Abstract
Rubinstein–Taybi syndrome (RTS) is a rare genetic disease that associates intellectual disability with somatic characteristics. We have conducted a study of the overall motor abilities of RTS participants. Static postural performance as well as gait parameters were somewhat decreased, although not significantly compared to typically developing (TD) participants. In contrast, the motor skills requiring a high level of visuomotor coordination were considerably degraded in RTS participants compared to TD participants. We also found that cognitive status was significantly correlated with performance for tasks requiring a higher level of visuomotor coordination in RTS but not TD participants. Our study demonstrates a reduction in the motor performance of RTS participants and a link between the level of intellectual disability and motor capacities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rubinstein-Taybi syndrome (RTS, OMIM #180849, #613684) is a rare (1/125,000) autosomal-dominant genetic disease classified as multiple congenital abnormalities-intellectual deficiency syndrome. It is characterized by distinctive facial features, broad and often angulated thumbs and enlarged toes, short stature, microcephaly, and moderate to severe intellectual disability (ID). Characteristic craniofacial features include downslanted palpebral fissures, low hanging columella, high palate, grimacing smile, and dental talon cusps. RTS is clinically heterogeneous with a wide spectrum of multiple congenital anomalies, including ocular anomalies, congenital heart defects, renal malformations, and brain anomalies (Hennekam 2006; Rubinstein and Taybi 1963). RTS patients also have skin anomalies with an increased risk of keloid formation and an increased predisposition to cancer (van de Kar et al. 2014). Their IQ scores range from 25 to 79 with an average of 36 (Hennekam et al. 1992). RTS participants also express a characteristic profile of repetitive behavior associated with elevated repetitive questioning and body stereotypy (Waite et al. 2015), although they do not have socio-behavioral difficulties compared with normal peers of the same developmental age (Galéra et al. 2009).
RTS is caused by mutations in two genes: mainly the CREBBP gene encoding for the cAMP response element binding protein (CREB-binding protein), and more rarely, the EP300 gene encoding for E1-Aassociated protein p300 (Coupry et al. 2002; Fergelot et al. 2016; Roelfsema et al. 2005). Both genes express homologous proteins belonging to the KAT3 family of lysine acetyltransferases, leading to transcriptional activity and gene expression via histone acetylation and chromatin remodeling. These two transcriptional coactivators play different roles in motor skill learning. CBP+/− conventional knockout mice show a significant deficit in motor coordination and learning in the rotarod task (Alarcon et al. 2004). CREB knock-out mice also have a motor learning deficit similar to that of CREB-binding domain mutant mice, but not to the same domain mutation of EP300, suggesting a more critical role of CREBBP for motor skill learning (Oliveira et al. 2006).
It is generally acknowledged that motor skills are an important factor not only for motor development, but also in the development of cognitive and/or socialof capabilities (Diamond 2000; Fischer 1980; Houwen et al. 2014; Piek et al. 2010). Although it remains unclear as to whether there are causal relationships between these variables, it has been suggested that motor and cognitive development are strongly inter-related (Diamond 2000). As reduced motor capabilities have frequently been obseved in developmental disorders closely associated with ID during childhood or in adulthood (Carmeli et al. 2008), they have been proposed to represent precocious indicators of neural vulnerability (for review see Levit-Binnun et al. 2013). These sensorimotor impairements in patients with ID or other neurodevelopmental disorders may have functional consequences, such as the finding that individuals with ID have an increase risk of falling compared to typically developing persons (Chiba et al. 2009; Sherrard et al. 2002). Several studies have related this increased fall risk to a reduction in postural performance (Blomqvist et al. 2013; Buderath et al. 2009; Dellavia et al. 2009; Gomes and Barela 2007; Lee et al. 2016; Molloy et al. 2003) which may therefore imapct on their dynamic balance during walking or running. Although RTS patients display several specific behavioral patterns including motor stereotypies and poor coordination and are known to have significant motor troubles in their daily life (Galera et al. 2009; Gotts and Liemohn 1977; Hennekam et al. 1992), no study has so far been conducted to evaluates their overall motor abilities.
In the present study, therefore, we investigated the motor capability of RTS patients in order to provide a motor profile that could be used both for a clinical evaluation and recommendations for care and possible specific rehabilitation. For this purpose we used a battery of tests that addressed several aspects of RTS motor activities ranging from low level abilities (posturography) to tasks requiring a higher level of visuospatial coordination. Postural balance control requires the integration of various somatosensory systems including visual, proprioceptive and vesibular informations (Molloy et al. 2003). We therefore addressed these various sensory modalities by comparing postural sway in eyes-open and eyes-closed conditions, since it has been recently reported that adolescents with ID do not rely more on vision to maintain postural balance than their peers without ID (Blomqvist et al. 2013).
The aim was to provide an overall portrait of motor competencies that was not restricted only to basic gait parameter analysis. A second aim of the study was to examine whether the cognitive abilities of participants were correlated with the execution of specific motor tasks.
Materials and Methods
Participants
Twenty-seven participants with Rubinstein–Taybi Syndrome (RTS) were initially involved in the study (mean age 14.7 ± 3 (SD) years; age range 11–20 years). Two participants were subsequently removed from the analysis because they refused to undergo all the proposed tasks. Individuals with RTS, which was diagnosed by two expert geneticists, were recruited from the Bordeaux University medical genetics department at the Bordeaux Pellegrin hospital in France and via a national patient association. Molecular testing (by gene sequencing) was performed on all participants. Eighteen participants with typical development (TD) were also recruited for this study from the local community as a control group that was matched for both age and gender (mean age 15.1 ± 2.5 (SD) years; age range 11–19 years). The characteristics for the two groups groups are detailed in Table 1, whereas Table 2 summarizes the most striking characteristics of the RTS subjects participating in in the present study. None of the participants were diagnosed with ASD. TD adult participants gave their written informed consent whereas parental permission was obtained for each child, with the protocolused being in accordance with ethical guide-lines approved by the local ethical research committee (Comité de Protection des Personnes Bordeaux A CPP N 2011/97). RTS participants passed all tests on the same day and in the cas of child participants in the presence of their parents.
Cognitive Evaluation
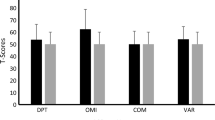
The non-verbal Leiter-R International Performance Scale (Leiter-3) was used to evaluate the cognitive, attentional and neuropsychological abilities of the participants. Cognitive evaluation was perfomed on all RTS participants and on a sub-set of nine TD participants. In the TD participants, the IQ was determined using the visualization and reasonning battery and Table 3 provides detailled participants’ scores for each Leiter subtests.
Posturography
Postural control was assessed using a force plate (AMTI, USA) designed to measure the forces and moments applied to its upper surface (Fig. 1a1; see also Blomqvist et al. 2013; Buderath et al. 2009; Dellavia et al. 2009; Gomes and Barela 2007; Lee et al. 2016; Molloy et al. 2003). Participants initially remained in a quiet and erect self-selected comfortable position for 30 s on a multi-component force platform to record the position of their center of pressure (COP i.e. the point of application of the sum of all forces applied to the force plate). This 30 s value was not only the minimum time required to allow correct assessment of postural performance, but was also chosen since for many RTS participants maintaining a static position throughout this period was already a challenging task. In an upright standing position, all individuals sway naturally in the antero-posterior and lateral direction, with the resulting shifts of the COP providing an index of a participant’s ability to maintain balance. Signals were filtered with a low-pass filter (2nd order, 10 Hz). Participants performed one trial with their eyes open (EO) and a second trial with their eyes closed (EC). The removal of visual inputs with eye closure increased the task difficulty and challenged the postural control since the latter can no longer rely on visual cues but only on proprioceptive and vestibular information (Dellavia et al. 2009; Suomi and Koceja 1994; Webber et al. 2004). The position of the feet was identical for both tests. Posturographic parameters were selected based on those proposed by Prieto et al. (1996). Sway area, the surface of the stabilogram that included the 95% confidence ellipse (Fig. 1a) and the distance travelled by the COP throughout the test period was calculated with a customized program in Matlab (Mathworks, Natick, USA). The higher the value of these two parameters, the more unstable a participants was.
Static posture. a, Experimental setup (a1) ; Representative stabilograms for Typical Development (TD) (a2) and Rubinstein-Taybi Syndrome (RTS) (a3) participants in the eyes open condition. The ellipse in each case indicates sway area (in mm2) that included 95% of Center of Pressure (COP) positions. b, Plots of mean amplitude values for sway area (b1) and COP displacement (b2) for TD (unfilled bars) and RTS participants (filled bars) in eyes open (left histogram pairs) and eyes closed (right histogram pairs) conditions. Error bars : mean ± standard deviation (SD); asterisk symbol indicates statistical significance at p < 0.05
Gait Analysis
Spatial and temporal gait parameters were recorded using a video motion system (Elite BTS Milan Italy, 100 Hz ; for details see Ceccato et al. 2009). Passive reflective markers were fixed to the feet of participants. Participants then had to walk at a self-selected speed in a 12 m walkway (room size 14 × 8 m). In order to produce a minimum of ten step cycles, five trials were monitored. The means of step length, stride length, step width, cadence (steps/min) and stride duration were computed by a Matlab routine. To take into account the developmental changes in height, we normalized gait parameters to each participant’s height (Hof 1996). We also calculated the coefficient of variation (CV = SD/mean as %) of each of these parameters in order to determine walking variability (Lord et al. 2011). The CV reflects the instantaneous variations that affect successive steps, with a higher CV value indicating greater walking irregularity.
Pointing Task
Pointing is a motor task that is both easy to perform and to standardize. It allows evaluating various aspects of fine motor skills and of visuomotor coordination in the programming of execution. Participants were seated in front of a touch screen (1024 × 768 pixels) placed on a desk with their forefinger on a sensor situated on the table’s surface. The distance between the sensor and the screen was adjusted to match the participant’s height. When the target (a circle) appeared on the screen, they had to touch it as quickly as possible, then move their hand back to the starting position. Each participant undertook a training session to ensure that he/she understood the instructions and could complete the task. Each participant performed 40 trials. The target position was unpredictable and randomized on the entire screen. The reaction time (RT) and movement duration (MD) were recorded. Data were again processed using Matlab.
Object Interception Task
Participants had to catch a ball thrown by a speed-selected ping-pong ball robot (Joola Shorty, Germany). The distance between the robot and the participant was adapted to the participant’s height. Some RTS participants (32%) were unable to catch the ball thrown by the robot because either they could not understand the instruction or were frightened by the machine. In this case the balls were thrown by the experimenter. No difference was observed in performance under the two conditions. Twenty trials were performed and the success rate was used to compare both groups. Results of these trials were classified into two according to ball intercept success : (1) ball capture; (2) ball capture or ball contact (see Results).
Statistics
Statistical analyses were performed using IBM SPSS Statistics software (IBM Corporation, USA) or Prism software (Graphpad, USA) and according to Laerd statistics recommendation (Laerd Statistics, London, UK). Unless otherwise specified, values are given as mean ± standard deviation of the mean (M ± SD) and were considered to be significantly different at p < 0.05. Outliers were removed according to SPSS procedure as described by Laerd Statistics. The Levene’s test was employed to assess equality of variance. For the gait analysis, the pointing task and the posturographic data, T-tests were used to determine differences between groups. When homogeneity of variances was not met, we used an unequal variance T-test. For the posturographic tasks, a two-way repeated mixed ANOVA was used to test the effect of the opened eyes versus closed eyes condition in both groups (RTS vs. TD). The p value was adjusted for multiple comparisons using Bonferoni correction. To assess the relationship between IQ and motor performance (both X and Y variables that were measured) we used the non-parametric Spearman rank correlation (correlation coefficient rho) since the sample size was less than 30 in each group. Otherwise, simple linear regression analyses were performed to determine if there were linear relationships between experimental variables and participant age.
Results
Posturography
Figure 1a2–a3 illustrates typical stabilograms determined during a 30 s trial in TD and RTS participants, respectively. The difference in the surface areas of the ellipses indicates that the RTS participants produced movements of larger amplitude than TD participants. To test for an increased dependency on visual cues in RTS participants we then computed the eyes closed versus eyes open ratio for both the sway area and COP displacement. It is noteworthy that in the two conditions used to challenge balance (i.e., eyes open vs. eyes closed), most RTS participants could complete the task. In both groups, furthermore, there was no significant correlation between participant height and the two postural indices (TD eyes open : height vs. sway area, rho = −0.2, p = 0.45; height vs. COP distance, rho = 0.18, p = 0.53 ; TD eyes closed : height vs. sway area, rho = 0.0, p = 1; height vs. COP distance, rho = −0.13, p = 0.63. RTS eyes open : height vs. sway area, rho = −0.28, p = 0.21; height vs. COP distance, rho = −0.41, p = 0.06 ; RTS eyes closed : height vs. sway area, rho = 0.09, p = 0.67; height vs. COP distance, rho = −0.32, p = 0.13). Figure 1b1, b2 presents mean values for sway area and COP displacement, respectively. There was no statistically significant interaction between the groups (RTS and TD) and conditions (eyes open or closed) with sway area, [F(1, 75) = 0.01, p = 0.92]. The main effect of group showed that there was a significant difference between TD and RTS in mean sway area, [F(1, 75) = 13.5, p < 0.001]. The main effect of the eyes open/eyes closed conditions showed that there was no significant difference in mean sway area [F(1, 75) = 0.023, p = 0.88]. There was no significant interaction between the groups (RTS and TD) and conditions (eyes open or closed) on COP distance [F(1, 74) = 0.00, p = 0.99]. The main effect of group showed that there was statistically significant difference between TD and RTS in mean COP distance [F(1, 74) = 6.58, p = 0.012]. Finally, the main effect of the eyes open/eyes closed conditions showed that there was a significant difference in COP distance [F(1, 7) = 4.24, p < 0.043].
Importantly also, there was no significant differences for both TD and RTS participants in the mean sway area ratio between eyes opened and closed conditions (EC/EOTD = 1.04 ± 0.46; EC/EORTS = 1 ± 0.68; p = 0.83) nor the mean COP distance ratio (EC/EOTD = 0.86 ± 0.13 s; EC/EORTS = 0.86 ± 0.19; p = 0.97), suggesting that the RTS participants were no more dependent on visual cues than TD participants and therefore were no less stable. Finally, there were no significant correlations between the two postural performance parameters and the IQ (TD eyes open: IQ vs. sway area, rho = 0.57, p = 0.35; IQ vs. COP distance, rho = 0.25, p = 0.60 ; TD eyes closed: IQ vs. sway area, rho = 0.50, p = 0.54; IQ vs. COP distance, rho = 0.34, p = 0.90 ; RTS : IQ vs. sway area, rho = −0.27, p = 0.44 ; IQ vs. COP distance, rho = −0.14, p = 0.90 ; RTS eyes closed: IQ vs. sway area, rho = 0.19, p = 0.78; IQ vs. COP distance, rho = −0.05, p = 0.98).
Gait Analysis
Table 4 presents the parameter values that were selected to describe locomotor abilities (Oberg et al. 1993). The average height of individuals in the TD group was significantly different from the RTS group (Table 1; p < 0.001). Therefore, as indicated in the Methods, we normalized the spatial variables related to participant height in order to obtain dimensionless data that allowed a direct comparison between participants. We also calculated right and left step length in order to detect any potential asymmetry during walking. Amongst the stepping parameters selected, there was a slight but not significant increase in cadence and step width in RTS participants (Table 4). However, there were virtually no changes in the parameters that were related to step length or step width as well as to stride duration. As a consequence of the increased cadence with the other parameters remaining unaltered, RTS participants walked at a slightly faster speed during testing. The cadence (rho = 0.84, p < 0.001) and stride length (rho = 0.41, p = 0.04) were strongly correlated to the age of RTS participants. No gait parameters were correlated to age in TD participants.
In order to test whether the observed clumsiness was due to intra-subject gait variability, we assessed the regularity of walking in both groups by computing the coefficient of variations. For all parameters, the coefficient of variation was significantly higher in the RTS group compared to the control group (Table 4). This finding therefore suggests that the typically apparent clumsiness of RTS patients does not originate from differences in gait parameters, but rather from a step-to-step variability that in turn affects all gait components.
Again there was no significant correlation between locomotor parameters and cognitive status. (TD: IQ vs. Cadence, rho = 0.37, p = 0.66; IQ vs. stride duration, rho = 0.37, p = 0.66; IQ vs. stride length, rho = 0.54, p = 0.26; IQ vs. step width, rho = 0.10, p = 0.80; RTS : IQ vs. Cadence, rho = 0.14, p = 0.52; IQ vs. stride duration, rho = −0.14, p = 0.52; IQ vs. stride length, rho = 0.43, p = 0.74; IQ vs. step width, rho = −0.23, p = 0.55).
Pointing Task
The observation that RTS participants could complete the pointing task allowed us to test visuomotor coordination as well as attentional capacities. Figure 2b shows that both mean reaction time (Fig. 2b1) and movement duration (Fig. 2b2) were significantly different between the two groups. Participants with RTS had a longer reaction time (RT_TD = 0.335 ± 0.015 s; RT_RTS = 0.454 ± 0.023; p = 0.006) as well as a longer movement duration (MD_TD = 0.32 ± 0.01 ; MD_RTS = 0.81 ± 0.07; p < 0.001) compared to TD participants. The simple linear regression illustrated in Fig. 2a indicates that there were no developmental changes in the age range studied (11–20 years) since the slopes for reaction time (Fig. 2a1) and movement duration (Fig. 2a2) were not significantly different from zero both in the TD (p = 0.7 for RT; p = 0.7 for MD) and RTS (p = 0.8 for RT; p = 0.5 for MD) groups.
Pointing task. a Developmental relationship between age and reaction time (RT, a1) and movement duration (MD, a2) for TD (filled circles) and RTS (unfilled circles) participants. There was no change over time in the age range studied. b Plots of mean amplitude values in reaction time (b1) and movement duration (b2) for TD and RTS participants. Participants with RTS had a longer RT and a longer MD movement duration than TD participants. **p < 0.01; ***p < 0.001. Error bars: mean ± SD
Furthermore, we found significant negative correlations between the scores for cognitive evaluation relative to reaction time (RT) and to movement duration (MD) (RT vs. IQ rho = −0.6, p < 0.003; RT vs. Attention rho = −0.4, p = 0.045 ; RT vs. Visuospatial rho = −0.45, p = 0.03 ; MD vs. IQ rho = 0.5, p = 0.01 ; MD vs. Attention rho = 0.54, p = 0.008) i.e. the greater the IQ the smaller the RT.
Interception
Despite the fact that they produce the appropriate movement towards grabbing the ball, several RTS participants had problems in actually catching it. Consequently, we used two measures: (1), the condition where participants effectively caught the ball (success rate intercept: SRI) or (2), where participants effectively caught or touched the ball that subsequently fell down (success rate intercept or touch: SRIT). The linear regression shown in Fig. 3a indicates that there were no significant developmental changes both in the TD and RTS groups in the age range studied, since the slopes for SRI (Fig. 3a1) and SRIT (Fig. 3a2) were not significantly different from zero. However, the slightly positive slope suggests that there was a slight trend towards an improvement with age in the RTS group. In both intercept conditions, the mean TD participant score was significantly higher (Fig. 3b1, b2). RTS participants also had a significantly higher score in the “intercept or touch the ball” condition, suggesting that they were aware of the task’s requirement and had tried to employ an appropriate gestural strategy.
Interception task. a Developmental changes with age in thrown ball intercepting ability. The values in a1 denote success rates for the actual capture of the ping-pong ball, whereas the scores in a2 relate to the grasping or only touching of the ping-pong ball. b, Plots of mean amplitude values for catching (b1) and grasping or touching (b2). Error bars : mean ± SD
We found a significant positive correlation between the ability of RTS participants to effectively catch the ball and their IQ (SRI vs. IQ rho = 0.43, p = 0.035) but not in TD participants (SRI vs. IQ rho = −0.55, p = 0.12). We did not find any correlation in RTS ot TD participants between cognitive evaluation and the accomplishment of intercepting or touching the ball.
Discussion
Impairment of Motor Abilities in RTS Participants
Several previous studies have reported that people with RTS have more difficulties than TD participants in performing motor tasks, and therefore are considered as being poorly coordinated or clumsy (Galera et al. 2009; Gotts and Liemohn 1977; Hennekam et al. 1992). Here, we report the first detailed study of the motor abilities of patients suffering from the RTS. In contrast to many previous investigations of motor skills in participants with ID using developmental scales such as Movement Assessment Battery for Children (MABC; Vuijk et al. 2010), Test of Gross Motor Development (TGMD-2; Hartman et al. 2010) and psychomotor testing (Bruininks–Oseretsky Test of Motor Proficiency (BOTMP ; Ghaziuddin and Butler 1998; Wuang and Su 2009), we employed an experimental range of motor tests that have been widely used in characterizing other pathologies. These included postugraphy analysis using force plate and quantification of locomotor performance by using motion capture devices (for review see Enkelaar et al. 2011). One novelty of our study is the combined employment of these approaches within the same study. It would now be relevant to compare how motor assessment such as through application of the MABC or other tests correlate with the results of the present study. One important initial finding of our study is the demonstration of a feasible experimental approach to investigating the motor characteristics of RTS patients from the age of 11 years old. In effect, among the 27 participants that were initially selected, only two were excluded from the study due to their inability to achieve the tasks through being over-turbulent and incapable of following the experimental guidelines (e.g., did not remain calm or could not stand upright on the force platform or they ran instead of walking…). This extremely high rate of successful participation is likely to be due to the chosen tasks being similar to those encountered by the individuals in their everyday lives.
The impairment of motor abilities appears to be a general feature of children with neurodevelopmental disorders (Agiovlasitis et al. 2009; Blomqvist et al. 2013; Buderath et al. 2009). Here, we used motor tests ranging from low level automatic activities to tasks that require a high level of visuomotor coordination. One noticeable finding was that all fields of motor activity were not similarly impaired in RTS participants. In most conditions, posturographic data did not reveal systematic significant differences between control and RTS groups, although there was a general trend for postural parameters to increase. As already mentioned above, RTS participants and other individuals with intellectual disability (Dellavia et al. 2009; Lee et al. 2016; Webber et al. 2004) have reduced postural performance compared to TD participants. However, part of this lower performance could be explained by their decreased ability to follow experimental guidelines, especially for postural measurement that required participants to remain still for at least 30 s. Another finding is that static postural performance is not more sensitive to visual cues in RTS participants than in TD participants, as indicated by the lack of difference in the eyes closed/eyes open ratio for the two postural parameters. This suggests in turn that both proprioceptive and vestibular systems undergo normal development in RTS participants, although contradictory findings on this issue have been previously reported. Several studies have claimed a higher dependency on visual cues for ID participants (Dellavia et al. 2009; Suomi and Koceja 1994; Webber et al. 2004). In contrast, and comparable to our results, Blomqvist et al. (2013) reported that adolescents with ID do not rely more on vision to maintain postural balance than participants without ID. Part of these differences may be related to the heterogeneity in the aetiology of ID, as proposed by Dellavia et al. (2009) who found differences between participants with Down syndrome and nonsyndromic ID, as well as to experimental conditions.
Similar to postural ability, gait parameters were barely affected by RTS since only the cadence and step width were slightly increased. That postural regulation is little affected is therefore also indirectly confirmed by the fact that RTS participants maintained a slightly faster stepping speed than TD participants. Indeed, an actual decrease in walking speed is one of the main adaptive strategies used in response to balance problems as observed in stroke patients (Eng and Tang 2007), Parkinson’s disease (Frenkel-Toledo et al. 2005), elderly people (Helbostad et al. 2010) and adolescents with idiopathic scoliosis (Mallau et al. 2007). It is more likely that since RTS participants have greater difficulties in remaining quiet and are more anxious, this would have led to an increase in activity. In contrast, the higher step-to-step variability indicated by the substantial increase in the coefficients of variation affecting all gait components indicate greater difficulties for RTS participants during active displacement.
Developmental Changes
With the exception of gait, we found that for all other motor abilities investigated here there were no significant changes through development in the age range studied (11–20 year old) in either group. This was the case for both low level motor activities (static posture) but also for pointing and object interception. Previous studies of age-related changes in the spatiotemporal characteristics of gait have established that adult velocities are reached by the age of 7–8 years (Froehle et al. 2013; Sutherland et al. 1980), while cadence is stabilized between 13–14 years old. The significant correlation between age (or height) and cadence and stride length in RTS participants is likely to be due to the fact that they have a delayed growth during childhood (Beets et al. 2014), as indicated here by the significant differences in height between the two groups (Table 1; See Text). As established by Froehle et al. (2013), cadence normally decreases from 8 to 13–14 years of age to values similar to adults, and cadence is highly dependent on the participant’. Moreover, developmental aspects of pointing strategies have already been addressed in children aged 5–11 years and in adult healthy participants (Bourgeois and Hay 2003). These authors reported that movement and reaction time decreased with age until an optimized strategy is reached at 11 years old. The RTS participants studied here presented significantly greater reaction times and movement durations, suggesting that the underlying components of motor responsiveness, i.e. planification and execution, are both impaired since reaction time contributes to the programming of movement initiation, whereas movement duration is involved in the execution of the ongoing response (Laszlo and Livesey 1977).
Motor Ability and Cognitive Status
Figure 4 presents the four motor tasks that were tested in our study, classified as a continuum from a low level automatic task (postural capability) to motor functions that require movement planning (intercepting a light ball). Interestingly, the IQ was not significantly correlated with the posturography and locomotor parameters. In contrast, we found a positive correlation between the cognitive status of the RTS participants and the tasks requiring a higher level of visuomotor coordination, as indicated by the finding that IQ was significantly correlated to the pointing task and interception task peformance. Altogether this suggests that the execution of the various motor tasks does not imply equivalent capabilities. Indeed, the relationships between motor and cognitive abilities are complexes especially in developmental discorders that are known to impact on planning functions (Carmeli et al. 2008; Horvat et al. 2003, 2013; Matson and Shoemaker 2009; Millan 2013). By testing walking capacities during dual-task performance, Allali et al. (2008) have shown the influence of executive functions on gait parameters in participants with dementia and impaired executive function (IEF) versus participants with dementia and intact executive function. There are several reports indicating that children with ID (including Down syndrome) had reduced motor abilities related to difficulties in processing sensory and environmental information when preparing and executing movements (Horvat et al. 2003; Virji-Babul and Brown 2004). Although individuals with Down syndrome display a typical walking pattern, there is a more pronounced motor performance degradation during tasks requiring greater levels of information processing and executive functions than that found in individuals without ID (Horvat et al. 2003). By testing postural stability with the same methodology as used in the present study Molloy et al. (2003) found that participants with autism have reduced postural abilities under conditions with modified visual or priprioceptive inputs. Interestingly, however, these authors found that postural stability was unrelated to symptom severity as measured by the Autism Behavior Checklist (ABC; Molloy et al. 2003). In a more recent review of the litterature, Levit-Binnun et al. (2013) drew up a conceptual framework in which secondary sensorimotor abnormalities could represent behavioral indicators of the brain’s abnormal architecture. In their study, they encompassed many disorders including autism spectrum disorders, attention-deficit/ hyperactivity disorder, dyslexia and learning disabilities, anxiety, bipolar affective disorders and they propose that sensorimotor dysfunctionnn may precociously reflect early state of the psypathology as an indicator of brain vulnerability (Levit-Binnun et al. 2013). The hypothesis of a developmental cross-relation between the two developmental domains (cognitive and motor) is therefore highlighted by the results of our study, even if the question of early motor development in Rubistein–Taybi still needs further exploration.
Several studies have reported that people with intellectual disability experience more falls that non-disabled persons (Chiba et al. 2009; Sherrard et al. 2002), and it has been suggested that this could be related to poor postural performance and gait problems (Blomqvist et al. 2013; Dellavia et al. 2009; for review see; Enkelaar et al. 2011; Gomes and Barela 2007; Suomi and Koceja 1994; Webber et al. 2004). To date, comparable data on falls are not available for RTS patients. Part of the motor problem encountered by our RTS participants in their daily lives may therefore not be directly related to actual postural or gait deficiencies, but rather, to attentional and/or visuomotor impairment. Consequently, their motor capabilities would benefit from therapeutic strategies that are centered around early physiotherapyist cares that would be mainly focuses mainly d on improving attention and visuomotor competencies.
Limitations
The present results should be interpreted in light of some limitations of the study. For example, maintaining an upright stance position during static posturographic assessment with eyes closed, independent of the sensorimotor difficulty of the task, may be influenced by an inherent anxiety-provoking component for participants with ID that in turn could partly bias actual measurement. We also noted that RTS participants had greater difficulties in understanding the overall goal of the experiments, and indeed, this is likely to be a general feature of people suffering from ID and thus represents a potential pitfall in the interpretation of such studies.
In conclusion, our study demonstrates a reduction in the motor performance of RTS participants compared to TD controls when a given motor skills is more dependent on the visuo-motor coordination. Moreover, our study reveals a link between the level of intellectual disability and motor capacity.
References
Agiovlasitis, S., McCubbin, J. A., Yun, J., Mpitsos, G., & Pavol, M. J. (2009). Effects of down syndrome on three-dimensional motion during walking at different speeds. Gait & Posture, 30, 345–350.
Alarcon, J. M., et al. (2004). Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron, 42, 947–959.
Allali, G., Assal, F., Kressig, R. W., Dubost, V., Herrmann, F. R., & Beauchet, O. (2008). Impact of impaired executive function on gait stability. Dementia and Geriatric Cognitive Disorders, 26, 364–369.
Beets, L., Rodriguez-Fonseca, C., & Hennekam, R. C. (2014). Growth charts for individuals with Rubinstein-Taybi syndrome. American Journal of Medical Genetics Part A, 164A, 2300–2309.
Blomqvist, S., Olsson, J., Wallin, L., Wester, A., & Rehn, B. (2013). Adolescents with intellectual disability have reduced postural balance and muscle performance in trunk and lower limbs compared to peers without intellectual disability. Research in Developmental Disabilities, 34, 198–206.
Bourgeois, F., & Hay, L. (2003). Information processing and movement optimization during development: Kinematics of cyclical pointing in 5- to 11-year-old children. Journal of Motor Behavior, 35, 183–195.
Buderath, P., et al. (2009). Postural and gait performance in children with attention deficit/hyperactivity disorder. Gait & Posture, 29, 249–254.
Carmeli, E., Bar-Yossef, T., Ariav, C., Levy, R., & Liebermann, D. G. (2008). Perceptual-motor coordination in persons with mild intellectual disability. Disability and Rehabilitation, 30, 323–329.
Ceccato, J. C., de Seze, M., Azevedo, C., & Cazalets, J. R. (2009). Comparison of trunk activity during gait initiation and walking in humans. PLoS ONE, 4, e8193.
Chiba, Y., et al. (2009). Risk of fall for individuals with intellectual disability. American Journal of Intellectual Development Disabilities, 114, 225–236.
Coupry, I., et al. (2002). Molecular analysis of the CBP gene in 60 patients with Rubinstein–Taybi syndrome. Journal of Medical Genetics, 39, 415–421.
Dellavia, C., Pallavera, A., Orlando, F., & Sforza, C. (2009). Postural stability of athletes in special olympics. Perceptual and Motor Skills, 108, 608–622.
Diamond, A. (2000). Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development, 71, 44–56.
Eng, J. J., & Tang, P. F. (2007). Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert Review of Neurotherapeutics, 7, 1417–1436.
Enkelaar, L., Smulders, E., van Schrojenstein Lantman-de Valk, H., Geurts, A. C. H., & Weerdesteyn, V. (2011). A review of balance and gait capacities in relation to falls in persons with intellectual disabilities. Research in Developmental Disabilities, 33, 291–306.
Fergelot, P., et al. (2016). Phenotype and genotype in 52 patients with Rubinstein–Taybi syndrome caused by EP300 mutations. American Journal of Medical Genetics Part A, 170(12), 3069–3082.
Fischer, K. W. (1980). A theory of cognitive-development—The control and construction of hierarchies of skill. Psychological Review, 87, 477–531.
Frenkel-Toledo, S., Giladi, N., Peretz, C., Herman, T., Gruendlinger, L., & Hausdorff, J. M. (2005). Effect of gait speed on gait rhythmicity in Parkinson’s disease: Variability of stride time and swing time respond differently. Journal of Neuroengineering and Rehabilitation, 2, 23.
Froehle, A. W., Nahhas, R. W., Sherwood, R. J., & Duren, D. L. (2013). Age-related changes in spatiotemporal characteristics of gait accompany ongoing lower limb linear growth in late childhood and early adolescence. Gait & Posture, 38, 14–19.
Galéra, C., et al. (2009). Socio-behavioral characteristics of children with Rubinstein–Taybi syndrome. Journal of Autism and Developmental Disorders, 39, 1252–1260.
Galera, C., et al. (2009). Socio-behavioral characteristics of children with Rubinstein–Taybi syndrome. Journal of Autism and Developmental Disorders, 39, 1252–1260.
Ghaziuddin, M., & Butler, E. (1998). Clumsiness in autism and Asperger syndrome: A further report. Journal of Intellectual Disability Research, 42(Pt 1), 43–48.
Gomes, M. M., & Barela, J. A. (2007). Postural control in down syndrome: The use of somatosensory and visual information to attenuate body sway. Motor Control, 11, 224–234.
Gotts, E. E., & Liemohn, W. P. (1977). Behavioral characteristics of three children with the broad thumb-hallux (Rubinstein–Taybi) syndrome. Biological Psychiatry, 12, 413–423.
Hartman, E., Houwen, S., Scherder, E., & Visscher, C. (2010). On the relationship between motor performance and executive functioning in children with intellectual disabilities. Journal of Intellectual Disability Research, 54, 468–477.
Helbostad, J. L., Sturnieks, D. L., Menant, J., Delbaere, K., Lord, S. R., & Pijnappels, M. (2010). Consequences of lower extremity and trunk muscle fatigue on balance and functional tasks in older people: A systematic literature review. BMC Geriatrics, 10, 56.
Hennekam, R. C., et al. (1992). Psychological and speech studies in Rubinstein–Taybi syndrome. American Journal of Mental Retardation, 96, 645–660.
Hennekam, R. C. (2006). Rubinstein–Taybi syndrome. European Journal of Human Genetics, 14, 981–985.
Hof, A. L. (1996). Scaling gait data to body size. Gait & Posture, 4, 222–223.
Horvat, M., Croce, R., Tomporowski, P., & Barna, M. C. (2013). The influence of dual-task conditions on movement in young adults with and without down syndrome. Research in Developmental Disabilities, 34, 3517–3525.
Horvat, M., Ramsey, V., Amestoy, R., & Croce, R. (2003). Muscle activation and movement responses in youth with and without mental retardation. Research Quarterly for Exercise and Sport, 74, 319–323.
Houwen, S., van der Putten, A., & Vlaskamp, C. (2014). A systematic review of the effects of motor interventions to improve motor, cognitive, and/or social functioning in people with severe or profound intellectual disabilities. Research in Developmental Disabilities, 35, 2093–2116.
Laszlo, J. I., & Livesey, J. P. (1977). Task complexity, accuracy, and reaction time. Journal of Motor Behavior, 9, 171–177.
Lee, K., Lee, M., & Song, C. (2016). Balance training improves postural balance, gait, and functional strength in adolescents with intellectual disabilities: Single-blinded, randomized clinical trial. Disability and Health Journal, 9, 416–422.
Levit-Binnun, N., Davidovitch, M., & Golland, Y. (2013). Sensory and motor secondary symptoms as indicators of brain vulnerability. Journal of Neurodevelopment Disorders, 5, 26.
Lord, S., Baker, K., Nieuwboer, A., Burn, D., & Rochester, L. (2011). Gait variability in Parkinson’s disease: An indicator of non-dopaminergic contributors to gait dysfunction? Journal of Neurology, 258, 566–572.
Mallau, S., Bollini, G., Jouve, J.L., & Assaiante, C. (2007). Locomotor skills and balance strategies in adolescents idiopathic scoliosis. Spine, 32, E14–E22.
Matson, J. L., & Shoemaker, M. (2009). Intellectual disability and its relationship to autism spectrum disorders. Research in Developmental Disabilities, 30, 1107–1114.
Millan, M. J. (2013). An epigenetic framework for neurodevelopmental disorders: From pathogenesis to potential therapy. Neuropharmacology, 68, 2–82.
Molloy, C. A., Dietrich, K. N., & Bhattacharya, A. (2003). Postural stability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 33, 643–652.
Oberg, T., Karsznia, A., & Oberg, K. (1993). Basic gait parameters: reference data for normal subjects, 10–79 years of age. Journal of Rehabilitation Research and Development, 30, 210–223.
Oliveira, A. M., Abel, T., Brindle, P. K., & Wood, M. A. (2006). Differential role for CBP and p300 CREB-binding domain in motor skill learning. Behavioral Neuroscience, 120, 724–729.
Piek, J. P., Barrett, N. C., Smith, L. M., Rigoli, D., & Gasson, N. (2010). Do motor skills in infancy and early childhood predict anxious and depressive symptomatology at school age? Human Movement Science, 29, 777–786.
Prieto, T. E., Myklebust, J. B., Hoffmann, R. G., Lovett, E. G., & Myklebust, B. M. (1996). Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Transactions on Bio-Medical Engineering, 43, 956–966.
Roelfsema, J. H., et al. (2005). Genetic heterogeneity in Rubinstein–Taybi syndrome: Mutations in both the CBP and EP300 genes cause disease. American Journal of Human Genetics, 76, 572–580.
Rubinstein, J. H., & Taybi, H. (1963). Broad thumbs and toes and facial abnormalities. A possible mental retardation syndrome. American Journal of Diseases of Children, 105, 588–608.
Sherrard, J., Tonge, B. J., & Ozanne-Smith, J. (2002). Injury risk in young people with intellectual disability. Journal of Intellectual Disability Research, 46, 6–16.
Suomi, R., & Koceja, D. M. (1994). Postural sway patterns of normal men and women and men with mental retardation during a two-legged stance test. Archives of Physical Medicine and Rehabilitation, 75, 205–209.
Sutherland, D. H., Olshen, R., Cooper, L., & Woo, S. L. (1980). The development of mature gait. The Journal of Bone and Joint Surgery, 62, 336–353.
van de Kar, A. L., et al. (2014). Keloids in Rubinstein–Taybi syndrome: A clinical study. The British Journal of Dermatology, 171, 615–621.
Virji-Babul, N., & Brown, M. (2004). Stepping over obstacles: Anticipatory modifications in children with and without down syndrome. Experimental Brain Research, 159, 487–490.
Vuijk, P. J., Hartman, E., Scherder, E., & Visscher, C. (2010). Motor performance of children with mild intellectual disability and borderline intellectual functioning. Journal of Intellectual Disability Research, 54, 955–965.
Waite, J., et al. (2015). Repetitive behavior in Rubinstein–Taybi syndrome: Parallels with autism spectrum phenomenology. Journal of Autism and Developmental Disorders, 45, 1238–1253.
Webber, A., Virji-Babul, N., Edwards, R., & Lesperance, M. (2004). Stiffness and postural stability in adults with down syndrome. Experimental Brain Research, 155, 450–458.
Wuang, Y. P., & Su, C. Y. (2009). Reliability and responsiveness of the Bruininks–Oseretsky test of motor proficiency-second edition in children with intellectual disability. Research in Developmental Disabilities, 30, 847–855.
Acknowledgments
We thank the patients and their family for their participation in this study, and the Association Française du syndrome de Rubinstein-Taybi. We thank Clément Bader and Paul Perez for their contribution to the conception of the Rubival protocol. Grants were obtained from Ministery of Health (APN/PHRC national 2011 Rubival), and from Fondation du syndrome de Rubinstein–Taybi (under the auspice of the Fondation de France).
Author Contributions
JRC and DL conceived of the study, participated in its design and coordination and drafted the manuscript; EB, EG and ED participated in the design and interpretation of the data, performed the measurement and performed the statistical analysis; MPB, CG, IG, EG, JVG, PF, SF, ET and BA participated in the coordination of the study and performed the measurement; MB and AA conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cazalets, J.R., Bestaven, E., Doat, E. et al. Evaluation of Motor Skills in Children with Rubinstein–Taybi Syndrome. J Autism Dev Disord 47, 3321–3332 (2017). https://doi.org/10.1007/s10803-017-3259-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-017-3259-1