Abstract
We evaluated the efficacy of vitamin D (VID), omega-3 long chain polyunsaturated fatty acids (omega-3 LCPUFA, OM), or both (VIDOM) on core symptoms of ASD. New Zealand children with ASD (n = 73; aged 2.5–8.0 years) received daily 2000 IU vitamin D3, 722 mg docosahexaenoic acid, both, or placebo. Outcome measures were Social Responsiveness Scale (SRS) and Sensory Processing Measure (SPM). Of 42 outcome measures comparisons (interventions vs. placebo), two showed greater improvements (P = 0.03, OM and VIDOM for SRS-social awareness) and four showed trends for greater improvements (P < 0.1, VIDOM for SRS-social communicative functioning, OM for SRS-total, VIDOM for SPM-taste/smell and OM for SPM-balance/motion). Omega-3 LCPUFA with and without vitamin D may improve some core symptoms of ASD but no definitive conclusions can be made.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder encompassing impairment in social and communicative functioning and repetitive/stereotypic interests and behaviours (RRB), with a wide range of variety of symptoms (American Psychiatric Association 2013). Sensory issues are considered as a distinctive criterion for RRB (American Psychiatric Association 2013). The symptoms clinically cause significant impairment in social, occupational and other functioning (American Psychiatric Association 2013). It is generally agreed that ASD can be caused by a combination of genetic and environmental factors, although specific causes are not clearly understood. Accordingly, there are no well-documented, mechanism-based pharmacological treatment agents, and those prescribed focus mainly on co-occurring problem behaviours (e.g. irritability and hyperactivity) rather than core symptoms of ASD (e.g. social communication and RRB) (Hendren 2013; McPheeters et al. 2011). Therefore, many parents/caregivers of children with ASD turn towards complementary and alternative treatments including vitamin D and omega-3 long chain polyunsaturated fatty acids (omega-3 LCPUFA), because they are dietary supplements and their administration, in appropriate doses, to children are considered to be safe and well-tolerated (Mazahery et al. 2017; Saad et al. 2018), and may increase the efficacy of pharmacological medications (Al-Haidar 2008).
There is accumulating data to support that vitamin D and omega-3 LCPUFA (especially eicosapentaenoic acid, EPA and docosahexaenoic acid, DHA) are important for brain function and structure and for neurotransmitter and glutamatergic systems, and both have immunomodulatory, anti-inflammatory and anti-oxidant properties (Cass et al. 2006; Mazahery et al. 2016a, b, 2017; Patrick and Ames 2015; Tang et al. 2016), making these nutrients powerful candidates, either alone or in combination, for the management of ASD.
Several reports suggest children with ASD have inadequate intakes of vitamin D and omega-3 LCPUFA (Bandini et al. 2010; Emond et al. 2010; Graf-Myles et al. 2013; Hertz-Picciotto et al. 2010; Marí-Bauset et al. 2015), as is reflected in significantly lower vitamin D and/or omega-3 LCPUFA status than their healthy counterparts (Mazahery et al. 2017; Wang et al. 2016).
Evidence to support the use of vitamin D, omega-3 LCPUFA, or both for the treatment of core symptoms of ASD is either inconclusive, inadequate or lacking (Mazahery et al. 2016, 2017). A recent review of evidence linking vitamin D and ASD identified six intervention trials with inconsistent findings (Mazahery et al. 2016); while some of the identified studies showed positive effects of vitamin D on core symptoms (Feng et al. 2017; Humble et al. 2010; Jia et al. 2015; Saad et al. 2015), others failed to confirm such effects (Azzam et al. 2015; Ucuz et al. 2015). Two more randomised controlled trials have been published since this review, and again the results are mixed (Kerley et al. 2017; Saad et al. 2018). Similarly, the results of omega-3 LCPUFA intervention trials in ASD have been somewhat variable; while all open label intervention trials (including one case study) along with three RCTs showed beneficial effects on some core symptoms of ASD (Amminger et al. 2007; Bent et al. 2014; Johnson et al. 2010; Johnson and Hollander 2003; Meguid et al. 2008; Meiri et al. 2009; Ooi et al. 2015; Patrick and Salik 2005; Yui et al. 2012), others failed to replicate those findings (Bent et al. 2011; Mankad et al. 2015; Voigt et al. 2014). Small sample size has been suggested to be the major limitation of these studies. The pooled analyses of RCTs have demonstrated that omega-3 LCPUFA might improve core symptoms of ASD (lethargy and repetitive behaviours) (Cheng et al. 2017; Horvath et al. 2017; Mazahery et al. 2017), though the authors of these meta-analyses precluded any definitive conclusions due to the limited number of studies, and small sample size and overall effects. To our knowledge there are no studies that have tested the efficacy of combination of vitamin D and omega-3 LCPUFA on ASD symptoms.
The primary objective of the study was to test the hypothesis that supplementation with vitamin D (VID), omega-3 LCPUFA (OM) or both (VIDOM) reduces core symptoms of ASD in children living in New Zealand. The secondary objective of the study was to test the relationship between change in biomarkers of vitamin D (serum 25(OH)D) and omega-3 LCPUFA (omega-3 index) and the change in core symptoms of ASD.
Methods
Study design and data collection methods for this study are described briefly below, with further details reported elsewhere (Mazahery et al. 2016).
The study was registered with the Australian New Zealand Clinical Trial Registry, ACTRN12615000144516. Ethical approval was granted by Health and Disability Ethics Committees, NZ, Reference NO. 14/NTA/113. All parents/caregivers were provided with an information sheet explaining the study protocol in detail and signed an informed consent form.
Study Participants
New Zealand children were included if they were between 2.5 and 8 years, had a medical diagnosis of ASD confirmed by a developmental paediatrician in accordance with the criteria listed in the Diagnostic and Statistical Manual of Mental Disorders, version five (DSM-5) (American Psychiatric Association 2013), and onset of symptoms after 18 months of age. Children were excluded if (1) they were diagnosed as having developmental delay since birth; (2) they failed to take corrective action for nutritional deficiencies; or (3) they had serum 25(OH)D ≥ 75 + 10 nmol/L (≥ 85 nmol/L) if they entered the trial in winter and ≥ 105 nmol/L + 10 nmol/L (≥ 115 nmol/L) if they entered the trial in summer. There is a consensus between the Ministry of Health and Cancer Society of New Zealand (Ministry of Health and Cancer Society of New Zealand 2012) and Institute of Medicine (Institute of Medicine 2011) that the minimum 25(OH)D concentrations for a better health outcome are at least 50 nmol/L. However, the Endocrine Society in the US propose concentrations of 75 nmol/L or more for multiple clinical outcomes (Holick et al. 2011). Two different cut-off points for exclusion were applied due to a large seasonal variation in serum 25(OH)D concentrations in New Zealand ranging from 30 nmol/L (Logan et al. 2013) to 44 nmol/L (Rockell et al. 2008). A 10 nmol/L variation was chosen because of the potential assay variability (Enko et al. 2014).
Study Design
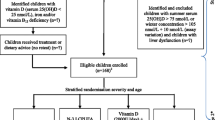
A 12-month randomised placebo controlled double-blind study design was used (Fig. 1). Children who met the initial inclusion criteria had a blood draw and were screened for nutritional deficiencies (including vitamin D, iron and vitamin B12 deficiencies). Prior to randomisation, those deficiencies were addressed. Refer to Table 1 for a list of nutritional deficiencies and the management strategies applied in this trial. Children were then randomly assigned to one of four treatment groups, each consuming four capsules per day for 12 months; vitamin D3 (2000 IU/day), DHA (722 mg/day), both, or placebo. The treatment materials were delivered in 750 mg gel capsules with a tear-off nozzle manufactured and supplied by Douglas Nutrition Ltd, Auckland, NZ. All study capsules were identical in appearance and were tasteless and colourless. A third party not involved in any aspect of the study was responsible for generating the randomisation sequence. Randomisation was stratified by age (2.5–5.0 years old and 5–8.0 years old) and severity of ASD (mild, moderate, and severe) and the sequence was generated using the Website Randomisation.com (http://www.randomization.com/) and random block design in blocks of 4 and 8. Researchers, children, and caregivers were blinded to treatment allocations until after data analysis.
Schematic diagram of study design and flow of participants through the study. VID (vitamin D), OM (omega-3 LCPUFA), VIDOM (vitamin D+ omega-3 LCPUFA). *Corrective action was taken for nutritional deficiencies before randomisation. **The reasons for drop out were parents/caregivers’ concerns about side effects and having a blood sample taken (VID 2; OM 1; VIDOM 0; placebo 1), family and time constraints (VID 3; OM 2; VIDOM 4; placebo 3), child disliking the supplement (VID 1; OM 0; VIDOM 4; placebo 2), not seeing any benefits (VID 1; OM 0; VIDOM 0; placebo 1), moving overseas (VID 2; OM 0; VIDOM 0; placebo 0), child being diagnosed with other medical conditions during the study period (VID 1; OM 0; VIDOM 0; placebo 0), and no reason (VID 3; OM 2; VIDOM 5; placebo 6)
Data were collected during participants’ visit to the Massey University Human Nutrition Research Unit (HNRU), NZ. Demographic and medical history were recorded at baseline. ASD related outcomes were assessed and height and weight were measured at baseline and after 12 months. Non-fasting venous blood samples were collected at the North Shore or Waitakere Hospitals in Auckland, NZ, on three occasions, baseline, 6 months and 12 months (endpoint). Caregivers completed weekly online surveys to collect information regarding adverse events, supplement and medication use, behavioural therapies and compliance. Compliance was calculated using cumulative pill counts at the end of the study, and adherence was measured as a percentage; (number of pills supplied minus number of pills not taken)/number of pills supplied × 100. Compliance was also confirmed by biomarker analysis (serum 25(OH)D and omega-3 index).
Data Collection
Standardised psychological tests were used to assess the core symptoms of ASD. Standardised instructions were given to all caregivers on how to complete the questionnaires during their visit to the HNRU.
Social Responsiveness Scale™, Second Edition (SRS-2) (Constantino and Gruber 2012): The SRS–2 is a quantitative approach to screen and diagnose social impairment and repetitive behaviour as a single trait in ASD. SRS provides scores for five treatment subscales (social awareness, social cognition, social communication, social motivation, and RRB) as well as scores for two DSM-5 compatible subscales, social communicative functioning (the composite score of social awareness, social cognition, social communication and social motivation) and RRB. The SRS has been found to discriminate children with ASD from typically developing children (Cholemkery et al. 2014), from children with oppositional defiant/conduct disorder (Cholemkery et al. 2014), and from other psychiatric patients with/without pervasive developmental disorders (Cholemkery et al. 2014). In a cross-cultural validity study of the SRS, Bolte et al. (2008) reported a good internal consistency, test–retest reliability and inter-rater reliability, and a good convergent validity with other ASD diagnostic tools (Bolte et al. 2008).
Sensory Processing Measures™ (SPM) (Parham et al. 2007): The SPM has been developed according to the sensory integration theory—that sensory issues affect higher levels of functioning such as social participation and planning and ideas (praxis) (Parham et al. 2007). The SPM assesses sensory processing in relation to vision, hearing, touch, body awareness (proprioception), and balance and motion (vestibular function) in children. The Home Form has been standardized on a demographically representative sample of 1051 typically developing children (Parham et al. 2007). The internal consistency and test–retest reliability of SPM has been documented (Brown et al. 2010; Parham et al. 2007). The SPM has been also validated in a group of children with additional needs (Brown and Subel 2013), and its convergent validity with other tools assessing sensory features in children with ASD has been documented (Dugas et al. 2017).
Biochemical Analysis
Nutritional biomarkers were assayed from a non-fasted venous blood sample. These included the following: full blood count, erythrocyte fatty acids, and 25(OH)D, calcium, albumin, iron studies (iron, iron binding capacity, ferritin, and transferrin saturation), vitamin B12 and folate. With the exception of erythrocyte fatty acids, all other biomarkers were analysed at North Shore Hospital (IANZ accredited). Erythrocyte fatty acids were analysed at the University of Wollongong, Australia (Mazahery et al. 2016). Omega-3 index is defined as the combined percentage of two omega-3 LCPUFA, EPA and DHA, in red blood cells (Harris and Von Schacky 2004). Although an omega-3 index for neurodevelopmental disorders has yet to be determined, levels < 4% are associated with greater risk of mortality from cardiovascular disease while 8–12% are shown to be protective (Harris and Von Schacky 2004). The omega-3 index has been reported to be 1.4%, 3.2%, and 6.5% in children with ASD, ADHD, and typical development, respectively, indicating that levels under 4% could also be considered as a risk factor for neuropsychological disorders (Parletta et al. 2016). Cut-offs of > 225 nmol/L and > 2.7 mmol/L were used to define hypervitaminosis D and hypercalcaemia, respectively.
Statistical Analysis
It was calculated that a total of 168 children (42 per treatment group) would be required to demonstrate a clinically significant difference at 80% power and 5% statistical significance. Sample size was calculated using a potential dropout rate of 20% and the following formula (Fox et al. 1998): N = 2α2K/(µ2–µ1)2 [N, sample size; α, SD SRS-total (primary outcome), 25 [from our 2015 pilot study, unpublished]; K, constant [7.9]; µ2–µ1, clinically significant difference in SRS-total means, 17 units (Singh et al. 2014)].
Statistical analysis was performed using IBM SPSS version 24.0 (Armonk, NY). A P-value 0.05 was considered significant and a P-value of < 0.1 as a trend for a relationship (due to large drop-out and potential loss of statistical power). The variables were tested for normality using the Kolmogorov–Smirnov, Shapiro–Wilk tests and normality plots. Non-normally distributed data were transformed into approximate normal distributions by logarithmic transformations. The data were reported appropriately as mean ± SD for normally distributed data and as median (25, 75 percentiles) for non-normally distributed data and as frequencies for categorical data. Baseline between group differences were examined using analysis of variance (ANOVA) for parametric data, the Kruskal–Wallis test for non-parametric data and X2-test for categorical data. A Bonferroni correction was applied for multiple comparisons (0.05/number of comparisons).
The primary analysis, comparing the effects of treatment on core symptoms of autism over 12-months, was conducted using a pair-wise mixed effects longitudinal models general linear mixed model design (variance components structure). Treatment (VID vs. placebo, OM vs. placebo, and VIDOM vs. placebo) and time (baseline and endpoint) were included as fixed effects, and participant was included as a random effect to account for the repeated measures within individuals. The interactions between treatments and time were tested with and without considering the potential effect of confounders. Potential confounding factors and effect modifiers investigated within the models were compliance, medication use, and therapy. Analysis was conducted on completers for each outcome measure using data from those who completed assessments at both timepoints (baseline and endpoint). No adjustments for multiple testing were made in order to avoid missing potentially important cause and effect relationship due to an inflation of the type II error for our final model (Rothman 1990). This approach has been adopted by others (Bent et al. 2014).
As an exploratory analysis, treatment groups were parameterised differently (all receiving vitamin D, including VID and VIDOM, vs. others and all receiving omega-3 LCPUFA, including OM and VIDOM, vs. others), and the data was reanalysed using the above-mentioned statistical analysis. The second parameterisation method was performed to investigate the effect of treatments or any synergy/antagonism between VID and OM (or any additive effect of both) on primary outcome measures. The goal of latter parameterisation was to answer the research question much more clearly. However, due to not being the pre-defined statistical method of the present trial, the findings of the latter analysis are considered as the exploratory findings.
The relationship between biochemical markers (25(OH)D and omega-3 index) (as continuous variables) and change in outcome measures were assessed using Pearson for normally distributed variables and Spearman correlations for non normally distributed variables. Also, the difference in change in outcome measures between low and high levels of biomarkers were assessed using independent samples t-test and Mann Whitney U test for normally and non normally distributed variables, respectively.
Results
Baseline Socio-Demographic and Behavioural Characteristics
The flow of participants through the study are summarised in Fig. 1. Over the 25-month period of recruitment (from January 2015 to February 2017), 309 families registered their interest in the study, of whom 190 families were either excluded or not enrolled. The children of remaining families (n = 119) were screened for nutritional deficiencies and high serum 25(OH)D concentrations. Out of 119 children, 33 were treated for nutritional deficiencies, of whom one was excluded due to failure to take corrective action for iron deficiency. A total of 117 children were randomised to treatment groups. Seventy-three children completed the study out of 117 who completed the baseline assessments, with a median follow-up of 54 (53, 55) weeks and compliance rate of 95% (92%, 96%). Out of 44 children who did not complete the study, 16 were lost to follow up and 28 dropped out. The reasons for dropping out were parents/caregivers’ concerns about side effects and having a blood sample taken (n = 4), family and time constraints (n = 12), child disliking the supplement (n = 7), not seeing any benefits (n = 2), moving overseas (n = 2), and child being diagnosed with other medical conditions during the study period (n = 1). The reasons for drop out were related to study supplements in 13–14% of drop outs in VID, VIDOM, and placebo groups, as compared with 3% in OM group, P = 0.48 (Fig. 1).
Baseline characteristics of children randomised (n = 117) did not differ significantly across treatment groups (Table 2). After exclusion of children who did not complete the study, the baseline characteristics were equally distributed across treatment groups (Supplemental Table 1). For a list of types of behavioural interventions and medications used by children over the study period refer to Supplemental Table 2.
Treatment Efficacy
The baseline, endpoint (after 12 months) and the changes in SRS-total behavioural scores and SRS domains of study populations across treatment groups are presented in Table 3. Both unadjusted and adjusted analysis of the scores on SRS-total and SRS domains revealed a significant effect of time. The analysis of the scores on SRS-total showed a non-significant trend for a greater improvement in OM group (P = 0.09 unadjusted for covariates and P = 0.08 adjusted for covariates) than placebo (Fig. 2a). The rate of positive response (at least a 30% improvement in SRS-total score) was 20–22% in active treatment groups (4/19, 5/23, and 3/15 children in VID, OM and VIDOM groups, respectively) and nil in the placebo group. Furthermore, while there was a decrease in the proportion of children with severe autism (level 3) after 12 months in all active treatment groups (VID, − 13%; OM, − 26%; and VIDOM, − 14%), the proportion increased by 6% in the placebo group (P = 0.02).
Graphical presentation of the pattern of change in SRS-total score (a), SRS-social communicative functioning (b), SRS-social awareness (c), and SRS-social motivation (d) over the study period (baseline and endpoint) within each of the treatment groups. a Significant effect of time point, F(1,73) = 14, P < 0.001 (unadjusted), and a trend for a greater improvement with omega-3 (OM) than placebo, F(1,69) = 3.1, P = 0.08 (adjusted for therapy, medication and compliance). b Significant effect of time point, F(1,73) = 16, P < 0.001 and a trend for a greater improvement with vitamin D+ omega-3 (VIDOM), F(1,69) = 3.4, P = 0.07 (adjusted for covariates) than placebo. c Significant effect of time point, F(1,73) = 8.0, P < 0.01, and a greater improvement with OM, F(1,69) = 5.0, P = 0.03 (adjusted for covariates) and VIDOM, F(1,69) = 5.0, P = 0.03 (adjusted for covariates) than placebo. d Significant effect of time point, F(1,69) = 2.6, P < 0.01, and a trend for a greater improvement with OM, F(1,69) = 2.6, P = 0.1 (adjusted for covariates) than placebo
The unadjusted analysis of the scores on SRS-social communicative functioning revealed no effect of active treatments. However, when the analysis was adjusted for covariates a trend for a greater improvement with VIDOM was identified than with placebo (P = 0.07) (Fig. 2b). Of all four SRS-social communicative functioning subdomains (social awareness, cognition, communication, and motivation), a significant effect of treatment groups was identified only for social awareness (OM vs. placebo: P = 0.06 unadjusted for covariates and P = 0.03 adjusted for covariates; and VIDOM vs. placebo: P = 0.04 unadjusted for covariates and P = 0.03 adjusted for covariates) and a trend for an effect of treatment groups for social motivation [OM vs. placebo: P = 0.06 unadjusted for covariates and P = 0.1 adjusted for covariates] subdomains (Fig. 2c, d).
The baseline, endpoint and the changes in SPM-total and subdomain scores of study populations across treatment groups are presented in Table 4. With the exception of SPM-taste and smell and SPM-balance and motion subdomains, the slopes of change over time for the SPM composite score as well as four other SPM subdomains (vision, hearing, touch, and body awareness) did not differ between active treatment and placebo groups. There was a possible relationship between VIDOM and SPM-taste and smell [F(1,74) = 3.8, P = 0.06 (unadjusted) and F(1,69) = 3.7, P = 0.06 (adjusted for covariates)] and between OM and SPM-balance and motion, which became weaker when the analysis was adjusted for covariates [F(1,74) = 3.3, P = 0.07 (unadjusted) and F(1,69) = 3.0, P = 0.09 (adjusted for covariates)].
In an exploratory fashion, the effect of treatments on the social participation domain of SPM was assessed (Table 4). The analysis of the scores revealed a trend for a greater improvement with VID (P = 0.06) and VIDOM (P = 0.08) than placebo.
Also, when other parameterisation method was used the relationship between OM and SRS-total (P = 0.06), SRS-social communicative functioning (P = 0.04), SRS-awareness (P = 0.008) and SRS-communication (P = 0.01), and the relationship between VID and SPM-social participation (P = 0.03) became stronger (based on P-value adjusted for covariates).
The analyses of serum 25(OH)D concentrations and omega-3 index showed a significant effect of time of follow-up (P < 0.01) and the interaction between time point and treatment groups (P < 0.01) (Fig. 3a, b). Significant increases were seen in mean serum 25(OH)D concentration in the VID and VIDOM groups and in the median omega-3 index in the OM and VIDOM groups. The proportion of children having serum 25(OH)D ≥ 75 nmol/L in the VID, VIDOM, OM, and placebo groups increased from 29, 33, 30, and 14% at baseline to 71, 100, 35, and 21% at endpoint, respectively.
Graphical presentation of the pattern of change in serum 25(OH)D concentration (a) and omega-3 index (b) over the study period (baseline, 6 months, and endpoint) for serum 25(OH)D concentration and baseline and endpoint for omega-3 index within each of the treatment groups. a Reference lines at 50 and 75 nmol/L (dotted lines) were added for clarification. Significant effect of time of the follow-up, F(2,42) = 27, P < 0.001, effect size = 0.6 large effect size, and the interaction between time point and treatment groups, F(6,82) = 4.4, P = 0.001 [from 64 ± 25, 63 ± 28, 62 ± 24 and 52 ± 28 nmol/L at baseline to 98 ± 38, 99 ± 17, 63 ± 20 and 61 ± 19 nmol/L at endpoint in the VID (n = 14), VIDOM (n = 9), OM (n = 20), and placebo (n = 14) groups, respectively]. b Reference lines at 4% and 8% (dotted lines) were added for clarification. Significant effect of time of the follow-up, F(1,62) = 62, P < 0.0001, and the interaction between time point and treatment groups, F(3,74) = 13, P < 0.0001 [from 4.7 (4.4, 5.6), 4.5 (4.0, 6.2), 5.3 (4.7, 6.3) and 4.8 (3.7, 8.0) at baseline to 9.6 (9.0, 11), 9.2 (7.3, 10), 4.7 (4.4, 5.5) and 4.0 (3.4, 5.8) at endpoint in OM (n = 19), VIDOM (n = 8), VID (n = 13) and placebo (n = 13) groups, respectively]
As the secondary findings, no relationship was observed between change in biochemical markers and change in any outcome measure parameters (r values ≤ 0.1 and P values > 0.1). However, a greater improvement in SRS-social awareness (− 1.5 ± 2.9 vs. − 0.3 ± 3.0, P = 0.09) and SPM-total (− 15 ± 19 vs. − 7.4 ± 19, P = 0.03) was seen in children with initial serum 25(OH)D concentration < 50 nmol/L (n = 26) than ≥ 50 nmol/L (n = 46), respectively. Greater improvement in SRS-total (− 23 ± 16 vs. − 9.9 ± 18, P = 0.05), SRS-social communicative functioning (− 19 ± 14 vs. − 9.4 ± 18, P = 0.03), SRS-communication (− 11 ± 8.3 vs. − 3.2 ± 7.7, P = 0.01), and SPM-social participation (− 3.2 ± 2.8 vs. − 0.2 ± 4.6, P = 0.05) was observed in children with endpoint serum 25(OH)D > 100 nmol/L (n = 9, all on vitamin D and VIDOM supplements) than ≤ 100 nmol/L (n = 49).
A single possible relationship was noted between endpoint omega-3 index and change in SPM-total (rs = − 0.24, P = 0.08). Further analysis showed children with an endpoint omega-3 index > 8% (n = 21, all from OM and VIDOM groups) had a trend for larger improvement in SPM-total score than those with endpoint omega-3 index ≤ 8% (n = 33), − 16 ± 20 vs. − 8.4 ± 20, respectively, P = 0.07.
Safety and Adverse Events
Among all the study groups, there were no significant changes in the levels of serum calcium, and there were no reports of serum calcium (corrected for albumin) ≥ 2.7 mmol/L (hypercalcaemia) and serum-25(OH)D > 225 nmol/L (hypervitaminosis D). A list of adverse events reported by parents/caregivers over the study period is presented in Supplemental Table 4. All the gastrointestinal symptoms appeared to be unrelated to treatments because they were equally distributed across all treatment groups. Six parents/caregivers (OM, 5 and VIDOM, 1) reported allergic reactions. The allergic reactions included rash (n = 4), facial pimple (n = 1) and red ear (n = 1) and were reported on a few occasions over the study period (more than 4 weeks though at irregular intervals).
Adequacy of Blinding
All parents/caregivers who completed the study responded to the question, ‘‘which study supplements do you think your child was consuming over the study period?’’ at the end of the study. The proportion of parents who incorrectly guessed the treatment groups was 62% (45/73). Assigned groups were incorrectly guessed by 52% (12/23) in OM, followed by 60% (9/15) in VIDOM, 68% (13/19) in VID and 69% (11/16) in placebo groups, P = 0.66.
Generalisability of Findings
The proportion of children who were lost to follow up/dropped out within each treatment group and sociodemographic and behavioural characteristics of completers and non-completers is presented in Supplemental Table 3. A larger proportion of children of Pacific ethnicity (85% vs. 0–40% of other ethnicities, P < 0.001) were non-completers. Approximately half of children who had confirmed clinical diagnosis of severe ASD (level 3) were lost to follow up/dropped out vs. 32% and 37% of children with mild (level 1) and moderate (level 2) ASD, respectively. Furthermore, non-completers had lower omega-3 index than completers (P = 0.001) at baseline.
Discussion
This work suggests the possible efficacy of omega-3 LCPUFA alone or in combination with vitamin D in managing some core symptoms of ASD, including social-communicative functioning and some domains of sensory issues. However, treatment with vitamin D alone did not lead to a statistically significant improvement in core symptoms of ASD. Although not a specific goal of this study, vitamin D was associated with a significantly greater improvement in social participation measured by SPM. Furthermore, both omega-3 index and vitamin D status significantly increased in response to omega-3 LCPUFA and vitamin D supplementation, a finding suggestive of a good compliance.
Evidence suggests that supplementation with omega-3 LCPUFA can positively affect neurodevelopment, modulate inflammatory responses, promote homeostasis, and have a positive effect on behaviours associated with mental disorders including ASD in animal models (Fortunato et al. 2017). The findings of the present trial are in accordance with one previous report in that omega-3 LCPUFA might be associated with an improvement in social interaction and communication domains (Yui et al. 2012). However, the findings are inconsistent with those of other trials, where no beneficial effect of omega-3 LCPUFA on symptoms of ASD was reported (Amminger et al. 2007; Bent et al. 2011, 2014; Mankad et al. 2015; Voigt et al. 2014). The inconsistent findings of the most omega-3 LCPUFA ASD RCTs and those of the present trial could be attributed to the study duration (12 months vs. 16–24 weeks), dose of omega-3 LCPUFA (722 mg/day vs. 200–700 mg/day), with some previous reports using a combination of EPA and DHA (Amminger et al. 2007; Mankad et al. 2015), and outcome measures used (each having unique psychometric properties). Evidence suggests that PUFA erythrocyte membrane reaches a steady state after 6 months (Katan et al. 1997) and longer study periods of 1 year might be needed to demonstrate behavioural changes in response to omega-3 LCPUFA supplementation (Raine et al. 2015). It is also well documented that erythrocyte omega-3 index increases in a dose-dependent manner in response to DHA and EPA supplementation (Flock et al. 2013), with DHA having a greater impact (Allaire et al. 2017).
Many repetitive behaviours seen in populations with ASD are related to seeking or avoiding sensory inputs and because of that sensory issues are considered as a distinct criterion under RRB domain in ASD diagnosis. Sensory issues and sensory overload have been shown to be affected by omega-3 LCPUFA treatment in animal models (Fedorova et al. 2009; Robson et al. 2010). In line with Boone et al. (2017) (Boone et al. 2017), the present trial found a trend for a greater improvement in some behaviours associated with sensory issues with omega-3 LCPUFA in children with ASD. However, Mankad et al. (2015) failed to confirm those findings (Mankad et al. 2015).
Vitamin D has been recently proposed to be an important component of ASD aetiology and symptoms management (Mazahery et al. 2016). However, only a few intervention trials have examined its efficacy in ASD, and the results from these trials are inconclusive. The findings of the current trial support those of one previous randomised controlled placebo trial where vitamin D was found to be ineffective in reducing core symptoms of ASD (Kerley et al. 2017), though are inconsistent with the findings of others (Saad et al. 2018). Compared with the latter trial (Saad et al. 2018), the present and Kerley’s et al.’s trials (2017) (Kerley et al. 2017) used lower doses of vitamin D (2000 IU/day vs. 300 IU/kg/day not to exceed 5000 IU/day), included both vitamin D deficient and sufficient children (vs. vitamin D sufficient children), had larger standard deviations for SRS-total (25–27 units vs. < 5 units), and had smaller sample sizes (< 20 vs. 55 children in vitamin D group analysed). Serum 25(OH)D has been shown to increase in a dose-dependent manner in response to vitamin D supplementation, with higher doses resulting in higher long-run concentrations in adults and children (Lewis et al. 2013; Mazahery et al. 2015), which is consistent with well-being in adults and better neurodevelopmental outcomes in children (Salas et al. 2018; Vieth et al. 2004). Although the daily dose of 2000 IU for 12 months was adequate to increase serum 25(OH)D concentration to levels at or above 75 nmol/L (that are considered sufficient) in most children, higher serum 25(OH)D concentrations and accordingly higher doses might be required to see beneficial effect on behavioural symptoms in children with ASD (Saad et al. 2018). Also, baseline vitamin D status is a significant predictor of circulating 25(OH)D in response to vitamin D supplementation (Mazahery and von Hurst 2015), and may modify the relationship between vitamin D supplementation and health outcomes. Furthermore, the small SD observed in Saad et al.’s trial (Saad et al. 2018) indicates there is a small amount of variance in ASD symptomatology and the study population is more homogenous (and likely to respond similarly to an intervention) than those of present and Kerley et al’s trials (Kerley et al. 2017). Finally, inconsistent with Kerley et al. and Saad et al. mean serum 25(OH)D concentration in the current trial increased in some children on placebo (n = 8; + 27 ± 12 nmol/L). This increase might have masked the difference in outcome measures between vitamin D and placebo.
There are speculations that vitamin D and omega-3 LCPUFAs may improve ASD symptoms because of their shared and complementary nutrient functions (Patrick and Ames 2015). However, no ASD intervention trials have examined the effect of both treatments and therefore no comparison can be made. The present trial found a non-significant positive effect of combined treatment on composite score of social interaction and communication, which was mainly attributed to the improvement in social awareness domain.
Vitamin D and omega-3 LCPUFA supplementation was not associated with any serious adverse events in this study, and others have demonstrated safety with larger doses of these supplements than those used here (Milte et al. 2012; Saad et al. 2018).
This is the first randomised controlled trial to our knowledge to study the efficacy of vitamin D, omega-3 LCPUFA, or both in children with confirmed clinical diagnosis of ASD. Previous studies have evaluated either vitamin D or omega-3 LCPUFA alone and no studies considered the efficacy of both on ASD symptoms. Moreover, major nutritional deficiencies were addressed prior to the trial entry to limit their potential confounding effects on outcome measure parameters. Also, the four treatment groups were well matched at baseline with no significant differences in demographics, behavioural characteristics and blood biomarkers of vitamin D and omega-3 LCPUFA. Furthermore, the study has the longest study duration (12 months), allowing us to examine effects on functional outcomes and accounting for seasonal variation. The good compliance rate as confirmed by increases in biomarkers is another strength of the present trial. Finally, children had low initial omega-3 index, and possibly a greater room for improvement.
This study is limited by a smaller sample than was originally proposed and the relatively high attrition rate. Although the study was designed to enrol more children and despite an intensive approximately 25-month recruitment, the present trial was only able to enrol 117 children (28–31 children per group) and retain 15–23 children per group at endpoint (an attrition rate of approximately 38%). Researchers often find recruitment and retention of participants in studies involving children and adolescents, particularly those with chronic medical conditions and neuropsychological disorders, challenging (Karlson and Rapoff 2009; van der Wurff et al. 2017). A recent report shows the attrition rate in paediatric intervention trials ranges between 0 and 54% (Karlson and Rapoff 2009), and the presence of psycho-behavioural concerns is associated with the greater drop-out rate (Bender et al. 2003). Similarly, the average attrition rate in omega-3 LCPUFA paediatric intervention trials has been reported to vary between 0% and 58% (35% in Australia) (van der Wurff et al. 2017), with studies involving participants with health conditions having higher attrition rate.
High attrition rate may compromise the internal, external, or statistical validity of randomised controlled trials (Sifers et al. 2002). Although the high attrition rate in the current study did not alter the random composition of groups and their equivalence in relation to demographic and behavioural characteristic, the possibility remains that some children might drop out due to the worse variation in their symptoms over time (natural variability). Furthermore, the external validity of the current study might be compromised because a larger proportion of non-completers were of Pacific ethnicity or were more likely to have severe autism and lower omega-3 index than completers (P < 0.001). Finally, statistical validity of the current study might have been compromised due to the reduced sample size and power. However, despite the small sample size, the present trial found a positive effect of omega-3 LCPUFA on ASD core symptoms. It is important to note that some patients do not adhere to medications/dietary supplements in the real life usual care, despite health professionals’ advice. The design of current study could be a replication of the real-life scenarios.
Conclusions
Our findings suggest the possible efficacy of supplementary omega-3 LCPUFA alone or in combination with vitamin D in the management of core symptoms of ASD in children. However, large attrition rates and resultant loss of statistical power preclude any definitive conclusion. Also, the interpretation of findings regarding vitamin D alone is difficult. Taking the adequate length and good compliance rate of the present trial into consideration, and in the light of well-documented, biological values of both nutrients and the extent of deficiencies in these nutrients in children with ASD, large and preferably more ASD friendly trials (e.g. using less invasive approaches for blood testing) with both vitamin D (but larger doses and in children who have lower vitamin D status) and omega-3 LCPUFA are warranted.
References
Al-Haidar, F. A. (2008). Parental attitudes toward the prescription of psychotropic medications for their children. Journal of Family & Community Medicine, 15(1), 35–42.
Allaire, J., Harris, W. S., Vors, C., Charest, A., Marin, J., Jackson, K. H., & Lamarche, B. (2017). Supplementation with high-dose docosahexaenoic acid increases the Omega-3 Index more than high-dose eicosapentaenoic acid. Prostaglandins, Leukotrienes and Essential Fatty Acids, 120, 8–14. https://doi.org/10.1016/j.plefa.2017.03.008.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, D.C.: American Psychiatric Association
Amminger, G. P., Berger, G. E., Schafer, M. R., Klier, C., Friedrich, M. H., & Feucht, M. (2007). Omega-3 fatty acids supplementation in children with autism: A double-blind randomized, placebo-controlled pilot study. Biological Psychiatry, 61(4), 551–553. https://doi.org/10.1016/j.biopsych.2006.05.007.
Azzam, H. M. E., Sayyah, H., Youssef, S., Lotfy, H., Abdelhamid, I. A., Elhamed, A., & Maher, S. (2015). Autism and vitamin D: An intervention study. Middle East Current Psychiatry, 22(1), 9–14. https://doi.org/10.1097/01.xme.0000457269.05570.78.
Bandini, L. G., Anderson, S. E., Curtin, C., Cermak, S., Evans, E. W., Scampini, R., & Must, A. (2010). Food selectivity in children with autism spectrum disorders and typically developing children. Journal of Pediatrics, 157(2), 259–264. https://doi.org/10.1016/j.jpeds.2010.02.013.
Bender, B. G., Ellison, M. C., Gleason, M., Murphy, J. R., Sundstrom, D. A., & Szefler, S. J. (2003). Minimizing attrition in a long-term clinical trial of pediatric asthma. Annals of Allergy Asthma and Immunology, 91(2), 168–176. https://doi.org/10.1016/s1081-1206(10)62173-4.
Bent, S., Bertoglio, K., Ashwood, P., Bostrom, A., & Hendren, R. L. (2011). A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. Journal of Autism and Devevlopmental Disorder, 41(5), 545–554. https://doi.org/10.1007/s10803-010-1078-8.
Bent, S., Hendren, R. L., Zandi, T., Law, K., Choi, J. E., Widjaja, F., & Law, P. (2014). Internet-based, randomized controlled trial of omega-3 fatty acids for hyperactivity in autism. Journal of the American Academy of Child and Adolescent Psychiatry, 53(6), 658–666. https://doi.org/10.1016/j.jaac.2014.01.018.
Bolte, S., Poustka, F., & Constantino, J. N. (2008). Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS). Autism Research, 1(6), 354–363. https://doi.org/10.1002/aur.49.
Boone, K. M., Gracious, B., Klebanoff, M. A., Rogers, L. K., Rausch, J., Coury, D. L., & Keim, S. A. (2017). Omega-3 and -6 fatty acid supplementation and sensory processing in toddlers with ASD symptomology born preterm: A randomized controlled trial. Early Human Development, 115, 64–70. https://doi.org/10.1016/j.earlhumdev.2017.09.015.
Brown, T., Morrison, I. C., & Stagnitti, K. (2010). The reliability of two sensory processing scales used with school-age children: Comparing the response consistency of mothers, fathers, and classroom teachers rating the same child. Journal of Occupational Therapy, Schools, & Early Intervention, 3(4), 331–347. https://doi.org/10.1080/19411243.2010.541775.
Brown, T., & Subel, C. (2013). Known-group validity of the infant toddler sensory profile and the sensory processing measure-preschool. Journal of Occupational Therapy, Schools, & Early Intervention, 6(1), 54–72. https://doi.org/10.1080/19411243.2013.771101.
Cass, W. A., Smith, M. P., & Peters, L. E. (2006). Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Annals of New York Academy of Sciences, 1074, 261–271. https://doi.org/10.1196/annals.1369.023.
Cheng, Y. S., Tseng, P. T., Chen, Y. W., Stubbs, B., Yang, W.-C., Chen, T. Y., & Lin, P. Y. (2017). Supplementation of omega 3 fatty acids may improve hyperactivity, lethargy, and stereotypy in children with autism spectrum disorders: A meta-analysis of randomized controlled trials. Neuropsychiatric Disease and Treatment, 13, 2531–2543. https://doi.org/10.2147/NDT.S147305.
Cholemkery, H., Kitzerow, J., Rohrmann, S., & Freitag, C. (2014). Validity of the social responsiveness scale to differentiate between autism spectrum disorders and disruptive behaviour disorders. European Child and Adolescent Psychiatry, 23(2), 81–93. https://doi.org/10.1007/s00787-013-0427-5.
Constantino, J., & Gruber, C. (2012). The social responsiveness scale, Second Edition (SRS-2). Los Angeles: Western Psychological Services.
Dugas, C., Simard, M.-N., Fombonne, E., & Couture, M. (2017). Comparison of two tools to assess sensory features in children with autism spectrum disorder. American Journal of Occupational Therapy, 72(1), 1–9. https://doi.org/10.5014/ajot.2018.024604.
Emond, A., Emmett, P., Steer, C., & Golding, J. (2010). Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics, 126(2), e337–e342. https://doi.org/10.1542/peds.2009-2391.
Enko, D., Fridrich, L., Rezanka, E., Stolba, R., Ernst, J., Wendler, I., & Halwachs-Baumann, G. (2014). 25-Hydroxy-vitamin D status: Limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clinica y Laboratorio, 60(9), 1541–1550. https://doi.org/10.7754/Clin.Lab.2014.131114.
Fedorova, I., Alvheim, A. R., Hussein, N., & Salem, N. Jr.. (2009). Deficit in prepulse inhibition in mice caused by dietary n–3 fatty acid deficiency. Behavioral Neuroscience. 123(6), 1218–1225. https://doi.org/10.1037/a0017446.
Feng, J., Shan, L., Du, L., Wang, B., Li, H., Wang, W., & Jia, F. (2017). Clinical improvement following vitamin D3 supplementation in autism spectrum disorder. Nutritional Neuroscience, 20(5), 284–290. https://doi.org/10.1080/1028415x.2015.1123847.
Flock, M. R., Skulas-Ray, A. C., Harris, W. S., Etherton, T. D., Fleming, J. A., & Kris-Etherton, P. M. (2013). Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: A dose-response randomized controlled trial. Journal of American Heart Association, 2(6), e000513. https://doi.org/10.1161/jaha.113.000513.
Fortunato, J. J., da Rosa, N., Laurentino, M., Goulart, A. O., Michalak, M., Borges, C., & Petronilho, F. (2017). Effects of ω-3 fatty acids on stereotypical behavior and social interactions in Wistar rats prenatally exposed to lipopolysaccarides. Nutrition, 35, 119–127. https://doi.org/10.1016/j.nut.2016.10.019.
Fox, N., Hunn, A., & Mather, N. (1998). Sampling. Trent Focus for Research and Development in Primary Health Care.
Graf-Myles, J., Farmer, C., Thurm, A., Royster, C., Kahn, P., Soskey, L., & Swedo, S. (2013). Dietary adequacy of children with autism compared with controls and the impact of restricted diet. Journal of Developmental and Behavioral Pediatrics, 34(7), 449–459. https://doi.org/10.1097/DBP.0b013e3182a00d17.
Harris, W. S., & Von Schacky, C. (2004). The Omega-3 Index: A new risk factor for death from coronary heart disease? Preventive Medicine, 39(1), 212–220. https://doi.org/10.1016/j.ypmed.2004.02.030.
Hendren, R. L. (2013). Autism: biomedical complementary treatment approaches. Child and Adolescent Psychiatry Clinics of North America, 22(3), 443–456. https://doi.org/10.1016/j.chc.2013.03.002. vi.
Hertz-Picciotto, I., Green, P. G., Delwiche, L., Hansen, R., Walker, C., & Pessah, I. N. (2010). Blood mercury concentrations in CHARGE Study children with and without autism. Environmental Health Perspectives, 118(1), 161–166. https://doi.org/10.1289/ehp.0900736.
Holick, M. F., Binkley, N. C., Bischoff-Ferrari, H. A., Gordon, C. M., Hanley, D. A., Heaney, R. P., & Weaver, C. M. (2011). Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism, 96(7), 1911–1930. https://doi.org/10.1210/jc.2011-0385.
Horvath, A., Lukasik, J., & Szajewska, H. (2017). Omega-3 fatty acid supplementation does not affect autism spectrum disorder in children: A systematic review and meta-analysis. Journal of Nutrition, 147(3), 367–376. https://doi.org/10.3945/jn.116.242354.
Humble, M. B., Gustafsson, S., & Bejerot, S. (2010). Low serum levels of 25-hydroxyvitamin D (25-OHD) among psychiatric out-patients in Sweden: relations with season, age, ethnic origin and psychiatric diagnosis. Journal of Steroid Biochemistry and Molecular Biology, 121(1–2), 467–470. https://doi.org/10.1016/j.jsbmb.2010.03.013.
Institute of Medicine. (2011). Dietary reference intakes for calcium and vitamin D. Washington, DC, USA: The National Academies Press.
Jia, F., Wang, B., Shan, L., Xu, Z., Staal, W. G., & Du, L. (2015). Core symptoms of autism improved after vitamin D supplementation. Pediatrics, 135(1), e196–e198. https://doi.org/10.1542/peds.2014-2121.
Johnson, C. R., Handen, B. L., Zimmer, M., & Sacco, K. (2010). Polyunsaturated fatty acid supplementation in young children with autism. Journal of Developmental and Physical Disabilities, 22(1), 1–10. https://doi.org/10.1007/s10882-009-9152-x.
Johnson, S. M., & Hollander, E. (2003). Evidence that eicosapentaenoic acid is effective in treating autism. Journal of Clinical Psychiatry, 64(7), 848–849. https://doi.org/10.4088/JCP.v64n0718c.
Karlson, C. W., & Rapoff, M. A. (2009). Attrition in randomized controlled trials for pediatric chronic conditions. Journal of Pediatric Psychology, 34(7), 782–793. https://doi.org/10.1093/jpepsy/jsn122.
Katan, M. B., Deslypere, J. P., van Birgelen, A. P., Penders, M., & Zegwaard, M. (1997). Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. Journal of Lipid Research, 38(10), 2012–2022.
Kerley, C. P., Power, C., Gallagher, L., & Coghlan, D. (2017). Lack of effect of vitamin D3 supplementation in autism: A 20-week, placebo-controlled RCT. Archives of Disease in Childhood, 102(11), 1030–1036. https://doi.org/10.1136/archdischild-2017-312783.
Lewis, R. D., Laing, E. M., Hill Gallant, K. M., Hall, D. B., McCabe, G. P., Hausman, D. B., & Weaver, C. M. (2013). A randomized trial of vitamin D3 supplementation in children: Dose-response effects on vitamin D metabolites and calcium absorption. The Journal of Clinical Endocrinology & Metabolism, 98(12), 4816–4825. https://doi.org/10.1210/jc.2013-2728.
Logan, V. F., Gray, A. R., Peddie, M. C., Harper, M. J., & Houghton, L. A. (2013). Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. British Journal of Nutrition, 109(6), 1082–1088. https://doi.org/10.1017/S0007114512002851.
Mankad, D., Dupuis, A., Smile, S., Roberts, W., Brian, J., Lui, T., & Anagnostou, E. (2015). A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Molecular Autism, 6, 18. https://doi.org/10.1186/s13229-015-0010-7.
Marí-Bauset, S., Llopis-González, A., Zazpe-García, I., Marí-Sanchis, A., & Morales-Suárez-Varela, M. (2015). Nutritional status of children with autism spectrum disorders (ASDs): A case–control study. Journal of Autism and Developmental Disordorder, 45(1), 203–212. https://doi.org/10.1007/s10803-014-2205-8.
Mazahery, H., Camargo, C. A. Jr., Conlon, C., Beck, K. L., Kruger, M. C., & von Hurst, P. R. (2016a). Vitamin D and autism spectrum disorder: A literature review. Nutrients, 8(4), 236. https://doi.org/10.3390/nu8040236.
Mazahery, H., Conlon, C., Beck, K. L., Kruger, M. C., Stonehouse, W., Camargo, C. A. Jr., & von Hurst, P. R. (2016b). Vitamin D and omega-3 fatty acid supplements in children with autism spectrum disorder: A study protocol for a factorial randomised, double-blind, placebo-controlled trial. Trials, 17(1), 295. https://doi.org/10.1186/s13063-016-1428-8.
Mazahery, H., Stonehouse, W., Delshad, M., Kruger, M. C., Conlon, C. A., Beck, K. L., & von Hurst, P. R. (2017). Relationship between long chain n–3 polyunsaturated fatty acids and autism spectrum disorder: Systematic review and meta-analysis of case–control and randomised controlled trials. Nutrients, 9(2), 28. https://doi.org/10.3390/nu9020155.
Mazahery, H., Stonehouse, W., & von Hurst, P. R. (2015). The effect of monthly 50,000 IU or 100,000 IU vitamin D supplements on vitamin D status in premenopausal Middle Eastern women living in Auckland. European Journal of Clinical Nutrition, 69(3), 367–372. https://doi.org/10.1038/ejcn.2014.264.
Mazahery, H., & von Hurst, P. R. (2015). Factors affecting 25-Hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients, 7(7), 5111–5142. https://doi.org/10.3390/nu7075111.
McPheeters, M. L., Warren, Z., Sathe, N., Bruzek, J. L., Krishnaswami, S., Jerome, R. N., & Veenstra-Vanderweele, J. (2011). A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics, 127(5), e1312–e1321. https://doi.org/10.1542/peds.2011-0427.
Meguid, N. A., Atta, H. M., Gouda, A. S., & Khalil, R. O. (2008). Role of polyunsaturated fatty acids in the management of Egyptian children with autism. Clinical Biochemistry, 41(13), 1044–1048. https://doi.org/10.1016/j.clinbiochem.2008.05.013.
Meiri, G., Bichovsky, Y., & Belmaker, R. H. (2009). Omega 3 fatty acid treatment in autism. Journal of Child and Adolescent Psychopharmacology, 19(4), 449–451. https://doi.org/10.1089/cap.2008.0123.
Milte, C. M., Parletta, N., Buckley, J. D., Coates, A. M., Young, R. M., & Howe, P. R. (2012). Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: A randomized controlled trial. Nutrition, 28(6), 670–677. https://doi.org/10.1016/j.nut.2011.12.009.
Ministry of Health and Cancer Society of New Zealand. (2012). Consensus Statement on Vitamin D and Sun Exposure in New Zealand. Wellington: Ministry of Health and Cancer Society of New Zealand.
Ooi, Y. P., Weng, S. J., Jang, L. Y., Low, L., Seah, J., Teo, S., & Sung, M. (2015). Omega-3 fatty acids in the management of autism spectrum disorders: Findings from an open-label pilot study in Singapore. European Journal of Clinical Nutrition, 69(8), 969–971. https://doi.org/10.1038/ejcn.2015.28.
Parham, L. D., Ecker, C., Miller-Kuhaneck, H., Henry, D. A., & Glennon, T. (2007). Sensory processing measure (SPM) manual. Los Angeles: Western Psychological Services.
Parletta, N., Niyonsenga, T., & Duff, J. (2016). Omega-3 and omega-6 polyunsaturated fatty acid levels and correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLOS ONE, 11(5), e0156432. https://doi.org/10.1371/journal.pone.0156432.
Patrick, L., & Salik, R. (2005). The effect of essential fatty acid supplementation on language development and learning skills in autism and Asperger’s syndrome. Autism Asperger’s Digestion, 39, 36–37.
Patrick, R. P., & Ames, B. N. (2015). Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB Journal, 29(6), 2207–2222. https://doi.org/10.1096/fj.14-268342.
Raine, A., Portnoy, J., Liu, J., Mahoomed, T., & Hibbeln, J. R. (2015). Reduction in behavior problems with omega-3 supplementation in children aged 8–16 years: A randomized, double-blind, placebo-controlled, stratified, parallel-group trial. Journal of Child Psychology and Psychiatry, 56(5), 509–520. https://doi.org/10.1111/jcpp.12314.
Robson, L. G., Dyall, S., Sidloff, D., & Michael-Titus, A. T. (2010). Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals. Neurobiology of Aging, 31(4), 678–687. https://doi.org/10.1016/j.neurobiolaging.2008.05.027.
Rockell, J. E. P., Skeaff, C. M., Venn, B. J., Williams, S. M., & Green, T. J. (2008). Vitamin D insufficiency in New Zealanders during the winter is associated with higher parathyroid hormone concentrations: Implications for bone health? New Zealand Medical Journal, 121(1286), 75–84.
Rothman, K. J. (1990). No adjustments are needed for multiple comparisons. Epidemiology, 1(1), 43–46.
Saad, K., Abdel-Rahman, A. A., Elserogy, Y. M., Al-Atram, A. A., Cannell, J. J., Bjorklund, G., & Ali, A. M. (2015). Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutritional Neuroscience., 19(8), 346–351. https://doi.org/10.1179/1476830515y.0000000019.
Saad, K., Abdel-Rahman, A. A., Elserogy, Y. M., Al-Atram, A. A., El-Houfey, A. A., Othman, H. A., & Abdel-Salam, A. M. (2018). Randomized controlled trial of vitamin D supplementation in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry, 59(1), 20–29. https://doi.org/10.1111/jcpp.12652.
Salas, A. A., Woodfin, T., Phillips, V., Peralta-Carcelen, M., Carlo, W. A., & Ambalavanan, N. (2018). Dose–response effects of early vitamin D supplementation on neurodevelopmental and respiratory outcomes of extremely preterm infants at 2 years of age: A randomized trial. Neonatology, 113(3), 256–262. https://doi.org/10.1159/000484399.
Sifers, S. K., Puddy, R. W., Warren, J. S., & Roberts, M. C. (2002). Reporting of demographics, methodology, and ethical procedures in journals in pediatric and child psychology. Journal of Pediatric Psychology, 27(1), 19–25. https://doi.org/10.1093/jpepsy/27.1.19.
Singh, K., Connors, S. L., Macklin, E. A., Smith, K. D., Fahey, J. W., Talalay, P., & Zimmerman, A. W. (2014). Sulforaphane treatment of autism spectrum disorder (ASD). Proceedings of the National Academy of Sciences, 111(43), 15550–15555. https://doi.org/10.1073/pnas.1416940111.
Tang, M., Zhang, M., Cai, H., Li, H., Jiang, P., Dang, R., & Wu, Y. (2016). Maternal diet of polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of hippocampus and influenced glutamatergic and serotoninergic systems of neonatal female rats. Lipids in Health and Disease, 15(1), 71. https://doi.org/10.1186/s12944-016-0236-1.
Ucuz, I. I., Dursun, O. B., Esin, İS., Özgeriş, F. B., Kurt, N., Kiziltunç, A., & Orbak, Z. (2015). The relationship between Vitamin D, autistic spectrum disorders, and cognitive development: Do glial cell line-derived neurotrophic factor and nerve growth factor play a role in this relationship? International Journal of Developmental Disabilities, 61(4), 222–230. https://doi.org/10.1179/2047387714Y.0000000054.
van der Wurff, I. S. M., Meyer, B. J., & de Groot, R. H. M. (2017). A review of recruitment, adherence and drop-out rates in omega-3 polyunsaturated fatty acid supplementation trials in children and adolescents. Nutrients. 9(5), 474. https://doi.org/10.3390/nu9050474.
Vieth, R., Kimball, S., Hu, A., & Walfish, P. G. (2004). Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutrition Journal, 3, 8. https://doi.org/10.1186/1475-2891-3-8.
Voigt, R. G., Mellon, M. W., Katusic, S. K., Weaver, A. L., Matern, D., Mellon, B., & Barbaresi, W. J. (2014). Dietary docosahexaenoic acid supplementation in children with autism. Journal of Pediatric Gastroenterology and Nutrition, 58(6), 715–722. https://doi.org/10.1097/mpg.0000000000000260.
Wang, T., Shan, L., Du, L., Feng, J., Xu, Z., Staal, W. G., & Jia, F. (2016). Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: A systematic review and meta-analysis. European Child & Adolescent Psychiatry, 25(4), 341–350. https://doi.org/10.1007/s00787-015-0786-1.
Yui, K., Koshiba, M., Nakamura, S., & Kobayashi, Y. (2012). Effects of large doses of arachidonic acid added to docosahexaenoic acid on social impairment in individuals with autism spectrum disorders: A double-blind, placebo-controlled, randomized trial. Journal of Clinincal Psychopharmacology, 32(2), 200–206. https://doi.org/10.1097/JCP.0b013e3182485791.
Funding
Partial funding for the study was provided by Massey University Strategic Innovation Fund, Massey University, New Zealand. Additional support was provided by Douglas Nutrition, Pty. Ltd., NZ who were supplying the active supplement and identical-appearing placebo, but who had no input into study design, implementation, data management, statistical analysis or reporting of results.
Author information
Authors and Affiliations
Contributions
PRvH: conceived and designed the study, acquired funding and ethics approval, and supervised the study; HM and OM: coordinated recruitment, participant management, and data collection; HM: conducted the data-analysis with supervision from BJ; HM: prepared the manuscript; CC, KLB, and MK: supervised the trial; WS: advised on the omega-3 fatty acid section and statistical analysis; CACJr: advised on the vitamin D section; BM: advised on the RBC fatty acids laboratory protocol; BT: advised on the autism section. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mazahery, H., Conlon, C.A., Beck, K.L. et al. A Randomised-Controlled Trial of Vitamin D and Omega-3 Long Chain Polyunsaturated Fatty Acids in the Treatment of Core Symptoms of Autism Spectrum Disorder in Children. J Autism Dev Disord 49, 1778–1794 (2019). https://doi.org/10.1007/s10803-018-3860-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-018-3860-y