Abstract
We investigated heart rate (HR) in infants at 3, 6, 9, and 12 months of age, at high (HRA) and low (LRC) familial risk for ASD, to identify potential endophenotypes of ASD risk related to attentional responses. HR was extracted from functional near-infrared spectroscopy recordings while infants listened to speech stimuli. Longitudinal analysis revealed that HRA infants and males generally had lower baseline HR than LRC infants and females. HRA infants showed decreased HR responses to early trials over development, while LRC infants showed increased responses. These findings suggest altered developmental trajectories in physiological responses to speech stimuli over the first year of life, with HRA infants showing less social orienting over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a developmental disorder that is characterized by persistent deficits in social communication and restricted, repetitive patterns of behavior (American Psychiatric Association 2013). Although the prevalence of autism in the general population is approximately 1:68 children (Baio 2014), it is far higher among infants with an older sibling; indeed, among such infants the overall recurrence rate is estimated to be anywhere between 18.7 and 47.5% for males (Messinger et al. 2015). Siblings and other first-degree relatives may in many cases share “endophenotypes,” or subtle traits intermediate between genes and clinical manifestations of a disorder, which are not themselves wholly predictive of ASD, but which nonetheless are risk factors for the development of ASD (Gottesman and Gould 2003; Viding and Blakemore 2007). Recent research conducted on infant siblings of children with ASD aims to identify such endophenotypes of ASD, which may shed light on the etiology of the disorder (Tager-Flusberg 2010).
One potentially unifying theory of ASD posits that the disorder may result from a deficit in the psychological and biological mechanisms which typically bias individuals toward preferentially orienting or attending to the social world (Chevallier et al. 2012). To investigate cognition and social attention developmentally, researchers commonly use some variant of looking time for non-verbal or infant populations (Aslin 2007). However, heart rate has also long been used as a physiological marker of attention that is likely superior to behavioral measures such as looking time or quieting, as it more directly measures the mechanisms underlying attentional control (Courage et al. 2006; Graham and Jackson 1970). Richards and Casey (1991) proposed a four-phase model to explain the changes in heart rate that accompany infants’ attention to environmental stimuli, typically in looking time studies. When infants first encounter a new stimulus, they experience a brief deceleration-acceleration response in heart rate, corresponding to the detection of change in the environment. Once they engage or orient themselves to a stimulus, heart rate undergoes a rapid deceleration from its pre-stimulus level. The third phase is that of “sustained attention,” during which heart rate remains at its decelerated level and the autonomic nervous system is activated to facilitate cognitive processing of the stimulus. This phase may be largely voluntary or subject-controlled. Finally, during the “attention termination” stage, the lowered heart rate begins to return to pre-stimulus levels. During this phase, although children may still be looking at a stimulus, they are no longer processing information about it, and they are also resistant to new stimulation (as cited in Courage et al. 2006).
In the current study we investigated heart rate changes in a longitudinal sample of infants at high and low familial risk for developing ASD, in order to identify potential endophenotypes of ASD risk related to attention and social orienting. Infants were studied at 3, 6, 9, and 12 months of age, and stimulus-related changes in their heart rates were quantified in terms of changes in inter-beat intervals (IBI). We first asked whether our sample of infants with an older sibling with an ASD diagnosis (high risk for autism; HRA), showed different trajectories of baseline heart rate development than infants with an older typically developing sibling (low risk controls; LRC) over the first year of life. Past research indicates that resting mean heart rate changes predictably over development, and may show significantly different patterns of maturation in children who were born prematurely (Glotzbach et al. 1994; Katona et al. 1980; Mrowka et al. 1996). Older children with ASD have been found, in different cases, to have elevated mean heart rates (Bal et al. 2010; Cohen and Johnson 1977; Kootz and Cohen 1981; Kushki et al. 2014, 2013; Ming et al. 2005; Watson et al. 2012), or not to differ significantly from their typical peers on measures of mean heart rate (Graveling and Brooke 1978; Lake et al. 1977).
Our second aim was to determine whether and at what ages HRA and LRC infants show differences in orienting and attention, as measured by IBI changes, in response to speech stimuli. In past studies, children with ASD have been found to show smaller (Daluwatte et al. 2013) or no heart rate changes (Corona et al. 1998; Levine et al. 2012; Sigman et al. 2003) to various (social and non-social) visual stimuli compared to their TD peers. Studies of heart rate responses to auditory stimuli in ASD are much less common, although a recent study of 6–21 year olds with ASD revealed that these individuals showed higher baseline heart rates than did their TD peers, but did not show any changes in heart rate in response to auditory stimuli (Porges et al. 2013). In this study we employed a speech processing paradigm developed by Gervain et al. (2008), in which infants listened passively to sequences of syllables containing either consecutive repetitions (e.g. ba-lo-lo, pe-na-na) or random non-repetitive sequences (e.g. pe-na-ku, ba-lo-ti). Prior work has shown that healthy (LRC) newborns, 7, and 9 month olds tested with this paradigm neurally differentiate the repetition and random sequences, thus showing evidence for the presence of an early mechanism for processing structural regularities in speech (Gervain et al. 2008; Wagner et al. 2011). In the present study, analyses of attention to the stimuli were carried out across all stimuli independent of condition as this distinction was not relevant to our aims of investigating overall auditory attention.
Given the profile of deficits that is characteristic of children at risk for ASD, we hypothesized that we might see attenuated heart rate responses to these speech stimuli in HRA infants, if they were failing to engage with relevant language-relevant stimuli from as early as the first year of life. We investigated this question using longitudinal statistical methods in order to take into account the developmental nature of our study population, as well as the developmental course of ASD development. Additionally, in keeping with past studies using these stimuli, we investigated changes in infants’ IBIs over the course of experimental exposure. Both Gervain et al. (2008) and Wagner et al. (2011) found evidence of neural changes to these stimuli over the course of an experiment (that is, comparing the first to the last four experimental blocks).
Methods
Participants
Infant participants were recruited into a longitudinal study before 3 months of age, and were classified as members of a group at high-risk of autism (HRA, n = 40) or a group of low-risk controls (LRC, n = 48). Members of the high-risk group had at least one older full sibling with a community diagnosis of ASD. All HRA probands had a pre-existing diagnosis of ASD, and in addition in most cases their diagnosis was confirmed independently in the project with the social communication questionnaire (SCQ; Rutter et al. 2003) and/or the autism diagnostic observation schedule (ADOS; Lord et al. 2008). Parents provided details of the ASD diagnosis including name and location of the diagnosing provider, date of diagnosis and the behaviors that supported the diagnosis. Members of the low-risk group were typically developing, had at least one older sibling and no siblings with an autism diagnosis, or first-degree relative with known ASD or other neurodevelopmental disorder. Additional inclusion criteria were gestational age of at least 36 weeks, no history of prenatal or postnatal medical or neurological disorders, and no known genetic disorders. Participants in the HRA group were excluded from further analysis if they had a positive ASD outcome at 3 years (n = 14) in order to focus the study on endophenotypes of ASD risk.Footnote 1 While all the LRC participants met the inclusion criteria at the time of enrollment, four participants were subsequently found to have a first-degree relative with known or suspected ASD and were excluded from the analysis.
Heart rate (HR) was assessed using a previously described method to extract HR from functional near-infrared spectroscopy (fNIRS) recordings (Perdue et al. 2014). Participants visited the lab over the first year of life at ages 3, 6, 9, and 12 months, and could optionally participate in the fNIRS study as part of a larger test battery involving EEG, behavioral assessments, and other measures. The number of usable fNIRS datasets for each group at each age is shown in Table 1 along with the number of trials and channels used to calculate HR. Participants in the HRA group contributed an average of 1.8 datasets, and participants in the LRC group contributed an average of 2.4 datasets, with a significant difference between the two groups (t(82) = 2.71, p = 0.008). Additional datasets (n = 61) were collected but excluded because they were unusable due to infant fussiness or measurement refusal (n = 4), technical problems (n = 3), too few trials completed to perform the full analysis (n = 21), or the HRA participant had a subsequent ASD diagnosis (n = 22 datasets, from 14 participants), or the LRC participant was found to have a first-degree relative with ASD (n = 11 datasets, from four participants). A total of 49 usable NIRS datasets were collected from the HRA group, and a total of 105 usable NIRS datasets were collected from the LRC group. The number of datasets excluded in each category is shown in Table 2. Informed consent was received from the caregivers before the study, and the study was approved by the local institutional review board.
Stimulus paradigm
An auditory stimulus paradigm was used, consisting of blocks of trisyllabic nonsense words with syllable structures in the form of “ABB” (e.g. ba-lo-lo) or “ABC” (e.g. ba-lo-ti), identical to those used in a prior study by Gervain et al. (2008). Stimuli were synthesized speech and all syllables had the same pitch (200 Hz). The auditory stimulus lasted for 16 s per block with ten “words” in each block and a random inter-word interval of 500–1500 ms. Block timing was experimenter-controlled, with at least 15 s between blocks, and blocks were presented when the infants had relatively low movement and seemed receptive. ABB and ABC blocks were presented in one of two pseudorandom sequences. Up to 28 total blocks were presented, and the experiment was stopped early if the infants became fussy. Blocks where other sounds or talking were present were marked and removed from the analysis. Infants were simultaneously presented with a video of moving shapes, and were seated on a caregiver’s lap in a dimly lit room during data collection. If infants became distracted or upset, an experimenter would calm them with bubbles or silent toys.
NIRS Data Collection and Heart Rate Extraction
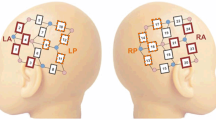
Heart rate was calculated from fNIRS data collected with a Hitachi ETG-4000, with wavelengths of 695 and 830 nm and a sampling frequency of 10 Hz. A 24-channel NIRS probe was used with bilateral temporal panels of 12 channels each, and a source-detector spacing of 3 cm. Heart rate extraction from the oxyhemoglobin NIRS signal was performed as described in Perdue et al. (2014). The heart rate extraction method combines estimates over all good channels and is not spatially specific, and every dataset had enough good channels to get a robust estimate of heart rate. Calculating HR from fNIRS data has been shown to be robust and accurate (Holper et al. 2016; Perdue et al. 2014; Trajkovic et al. 2011).
Heart Rate Statistical Methods
Three metrics were calculated to operationalize our measures of interest including baseline heart rate, overall attentional response, and change in attentional response over the course of the experiment. Baseline heart rate was calculated as a mean over a 2 s pre-stimulus interval for all trials, and is used as a measure unrelated to the social stimulus. Participants’ baseline heart rates were averaged over all pre-stimulus periods in the experiment. Stimulus-related analyses were conducted on the inter-beat intervals (IBIs). IBI change during stimulus presentation was calculated for each block by baseline correcting over the 2 s immediately prior to the onset of the stimulus block in order to determine the stimulus-related heart rate change from baseline. Responses to each trial were quantified as the mean IBI response from 0 to 20 s after stimulus presentation began. Overall attentional responses to the speech stimuli were defined as the mean IBI response to all trials (maximum of 14). Attentional changes over the course of the experiment were addressed by calculating mean response to early trials (first four) and late trials (last four), similar to the analysis of cortical hemodynamic responses in Gervain et al. (2008) and Wagner et al. (2011).
Heart rate responses were modeled longitudinally from study timepoints at 3, 6, 9, and 12 months using linear regression conducted in R (Pinheiro et al. 2016; R Core Team 2016). The regression model for each metric contained age, risk group, and gender. The models excluded stimulus condition of ABB or ABC as hypotheses about differences in attention by condition were beyond the scope of this paper. Follow up analyses tested for interaction effects between age and group or gender and group in order to test for differing developmental trajectories between the two risk groups.
Results
Baseline Heart Rate
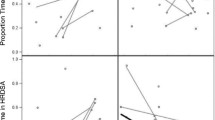
Longitudinal linear regression analysis of baseline HR of HRA and LRC infants modeling age, group, and gender revealed relationships between baseline HR and group (b = 3.48 such that the LRC group had higher baseline HR at all developmental time points, t(150) = 2.97, p= 0.0034), between baseline HR and age (b = −0.86 such that baseline HR decreased with age, t(150) = 6.34, p < 0.0001), and between baseline HR and gender (b = −2.36 such that males had lower baseline HR, t(150) = 2.21, p = 0.029). Estimated marginal means showing the change in baseline HR with age for each group are shown in Fig. 1.
Follow-up analyses revealed no modifying group × age interactions (b = −0.32 for LRC × age, t(149) = 1.12, p = 0.26) or group × gender interactions (b = −1.53 for LRC × male, t(149) = 0.65, p = 0.52). Baseline heart rates of the two groups and sexes differed significantly and by the same amount at all ages. This result suggests that although there may not be differences in the trajectories of baseline heart rate change between these two groups, there may be differences in absolute mean heart rate between HRA and LRC infants, and males and females, which persist across the first year of life. Specifically, HRA infants tended to have lower baseline heart rates than LRC infants, and males had lower heart rates than females.
Mean IBI Responses
Longitudinal linear regression analysis of mean IBI changes modeling age, group, and gender revealed a relationship between mean IBI and group (b = 2.17 such that LRC was larger, t(300) = 2.66, p = 0.0082), that was not modified by a group × age interaction (b = −0.01 for LRC × age, t(299) = 0.03, p = 0.98). Estimated marginal means depicting group differences are shown in Fig. 2. IBI changes to speech stimuli differed for HRA and LRC infants across all ages, regardless of sex. HRA showed generally smaller IBI changes to speech stimuli than did LRC infants.
IBI First 4 Responses
The IBI time course responses to the stimuli are shown in Fig. 3. After the stimulus onset, there is an increase in IBI reflecting a heart rate deceleration, which generally lasts for the duration of the stimulus presentation. Larger differences in the HR from baseline are seen to the first four stimuli as compared to the last four. HR responses were collapsed over the stimulus time window by averaging and used for further statistical analysis. Longitudinal linear regression analysis of IBI changes in the first four experimental blocks modeling age, group, and gender revealed an effect of group (b = 3.86 such that LRC had larger IBI responses to the speech stimuli, t(300) = 3.12, p = 0.002), that was modified by a group × age interaction (b = 0.79 for LRC × age, t(299) = 2.12 p = 0.035), such that LRC infants showed increasing IBI changes to these first 4 stimulus blocks over the course of development, whereas HRA infants showed a lowering of IBI responses with increasing age. Estimated marginal means by group over age are shown in Fig. 4.
IBI Last 4 Responses
Longitudinal linear regression analysis of IBI changes in the last experimental blocks modeling age, group, and gender revealed an effect of group (b = 2.06 such that the LRC group had higher IBI responses, t(300) = 2.50, p = 0.013) and gender (b = −1.56 such that males had lower IBI responses, t(300) = 2.10, p = 0.036). There was no age effect (b = 0.15, t(300) = 1.10, p = 0.27). The linear regression model found that the LRC group has more positive IBI responses to the last four blocks than the HRA group, and males had more negative IBI responses than females. Estimated marginal means separating group and gender are shown in Fig. 4. The estimates indicated positive IBI responses (HR deceleration) to the last four stimuli for LRC females and negative IBI responses (HR acceleration) to the last four stimuli for HRA males, with LRC males and HRA females falling between them.
Discussion
In this study, we investigated baseline heart rate and heart rate responses to speech stimuli in a sample of infants at high and low familial risk for ASD over the first year of life. Longitudinal analysis of baseline heart rate data at 3, 6, 9, and 12 months of age revealed significant relations among group, age, and gender and baseline heart rate variables, such that, on average, HRA infants had lower baseline heart rates than LRC infants, and males in general had lower baseline heart rates than females at all time points, although all infants’ baseline heart rates decreased over the first year of life. These results replicate those of past studies, in which typically developing individuals’ heart rates decrease over development (Fracasso et al. 1994; Richards and Cameron 1989). These findings also suggest that although there may not be differences in the developmental trajectories of resting heart rate between HRA and LRC infants, there appear to be differences in mean resting heart rate between these two groups, as well as the two genders, which persist across the first year of life. Our finding of significantly lower average baseline heart rates in HRA infants over all measured ages actually stands in contrast to much previous work, which has found evidence of increased mean heart rate in older participants with ASD (Bal et al. 2010; Daluwatte et al. 2013; Kushki et al. 2013, 2014; Ming et al. 2005; Watson et al. 2012). Several explanations may account for this discrepancy. For example, it may be the case that individuals at risk for ASD have atypical resting heart rates, but that the nature of this atypicality may change over development. As this is the first known study of heart rate in infants at risk for ASD, future research might investigate this hypothesis.
Alternatively, given the heterogeneity in severity, behavioral manifestation, and putative underlying causes of ASD, it is possible that our finding of lower average baseline heart rates in this sample may indicate an endophenotype of ASD risk that is specific to infants with affected older siblings, and thus indicates a distinct mechanism of ASD risk. Future research should investigate the role of autonomic system function potentially underlying these heart rate differences, in order to determine whether atypicalities in physiological arousal are an endophenotype of ASD specific to infant siblings (and perhaps related either to distinct genetic risk factors, or to the social or environmental effects of having an older sibling with ASD), and/or whether there is significant variation in the arousal of infant siblings of children with ASD, relative to matched low ASD risk populations.
Further compounding this potential heterogeneity within ASD endophenotypes is our finding of gender-based differences in heart rate, which are aligned with and extend the findings of past work, in which males were found to have lower baseline heart rates than females from 1 year of age through to adulthood (20 years of age; (Silvetti et al. 2001)). Given that males are more likely to develop ASD than females, and that the sibling recurrence rate of ASD is much higher in males than in females (Werling and Geschwind 2015), it is possible that heart rate and physiological arousal in ASD might appear or function differently in males and females. Future research into ASD endophenotypes—including those investigating heart rate and metrics of physiological arousal—may benefit from a focus on sex-specific mechanisms of risk and development.
HRA and LRC infants’ heart rate responses to speech stimuli were also significantly different from each other across all ages, when these responses were collapsed over all trials presented. Specifically, HRA infants showed smaller IBI changes (less heart rate deceleration) to the speech stimuli than LRC infants. This finding supports our hypothesis, and aligns with past autism literature, suggesting that individuals with ASD show smaller heart rate changes to both social and non-social visual stimuli than TD individuals (Daluwatte et al. 2013); our finding also extends this phenomenon to the auditory realm, to children who have familial ASD risk, and to time points within the first year of life. It also suggests a mechanism by which children at high risk for ASD might lose out on potentially language-relevant learning opportunities—if these attenuated changes in heart rate either give way to, or result from, reduced attentional capacity for speech or social stimuli. Further research is warranted to determine the direction of this relationship, as well as to determine whether the observed phenomenon is specific to speech stimuli, or more generally, environmental stimuli that are social in nature. However, the current findings indicate that attenuated heart rate changes to speech stimuli, which are fairly easy to measure and are detectable at multiple time points within the first year of life (including as young as 3 months of age), may thus be a potentially promising endophenotype of ASD risk.
In keeping with past literature using these speech stimuli, we also investigated differences in infants’ IBIs between the first and last four trials within a single experimental session or time point. Longitudinal analyses of IBI responses to the first four experimental blocks revealed that LRC infants showed increased heart rate deceleration to these first four stimulus blocks as they got older, whereas HRA infants showed decreasing heart rate deceleration as they got older. These results indicate that whereas LRC infants’ attention to our initial speech stimuli increased with increasing age, HRA infants’ attention to these initial stimuli likely decreased with increasing age. Again, these results point to a potential endophenotype of ASD risk, and in particular, suggest a mechanism that may underlie previously established language difficulties in children at familial risk for ASD. HRA infants appear to decrease attention, whereas LRC infants appear to increase attention to the initially presented speech stimuli as they get older. Close examination of Fig. 3 suggests that HRA infants’ decreased attention with age appears to be due to a decrease in their “sustained attention” rather than orientation to the stimuli (particularly between 3 and 9 months, while the rapid heart rate deceleration that indicates orientation to a stimulus actually appears to get stronger with age, heart rate returns to baseline levels more quickly with increasing age), while LRC infants show increases in both their orienting responses and their sustained attention with age (heart rate decelerates strongly shortly after stimulus onset, but does not return to baseline until between 20 and 30 s after stimulus onset after 6 months of age in the LRC sample). By their decreased attention to these initial stimuli with age, perhaps due to a lack of perceived relevance, HRA infants may spend less time processing the stimuli to which LRC infants dedicate cognitive resources, and in so doing, may lose out on learning opportunities that may form the foundation of later language skills. The age-related decrease in IBI responses to these initial experimental blocks thus seems to represent a kind of “tuning out” of these stimuli, in this group of infants. Future research investigating whether and what kinds of stimuli HRA infants instead tune into might provide important qualitative information about the differences in developmental trajectories between children at low and high familial ASD risk (e.g. visual geometric repetition (Pierce et al. 2016); audiovisual synchrony (Klin et al. 2009)).
Finally, longitudinal analysis of IBI changes over the last four experimental blocks revealed that across all ages, HRA infants show more negative IBI responses to these stimuli. In this case, HRA infants showed increased acceleration, rather than decreased deceleration, in their heart rate responses to the final speech stimulus blocks within an experiment. Further research, for example using physiological measures of stress such as salivary cortisol or galvanic skin resistance measures, might reveal whether these increased heart rate responses indicate that the speech stimuli have become aversive to HRA infants over repeated exposure. Such a finding might, in part, explain why HRA infants show decreasing attention to these stimuli at subsequent developmental time points.
An additional gender effect within the data, such that males across both risk groups showed smaller IBI changes to the last four stimulus blocks within an experiment, suggests gender differences in disengagement from the presented stimuli. Future research into this gender difference might be able to disentangle whether it reflects increased habituation, or an increased aversive response to the experimental stimuli.
Overall, the results herein suggest potential mechanisms by which established differences in speech, language, or other aspects of social development, between HRA and LRC—as well as male and female—infants may occur, and lay the foundation for future work which may explore the direct links between early attention or social orienting, and later social behaviors. Future research investigating the physiological phenomena explored herein, in those infants who go on to receive an ASD diagnosis,Footnote 2 may also lay the foundation for discovery of predictors of developing ASD earlier than is currently possible using traditional clinical methods.
Notes
To rule out the possibility that confounding cognitive factors may explain any findings of differing psychological arousal to speech stimuli in HRA infants, we examined available Mullen data, and found that neither verbal nor non-verbal developmental quotients (DQ) were significantly different between HRA and LRC infants (p (verbal DQ, 6 months) = 0.57, p (verbal DQ, 12 months) = 0.22, p (non-verbal DQ, 6 months) = 0.69, p (non-verbal DQ, 12 months) = 0.36) or males and females (p (verbal DQ, 6 months) = 0.71, p (verbal DQ, 12 months) = 0.18, p (non-verbal DQ, 6 months) = 0.92, p (non-verbal DQ, 12 months) = 0.12; see Table 1).
At the time of this manuscript such analyses are not possible (models including gender did not converge) as of the 66 infants who have completed the study and received final clinical judgments, only three females had received an ASD diagnosis.
References
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5 ® ). Washington, D.C.: American Psyciatric Association. doi:10.1002/gps.
Aslin, R. N. (2007). What’s in a look? Developmental Science, 10(1), 48–53. doi:10.1111/j.1467-7687.2007.00563.x.
Baio, J. (2014). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Center for Disease Control and Prevention Surveillance Summaries, 63(2), 1–24.
Bal, E., Harden, E., Lamb, D., Van Hecke, A. V., Denver, J. W., & Porges, S. W. (2010). Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders, 40(3), 358–370. doi:10.1007/s10803-009-0884-3.
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S., & Schultz, R. T. (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16(4), 231–239. doi:10.1016/j.tics.2012.02.007.
Cohen, D. J., & Johnson, W. T. (1977). Cardiovascular correlates of attention in normal and psychiatrically disturbed children. Blood pressure, peripheral blood flow, and peripheral vascular resistance. Archives of General Psychiatry, 34(5), 561–567.
Corona, R., Dissanayake, C., Arbelle, S., Wellington, P., & Sigman, M. (1998). Is affect aversive to young children with autism? Behavioral and cardiac responses to experimenter distress. Child Development, 69(6), 1494–1502.
Courage, M. L., Reynolds, G. D., & Richards, J. E. (2006). Infants’ attention to patterned stimuli: Developmental change from 3 to 12 months of age. Child Development, 77(3), 680–695. doi:10.1111/j.1467-8624.2006.00897.x.
Daluwatte, C., Miles, J. H., Christ, S. E., Beversdorf, D. Q., Takahashi, T. N., & Yao, G. (2013). Atypical pupillary light reflex and heart rate variability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 43(8), 1910–1925. doi:10.1007/s10803-012-1741-3.
Fracasso, M. P., Porges, S. W., & Lamb, M. E. (1994). Cardiac activity in infancy: Reliability and stability of individual differences. Infant Behavior and Development, 17(3), 277–284. doi:10.1016/0163-6383(94)90006-X.
Gervain, J., Macagno, F., Cogoi, S., Peña, M., & Mehler, J. (2008). The neonate brain detects speech structure. Proceedings of the National Academy of Sciences of the United States of America, 105(37), 14222–14227. doi:10.1073/pnas.0806530105.
Glotzbach, S. F., Edgar, D. M., Boeddiker, M., & Ariagno, R. L. (1994). Biological rhythmicity in normal infants during the first 3 months of life. Pediatrics, 94(4), 482–488.
Gottesman, I. I., & Gould, T. D. (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. The American Journal of Psychiatry, 160(4), 636–645. doi:10.1176/appi.ajp.160.4.636.
Graham, F. K., & Jackson, J. C. (1970). Arousal systems and infant heart rate responses. Advances in Child Development and Behavior, 5, 59–117.
Graveling, R. A., & Brooke, J. D. (1978). Hormonal and cardiac response of autistic children to changes in environmental stimulation. Journal of Autism and Childhood Schizophrenia, 8(4), 441–455.
Holper, L., Seifritz, E., & Scholkmann, F. (2016). Short-term pulse rate variability is better characterized by functional near-infrared spectroscopy than by photoplethysmography. Journal of Biomedical Optics, 21(9), 91308–091308. doi:10.1117/1.JBO.21.9.091308.
Katona, P. G., Frasz, A., & Egbert, J. (1980). Maturation of cardiac control in full-term and preterm infants during sleep. Early Human Development, 4(2), 145–159.
Klin, A., Lin, D. J., Gorrindo, P., Ramsay, G., & Jones, W. (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature, 459(7244), 257–261. doi:10.1038/nature07868.
Kootz, J. P., & Cohen, D. J. (1981). Modulation of sensory intake in autistic children: cardiovascular and behavioral indices. Journal of the American Academy of Child Psychiatry, 20(4), 692–701.
Kushki, A., Brian, J., Dupuis, A., & Anagnostou, E. (2014). Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism, 5(1), 39. doi:10.1186/2040-2392-5-39.
Kushki, A., Drumm, E., Pla Mobarak, M., Tanel, N., Dupuis, A., Chau, T., & Anagnostou, E. (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PloS One, 8(4), e59730–e59710. doi:10.1371/journal.pone.0059730.
Lake, C. R., Ziegler, M. G., & Murphy, D. L. (1977). Increased norepinephrine levels and decreased dopamine-beta-hydroxylase activity in primary autism. Archives of General Psychiatry, 34(5), 553–556.
Levine, T. P., Sheinkopf, S. J., Pescosolido, M., Rodino, A., Elia, G., & Lester, B. (2012). Physiologic arousal to social stress in children with autism spectrum disorders: A pilot study. Research in Autism Spectrum Disorders, 6(1), 177–183. doi:10.1016/j.rasd.2011.04.003.
Lord, C., Rutter, M., Dilavore, P., & Risi, S. (2008). ADOS: Autism diagnostic observation schedule. Boston: Hogrefe.
Messinger, D. S., Young, G. S., Webb, S. J., Ozonoff, S., Bryson, S. E., Carter, A., et al. (2015). Early sex differences are not autism-specific: A baby siblings research consortium (BSRC) study. Frontiers in Psychology, 6(1), 1–12. doi:10.1186/s13229-015-0027-y.
Ming, X., Julu, P. O. O., Brimacombe, M., Connor, S., & Daniels, M. L. (2005). Reduced cardiac parasympathetic activity in children with autism. Brain & Development, 27(7), 509–516. doi:10.1016/j.braindev.2005.01.003.
Mrowka, R., Patzak, A., Schubert, E., & Persson, P. (1996). Linear and non-linear properties of heart rate in postnatal maturation. Cardiovascular research 31(3), 447–454.
Perdue, K. L., Westerlund, A., McCormick, S. A., & Nelson, C. A. III (2014). Extraction of heart rate from functional near-infrared spectroscopy in infants. Journal of Biomedical Optics, 19(6), 067010–067010. doi:10.1117/1.JBO.19.6.067010.
Pierce, K., Marinero, S., Hazin, R., McKenna, B., Barnes, C. C., & Malige, A. (2016). Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biological Psychiatry, 79(8), 657–666. doi:10.1016/j.biopsych.2015.03.032.
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., R Core Team. (2016). Linear and nonlinear mixed effects models [R package nlme version 3.1-128]. http://CRAN.R-project.org/package=nlme.
Porges, S. W., Macellaio, M., Stanfill, S. D., McCue, K., Lewis, G. F., Harden, E. R., et al. (2013). Respiratory sinus arrhythmia and auditory processing in autism: modifiable deficits of an integrated social engagement system? International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 88(3), 261–270. doi:10.1016/j.ijpsycho.2012.11.009.
R Core Team. (2016). R: A Language and Environment for Statistical Computing. Vienna: R Core Team. https://www.R-project.org/.
Richards, J. E., & Cameron, D. (1989). Infant heart-rate variability and behavioral developmental status. Infant Behavior and Development, 12(1), 45–58. doi:10.1016/0163-6383(89)90052-0.
Richards, J. E., & Casey, B. J. (1991). Heart rate variability during attention phases in young infants. Psychophysiology, 28(1), 43–53. doi:10.1111/j.1469-8986.1991.tb03385.x.
Rutter, M., Bailey, A., & Lord, C. (2003). The Social Communication Questionnaire. Torrance, CA: Western Psychological Services.
Sigman, M., Dissanayake, C., Corona, R., & Espinosa, M. (2003). Social and cardiac responses of young children with autism. Autism : The International Journal of Research and Practice, 7(2), 205–216.
Silvetti, M. S., Drago, F., & Ragonese, P. (2001). Heart rate variability in healthy children and adolescents is partially related to age and gender. International Journal of Cardiology, 81(2–3), 169–174.
Tager-Flusberg, H. (2010). The origins of social impairments in autism spectrum disorder: Studies of infants at risk. Neural Networks : The Official Journal of the International Neural Network Society, 23(8–9), 1072–1076. doi:10.1016/j.neunet.2010.07.008.
Trajkovic, I., Scholkmann, F., & Wolf, M. (2011). Estimating and validating the interbeat intervals of the heart using near-infrared spectroscopy on the human forehead. Journal of Biomedical Optics, 16(8), 087002-087002-9. doi:10.1117/1.3606560.
Viding, E., & Blakemore, S.-J. (2007). Endophenotype approach to developmental psychopathology: implications for autism research. Behavior Genetics, 37(1), 51–60. doi:10.1007/s10519-006-9105-4.
Wagner, J. B., Fox, S. E., Tager-Flusberg, H., & Nelson, C. A. (2011). Neural processing of repetition and non-repetition grammars in 7-and 9-month-old infants. Frontiers in Psychology. doi:10.3389/fpsyg.2011.00168/abstract.
Watson, L. R., Roberts, J. E., Baranek, G. T., Mandulak, K. C., & Dalton, J. C. (2012). Behavioral and physiological responses to child-directed speech of children with autism spectrum disorders or typical development. Journal of Autism and Developmental Disorders, 42(8), 1616–1629. doi:10.1007/s10803-011-1401-z.
Werling, D. M., & Geschwind, D. H. (2015). Recurrence rates provide evidence for sex-differential, familial genetic liability for autism spectrum disorders in multiplex families and twins. Molecular Autism, 6(1), 63. doi:10.1186/s13229-015-0004-5.
Acknowledgments
We are extremely grateful to the families for their invaluable contribution to the Infant Sibling Project. We would also like to acknowledge the Infant Sibling Project staff—Tara Augenstein, Lauren Baczewski, Leah Casner, Kristin Concannon, Frances Cooley, Morgan Crossman, Kerri Downing, Mary Kate Driscoll, Sharon Fox, Linnea Joffe-Nelson, Kristina Joas, Brandon Keehn, Jack Keller, Nina Leezenbaum, Vanessa Loukas, Rhiannon Luyster, Stephanie Marshall, Sarah Mumanachit, Anne Seery, Meagan Thompson, Vanessa Vogel-Farley, Jennifer Wagner, and Anne-Marie Zuluaga—for their assistance in data acquisition and helpful advice.
Author Contributions
CAN, HTF conceived and designed the Infant Sibling Project. KLP, CAN designed the HR study. KLP, LAE analyzed data. KLP, LAE, CAN, HTF participated in the interpretation of the data. KLP, LAE drafted the manuscript. CAN, HTF critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This research was supported by National Institutes of Health Grant R01-DC010290 to Helen Tager-Flusberg and Charles A. Nelson and The Simons Foundation (137186) to Charles A. Nelson.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Katherine L. Perdue, Laura A. Edwards, Helen Tager-Flusberg, and Charles A. Nelson declared that they have no conflict of interest.
Ethical Approval
All study procedures were monitored and approved by the Boston Children’s Hospital Institutional Review Board under IRB protocol X10-02-0083. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Perdue, K.L., Edwards, L.A., Tager-Flusberg, H. et al. Differing Developmental Trajectories in Heart Rate Responses to Speech Stimuli in Infants at High and Low Risk for Autism Spectrum Disorder. J Autism Dev Disord 47, 2434–2442 (2017). https://doi.org/10.1007/s10803-017-3167-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-017-3167-4