams, C. M., & Hasan, N. T. (2009). Gender differences in alexithymia. Psychology of Men & Masculinity, 10(3), 190. https://doi.org/10.1037/a0015652.
Lewis, G. J., Lefevre, C. E., & Young, A. W. (2016). Functional architecture of visual emotion recognition ability: A latent variable approach. Journal of Experimental Psychology: General, 145(5), 589. https://doi.org/10.1037/xge0000160.
López, B., Donnelly, N., Hadwin, J., & Leekam, S. (2004). Face processing in high-functioning adolescents with autism: Evidence for weak central coherence. Visual Cognition, 11(6), 673–688. https://doi.org/10.1080/13506280344000437.
Lord, C., & Rutter, M. (2003). Social communication questionnaire (SCQ). Torrance, CA: Western Psychological Services.
Lord, C., Rutter, M., DiLavore, P., Risi, S., Gotham, K., & Bishop, S. (2012). Autism diagnostic observation schedule second edition (ADOS-2) manual (part 1): Modules 1–4. Torrance, CA: Western Psychological Services.
Loth, E., Garrido, L., Ahmad, J., Watson, E., Duff, A., & Duchaine, B. (2018). Facial expression recognition as a candidate marker for autism spectrum disorder: How frequent and severe are deficits? Molecular Autism, 9(1), 7. https://doi.org/10.1186/s13229-018-0187-7.
Lovibond, S. H., & Lovibond, P. F. (1995). Manual for the depression anxiety stress scales (DASS). Sydney: Psychology Foundation of Australia.
Lozier, L. M., Vanmeter, J. W., & Marsh, A. A. (2014). Impairments in facial affect recognition associated with autism spectrum disorders: A meta-analysis. Development and Psychopathology, 26(4ptl), 933–945. https://doi.org/10.1017/s0954579414000479.
Lundström, S. (2011). Autistic-like traits. Lund: Department of Clinical Sciences, Lund University.
Lundström, S., Chang, Z., Kerekes, N., Gumpert, C. H., Råstam, M., Gillberg, C., et al. (2011). Autistic-like traits and their association with mental health problems in two nationwide twin cohorts of children and adults. Psychological Medicine, 41(11), 2423–2433. https://doi.org/10.1017/S0033291711000377.
Lundström, S., Chang, Z., Råstam, M., Gillberg, C., Larsson, H., Anckarsäter, H., et al. (2012). Autism spectrum disorders and autistic-like traits: Similar etiology in the extreme end and the normal variation. Archives of General Psychiatry, 69(1), 46–52. https://doi.org/10.1001/archgenpsychiatry.2011.144.
Milosavljevic, B., Leno, V. C., Simonoff, E., Baird, G., Pickles, A., Jones, C. R., et al. (2016). Alexithymia in adolescents with autism spectrum disorder: Its relationship to internalising difficulties, sensory modulation and social cognition. Journal of Autism and Developmental Disorders, 46(4), 1354–1367. https://doi.org/10.1007/s10803-015-2670-8.
Palermo, R., Jeffery, L., Lewandowsky, J., Fiorentini, C., Irons, J. L., Dawel, A., et al. (2018). Adaptive face coding contributes to individual differences in facial expression recognition independently of affective factors. Journal of Experimental Psychology: Human Perception and Performance, 44(4), 503–517. https://doi.org/10.1037/xhp0000463.
Palermo, R., O’Connor, K. B., Davis, J. M., Irons, J., & McKone, E. (2013). New tests to measure individual differences in matching and labelling facial expressions of emotion, and their association with ability to recognise vocal emotions and facial identity. PLoS ONE, 8(6), e68126. https://doi.org/10.1371/journal.pone.0068126.
Parker, P. D., Prkachin, K. M., & Prkachin, G. C. (2005). Processing of facial expressions of negative emotion in alexithymia: The influence of temporal constraint. Journal of Personality, 73(4), 1087–1107. https://doi.org/10.1111/j.1467-6494.2005.00339.x.
Pellicano, E., & Burr, D. (2012). When the world becomes ‘too real’: A Bayesian explanation of autistic perception. Trends in Cognitive Sciences, 16(10), 504–510. https://doi.org/10.1016/j.tics.2012.08.009.
Preece, D., Becerra, R., Allan, A., Robinson, K., & Dandy, J. (2017). Establishing the theoretical components of alexithymia via factor analysis: Introduction and validation of the attention-appraisal model of alexithymia. Personality and Individual Differences, 119, 341–352. https://doi.org/10.1016/j.paid.2017.08.003.
Prkachin, G. C., Casey, C., & Prkachin, K. M. (2009). Alexithymia and perception of facial expressions of emotion. Personality and Individual Differences, 46(4), 412–417. https://doi.org/10.1016/j.paid.2008.11.010.
Qualtrics (2005; copyright 2019). Qualtrics (Version 2016–2017). Provo, UT, USA: Qualtrics.
Rhodes, G., Burton, N., Jeffery, L., Read, A., Taylor, L., & Ewing, L. (2017). Facial expression coding in children and adolescents with autism: Reduced adaptability but intact norm-based coding. British Journal of Psychology, 109(2), 204–218. https://doi.org/10.1111/bjop.12257.
Rhodes, G., Jeffery, L., Taylor, L., & Ewing, L. (2013). Autistic traits are linked to reduced adaptive coding of face identity and selectively poorer face recognition in men but not women. Neuropsychologia, 51(13), 2702–2708. https://doi.org/10.1016/j.neuropsychologia.2013.08.016.
Rhodes, G., Jeffery, L., Taylor, L., Hayward, W. G., & Ewing, L. (2014). Individual differences in adaptive coding of face identity are linked to individual differences in face recognition ability. Journal of Experimental Psychology: Human Perception and Performance, 40(3), 897. https://doi.org/10.1037/a0035939.
Rhodes, G., Pond, S., Burton, N., Kloth, N., Jeffery, L., Bell, J., et al. (2015). How distinct is the coding of face identity and expression? Evidence for some common dimensions in face space. Cognition, 142, 123–137. https://doi.org/10.1016/j.cognition.2015.05.012.
Robertson, C. E., & Baron-Cohen, S. (2017). Sensory perception in autism. Nature Reviews Neuroscience, 18(11), 671. https://doi.org/10.1038/nrn.2017.112.
Ronald, A., Happé, F., Bolton, P., Butcher, L. M., Price, T. S., Wheelwright, S., et al. (2006). Genetic heterogeneity between the three components of the autism spectrum: A twin study. Journal of the American Academy of Child and Adolescent Psychiatry, 45(6), 691–699. https://doi.org/10.1097/01.chi.0000215325.13058.9d.
Ronald, A., Happé, F., & Plomin, R. (2005). The genetic relationship between individual differences in social and nonsocial behaviours characteristic of autism. Developmental Science, 8(5), 444–458. https://doi.org/10.1111/j.1467-7687.2005.00433.x.
Russell-Smith, S. N., Maybery, M. T., & Bayliss, D. M. (2011). Relationships between autistic-like and schizotypy traits: An analysis using the autism spectrum quotient and Oxford-Liverpool inventory of feelings and experiences. Personality and Individual Differences, 51(2), 128–132. https://doi.org/10.1016/j.paid.2011.03.027.
Schmidt, F. L., & Hunter, J. E. (1996). Measurement error in psychological research: Lessons from 26 research scenarios. Psychological Methods, 1(2), 199. https://doi.org/10.1037/1082-989X.1.2.199.
Schultz, R. T. (2005). Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23(2–3), 125–141. https://doi.org/10.1016/j.ijdevneu.2004.12.012.
Skinner, A. L., & Benton, C. P. (2010). Anti-expression aftereffects reveal prototype-referenced coding of facial expressions. Psychological Science, 21(9), 1248–1253. https://doi.org/10.1177/0956797610380702.
Tabachnick, B. G., & Fidell, L. S. (2007). Using multivariate statistics. Boston, MA: Pearson Education.
Taylor, G. J., Bagby, R. M., & Parker, J. D. (1999). Disorders of affect regulation: Alexithymia in medical and psychiatric illness. Cambridge, NY: Cambridge University Press.
Uljarevic, M., & Hamilton, A. (2013). Recognition of emotions in autism: A formal meta-analysis. Journal of Autism and Developmental Disorders, 43(7), 1517–1526. https://doi.org/10.1007/s10803-012-1695-5.
Van de Cruys, S., Evers, K., Van der Hallen, R., Van Eylen, L., Boets, B., de-Wit, L., et al. (2014). Precise minds in uncertain worlds: Predictive coding in autism. Psychological Review, 121(4), 649. https://doi.org/10.1037/a0037665.
West, S. G., Finch, J. F., & Curran, P. J. (1995). Structural equation models with nonnormal variables: Problems and remedies. In R. H. Hoyle (Ed.), Structural equation modeling: Concepts, issues, and applications (pp. 56–75). Thousand Oaks, CA: Sage.
Acknowledgements
We thank Murray Mayberry, Gilles E. Gignac, Markus Neumann and Laura M. Engfors for their valuable advice. We also thank the participants and undergraduate students who contributed to data collection. EB conceived of the study, participated in its design, collected some data, performed the statistical analysis and drafted the manuscript; RP conceived of the study, participated in its design and helped to draft and edit the manuscript; GR conceived of the study and participated in its design; NB conceived of the study, participated in its design and coordinated most of the data collection; LJ conceived of the study; participated in its design and helped to draft and edit the manuscript. All authors read and approved the final manuscript.
Funding
This research is supported by Australian Research Council Centre of Excellence Grant CE110001021 and a Research Training Program Stipend to EB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bothe, E., Palermo, R., Rhodes, G. et al. Expression Recognition Difficulty Is Associated with Social But Not Attention-to-Detail Autistic Traits and Reflects Both Alexithymia and Perceptual Difficulty. J Autism Dev Disord 49, 4559–4571 (2019). https://doi.org/10.1007/s10803-019-04158-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-019-04158-y