Abstract
As the link between maternal obesity and risk of autism among offspring is unclear, the present study assessed this association. A systematic search of an electronic database was performed to identify observational studies that examined the association between maternal obesity and autism. The outcome measures were odds ratios comparing offspring autism risk between obese and normal-weight mothers. Five observational studies were included in the meta-analysis. A fixed-effects model was used since low heterogeneity was observed between studies. The pooled adjusted odds ratio was 1.47 (95 % CI 1.24–1.74). The meta-analysis results support an increased risk of autism spectrum disorder in children of women who were obese during pregnancy. However, further study is warranted to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is defined as a severe and pervasive neurobiological disorder characterized by developmental delays, communication difficulties, deficits in social functioning, and repetitive behaviours (American Psychiatric Association 2013). ASD diagnoses have substantially increased since the 1960s, and the latest study reports that nearly one in 68 children aged 8 years in the United States (US) met criteria for ASD in 2010, and a similar prevalence (1–1.2 %) exists in the United Kingdom (UK) (Baird et al. 2006; Baron-Cohen et al. 2009; CDC 2014). While approximately 25 % of ASD cases can be attributed to known factors, and ASD prevalence may be affected by the changes in autism diagnostic criteria found in DSM-V, most cases are believed to be of multifactorial aetiology, with genetic and environmental risk factors acting together (Kim et al. 2014; Miles 2011; Newschaffer et al. 2007; Sandin et al. 2014). Given the prevalent and pervasive impairments in individuals with ASD (Simonoff et al. 2008), this disorder impacts not only their education and health, but also their social outcomes (Baron-Cohen et al. 2009; Bolton et al. 1998). Moreover, ASD is reportedly associated with a heavy financial burden in the US and UK (Buescher et al. 2014). Thus, it is imperative to take some measures towards understanding the risk factors for ASD development, which has become an important public health issue.
Nearly 60 % of women of childbearing age are overweight, one-third are obese, and 16 % have metabolic syndrome in the US (Ervin 2009; Flegal et al. 2010). Numerous studies have indicated a probable association between prenatal risk factors and incidence of ASD in children (Gardener et al. 2009; Man et al. 2015; Rosen et al. 2014; Xu et al. 2014). However, a growing number of studies assessing maternal obesity and risk of ASD in offspring have yielded inconsistent outcomes (Dodds et al. 2011; Krakowiak et al. 2012; Moss and Chugani 2014; Reynolds et al. 2014; Suren et al. 2014).
In this study, we conducted a meta-analysis of published observational studies to systematically evaluate whether maternal obesity is a risk factor of ASD in children and have quantitatively summarized the data.
Methods
Search Strategy
A systematic literature search was conducted via Pubmed (1946 to February 2014) and Embase (1976 to February 2014) using Medical Subject Headings or Emtree headings and key words to find citations related to maternal obesity and ASD in offspring. The following search terms were used: obesity/overweight/obese/and autism/Autism Spectrum Disorder/Asperger’s/autistic. There is no restriction to the language in our initial literature search. In addition, hand searching was conducted on the reference lists of all selected studies, as well as reviews, to identify additional relevant studies that might have met our inclusion criteria as stated below.
Two investigators (LL and ZD) independently performed the electronic literature search with a standardized approach. Any inconsistencies between the scanning outcomes of the two authors were examined by the primary author (LYM) to reach a consensus.
Study Selection and Inclusion Criteria
Eligibility of publications was independently assessed by two investigators (LL and ZD). They first screened titles and abstracts, and full texts were retrieved to identify relevant studies. A study was eligible for inclusion if all the following criteria were met: (1) the study was a cohort or a case–control study; (2) the study assessed the association between maternal obesity and risk of ASD in offspring; (3) the outcome of interest was risk of developing ASD; (4) the data of odds ratio (OR) or risk ratio (RR) estimates with associated 95 % CIs or the data needed to calculate these effect estimates were provided or included in the study.
Data Extraction
Data from the included studies were independently extracted by two authors (LL and ZD) using a standardized data collection form, and discrepancies were resolved by the primary author (LYM). The following data were extracted: first author’s name, year of publication, country of study, study design, data source, sample size, assessment method of ASD, maternal obesity criteria, adjusted variables, and ORs with corresponding 95 % CI.
Quality Assessment
The quality assessment of the selected observational studies was conducted by using an improved Newcastle-Ottawa Scale (Wells et al. 2013), which is widely used in meta-analysis. Three main study aspects were evaluated: selection, comparability, and exposure. Each satisfactory answer was assigned one ‘star’ and studies with 6 or more stars were considered of high quality.
Two authors (LL and ZD) conducted the quality assessment independently, and the final score of each included study was adjudicated by the primary author (LYM) after reviewing the original paper.
Statistical Analysis
The ORs or RRs with the corresponding 95 % CI extracted from each eligible study were used as the common measure of association among studies, and the OR was assumed to approximate the RR, as the incidence of ASD was sufficiently low for the rare disease assumption (<10 %) (Greenland 1987). Heterogeneity across studies was assessed using the I2 statistic, and I2 values of 25, 50, and 75 % were interpreted as low, medium, and high heterogeneity, respectively (Higgins et al. 2003). If significant heterogeneity was detected (I2 > 50 %), the DerSimonian and Laird random-effect model was used to calculate the summary RRs and 95 % CI; otherwise, the fixed effect model was applied in the meta-analysis. Sensitivity analyses were performed to further examine the robustness of the estimated results by sequentially excluding each study and rerunning the meta-analysis.
Potential publication bias was assessed using the Egger’s or Begg’s regression model, and by visually examining funnel plot asymmetry. In addition, the Duval and Tweedie nonparametric ‘trim and fill’ method, which is used to estimate missing studies, was applied to adjust the funnel plot and recalculate the pooled RRs. All statistical analyses were conducted using Stata statistical software (version 12.0; Stata Corporation, College Station, TX, USA).
Results
Literature Search
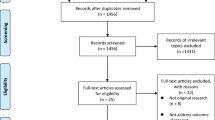
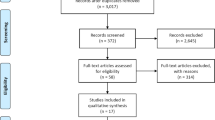
A total of 1000 citations were yielded in the initial search, with 278 from Pubmed and 722 from Embase. After excluding 156 duplicate studies and 828 irrelevant topics, 16 papers were identified on maternal obesity and ASD risk in offspring and were subjected to a detailed evaluation. Subsequently, seven studies were excluded because of irrelevant outcomes, including one study assessing maternal weight gain and ASD (Bilder et al. 2013). Three reviews, and two news report without any eligible data were excluded as well. One study was retrieved by manual search after reviewing the bibliographies of all evaluated full-text articles. Finally, five studies fulfilled all the inclusion criteria and were included in the meta-analysis. A detailed flow chart of the search and selection process is presented in Fig. 1.
Study Characteristics
The baseline characteristics of the 5 selected studies are presented in Table 1. All of the studies were published recently; all studies but Reynolds et al., which is a hospital cohort of very preterm infants (gestational age ≤30 weeks) followed to age 2 years, were population-based studies; three studies were conducted in the United States, one in Canada, and one in Norway. All studies, comprising four cohort studies and one case–control study, applied multivariable models to compute ORs or RRs with a corresponding 95 % CI for maternal obesity and risk of ASD in offspring. Pre-pregnancy body mass index (BMI) was calculated from height and weight, and a maternal BMI ≥ 30 was classified as obese in four studies; however, one study defined ‘obese’ as a pre-pregnancy weight more than 90 kilograms.
Several different assessment and/or diagnostic methods were applied to ascertain the cases of ASD. The 9th and 10th revisions of the International Statistical Classification of Diseases and Related Health Problems (ICD-9 and ICD-10) were used in 2 studies to identify cases [Dodds et al. used ICD-9 code 299 or ICD-10 code F84 while Suren et al. used ICD-10 excluded Rett (F84.2) and childhood disintegrative disorder (F84.3)]. One study used both the autism diagnostic interview-revised (ADI-R) and the autism diagnostic observation schedule (ADOS), which are gold standard assessments for ASD. One study used the Modified Checklist for ASD in Toddlers (M-CHAT), which is a screening tool to identify children 12–36 months of age who are at risk for developing ASD based on parent-report. Finally, in another study, parents were asked whether a doctor had indicated that their preschool or kindergarten child had autism.
Maternal Obesity and the Risk of Offspring ASD
A forest plot of the meta-analysis is presented in Fig. 2. The pooled estimated OR for ASD risk was significantly increased in the offspring of obese mothers compared with mothers of normal weight (OR 1.47, 95 % CI 1.24–1.74), with moderate heterogeneity (I2 = 46.0 %, p = 0.116) across studies. However, a subgroup analysis was not performed due to the limited number of studies.
Sensitivity Analyses
To test the stability of the results, each study was excluded iteratively. The results of our sensitivity analysis indicated that none of the exclusions of any specific study conspicuously changed the trend of our pooled estimated results, which revealed that maternal obesity is positively associated with ASD in offspring. However, the relatively maximal change in the pooled estimate was presented after excluding the study by Dodds (Fig. 3), and the recalculated OR was 1.34 (95 % CI 1.04–1.73) with medium heterogeneity (I2 = 51.4 %, p = 0.088). Besides, the heterogeneity was effectively decreased after excluding the study by Suren, the recalculated OR was 1.58 (95 % CI 1.31–1.91) with low heterogeneity (I2 = 33.0 %, p = 0.214).
Publication Bias
Publication bias was first assessed by the Egger’s test (p = 0.949) and the Begg’s Test (p = 1.000), and no obvious publication bias was detected. However, the asymmetry was revealed by the shape of the funnel plot. Thus, the trim and fill method was applied to further assess publication bias, indicating that one probable and potential study with unpublished results was missing by using the random effect model (Fig. 4). In addition, the adjusted pooled result exhibited a similar trend (OR 1.27, 95 % CI 1.01–1.61). Overall, there was no significant evidence of publication bias according to the results above. However, the possibility of publication bias still exists, as the number of studies was insufficient for the tests.
Discussion
Our current meta-analysis is based on recently published epidemiologic data pertaining to ASD risk in children of obese mothers. Our findings suggest that maternal obesity is associated with ASD risk in offspring. Compared with normal-weight mothers, obese women were 47 % more likely to have a child with ASD.
Some uncontrolled variables and various methodological limitations present in all or some of the studies included in the meta-analysis may have biased our results and contributed to the heterogeneity. First, the diverse age range and characteristics of the children in the studies may have affected ASD validation; for instance, one (Moss and Chugani 2014) of the five included studies reported a negative association between maternal obesity and risk of ASD in children, however, a positive association was indicated in samples of only very low birth weight children in this study. Second, one study focused on autism, and four studies examined ASD; however, we included all of them because autism is included in ASD, but the OR were not adjusted for this confound in the study. Third, the different ASD definition applied in studies may have resulted in bias due to diverse criteria, for instance, Suren et al. excluded Rett’s disorder and childhood disintegrative disorder, while Dodds et al. included both of them. The measures of diagnosing ASD in several studies may have affected the results as the data collected from self-reports were not valid. Fourth, some other characteristics of included studies may have brought about bias, such as follow-up periods, population, and covariates. The follow-up durations in several studies were not long enough to capture all potential cases. Most studies’ data source was population-based, except one was hospital-based with a relatively small sample size (Reynolds et al. 2014). Some important covariates and confounding factors were not adjusted in some studies, such as maternal age and psychiatric illness in the multivariable model. Besides, downstream factors of maternal obesity were inappropriately adjusted in some studies, such as birthweight and growth of infants. Age ranges among studies varied, but they were appropriate in their respective criterion. Last but not least, the exposure contrast among studies may have resulted in discrepant findings. Four out of 5 studies used BMI ≥ 30 to define maternal obesity, with one using a dichotomous variable [obese (BMI ≥ 30) vs. not obese (BMI < 30)]; two using obese vs. normal (BMI: 18.5, 24.9); one comparing obese mothers to mothers with BMI < 25. Dodds et al. used pre-pregnancy weight ≥90 kg to define obesity and compared them to mothers with weight <90 kg. These data in two studies (Dodds et al. 2011; Moss and Chugani 2014) were ascertained from self-report rather than medical record. All of the limitations above can alter the estimates, which were pooled in the meta-analysis.
It has been universally acknowledged that obesity is a significant risk factor for several diseases, such as hypertension and diabetes (type 2 and gestational), and is associated with insulin resistance and chronic inflammation (Olefsky and Glass 2010; Zavalza-Gómez et al. 2008). Therefore, the adverse foetal development may result from poorly regulated maternal glucose levels in a diabetic or possibly pre-diabetic pregnancy. Elevated glucose levels could lead to chronic foetal hyperinsulinemia, which could promote oxygen consumption and metabolism in the foetus and induce chronic intrauterine tissue hypoxia (Eidelman and Samueloff 2002). Furthermore, foetal iron deficiency could result from inherent biological responses to chronically elevated glucose (Georgieff 2008). Both hypoxia and iron deficiency in the foetus have a tremendous influence on neurodevelopment in humans (Georgieff 2006). Several inflammatory cytokines are elevated in obese individuals, including interleukin[IL]-1beta, IL-6, and tumor necrosis factor-alpha (Das 2001), and the role of cytokines in ASD has been suggested in several studies (Atladóttir et al. 2010; Pardo et al. 2005). In addition, the obese pregnant women have higher levels of estrogen, cortisol, free fatty acids, and oxidative stress compared with lean pregnant women, and these physiological differences may contribute to an increased ASD risk (Catalano et al. 2003; Chen and Scholl 2005; Kyrou and Tsigos 2009; Ramsay et al. 2002; Zumoff and Strain 1994).
There are several limitations in our study. First, the meta-analysis was insufficiently performed due to the limited number of eligible studies, which may have influenced the statistical results. While we included both cohort and case–control studies, we were still unable to conduct a subgroup analysis by study design because the number of estimates in each subgroup would be insufficient to permit meta-analysis. Second, the quality and robustness of meta-analysis is proportional to that of the individual studies included. Among the five included studies, one (Reynolds et al. 2014) was based on a small sample (<500), and the ascertainment of ASD in children in two studies (Moss and Chugani 2014; Reynolds et al. 2014) was dependent on parental response, which is not entirely objective, because the study report relied on the memory and honesty of subjects. Furthermore, though low heterogeneity across studies was detected, we were still unable to fully exclude potential heterogeneity. Differences in study periods, statistical methods and choice of covariates, and inconsistent ASD criteria were potential sources of heterogeneity. Finally, publication bias cannot be ruled out in our analysis. In the test of publication bias, some asymmetry in the funnel plot was observed; and in the ‘trim and fill’ method used to adjust the funnel plot, one possible missing study was retrieved. In general, studies with a small sample size and negative results are inclined to be unpublished. However, the pooled estimated OR was only slightly altered, which indicated the robustness of our findings.
To the best of our knowledge, this is the first meta-analysis of maternal obesity and ASD risk in offspring. One strength of our study is the inclusion of all the relevant observational studies concerning maternal obesity and ASD incidence in children. All available data were presented in a systematic and quantitative fashion. Besides, in the quality assessment by the Newcastle–Ottawa Scale, most studies were identified as high quality, which could probably enhance the robustness of our analysis (Table 2). Sensitivity analysis was performed to investigate whether any particular study could change the results. However, the findings were generally robust.
In conclusion, this comprehensive meta-analysis reveals that maternal obesity might increase ASD risk in children. Our findings should be treated with caution due to the limited number of available studies. Further studies, particularly prospective cohort studies based on a large population, are recommended to confirm the results. Moreover, future replication studies in this area should have more accurate measures of exposure and outcome, and better control of confounding factors. We suggest that other confounding variables such as biomarkers of inflammation and glucose regulation should be confirmed to make the results more accurate and valid.
References
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association.
Atladóttir, H. Ó., Thorsen, P., Østergaard, L., Schendel, D. E., Lemcke, S., Abdallah, M., & Parner, E. T. (2010). Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(12), 1423–1430.
Baird, G., Simonoff, E., Pickles, A., Chandler, S., Loucas, T., Meldrum, D., & Charman, T. (2006). Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: The special needs and autism project (SNAP). Lancet, 368(9531), 210–215.
Baron-Cohen, S., Scott, F. J., Allison, C., Williams, J., Bolton, P., Matthews, F. E., & Brayne, C. (2009). Prevalence of autism-spectrum conditions: UK school-based population study. British Journal of Psychiatry, 194(6), 500–509.
Bilder, D. A., Bakian, A. V., Viskochil, J., Clark, E. A., Botts, E. L., Smith, K. R., et al. (2013). Maternal prenatal weight gain and autism spectrum disorders. Pediatrics, 132(5), e1276–e1283.
Bolton, P., Pickles, A., Murphy, M., & Rutter, M. (1998). Autism, affective and other psychiatric disorders: patterns of familial aggregation. Psychological Medicine, 28(02), 385–395.
Buescher, A. V., Cidav, Z., Knapp, M., & Mandell, D. S. (2014). Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatrics, 168(8), 721–728.
Catalano, P. M., Kirwan, J. P., Haugel-de Mouzon, S., & King, J. (2003). Gestational diabetes and insulin resistance: role in short-and long-term implications for mother and fetus. Journal of Nutrition, 133(5), 1674S–1683S.
CDC. (2014). Prevalence of autism spectrum disorders among children aged 8 years: Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveillance Summaries, 63(2), 1–22.
Chen, X., & Scholl, T. O. (2005). Oxidative stress: changes in pregnancy and with gestational diabetes mellitus. Current Diabetes Reports, 5(4), 282–288.
Das, U. (2001). Is obesity an inflammatory condition? Nutrition, 17(11), 953–966.
Dodds, L., Fell, D. B., Shea, S., Armson, B. A., Allen, A. C., & Bryson, S. (2011). The role of prenatal, obstetric and neonatal factors in the development of autism. Journal of Autism and Developmental Disorders, 41(7), 891–902.
Eidelman, A. I., & Samueloff, A. (2002) The pathophysiology of the fetus of the diabetic mother. Seminars in perinatology. Elsevier (pp 232–236).
Ervin, R. B. (2009). Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States. National Health Statistics Reports, 13, 1–8.
Flegal, K. M., Carroll, M. D., Ogden, C. L., & Curtin, L. R. (2010). Prevalence and trends in obesity among US adults, 1999–2008. JAMA, 303(3), 235–241.
Gardener, H., Spiegelman, D., & Buka, S. L. (2009). Prenatal risk factors for autism: Comprehensive meta-analysis. British Journal of Psychiatry, 195(1), 7–14.
Georgieff, M. K. (2006). The effect of maternal diabetes during pregnancy on the neurodevelopment of offspring. Minnesota Medicine, 89(3), 44–47.
Georgieff, M. K. (2008). The role of iron in neurodevelopment: Fetal iron deficiency and the developing hippocampus. Biochemical Society Transactions, 36(Pt 6), 1267.
Greenland, S. (1987). Quantitative methods in the review of epidemiologic literature. Epidemiologic Reviews, 9(1), 1–30.
Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. British Medical Journal, 327(7414), 557.
Kim, Y. S., Fombonne, E., Koh, Y. J., Kim, S. J., Cheon, K. A., & Leventhal, B. L. (2014). A comparison of DSM-IV pervasive developmental disorder and DSM-5 autism spectrum disorder prevalence in an epidemiologic sample. Journal of the American Academy of Child and Adolescent Psychiatry, 53(5), 500–508.
Krakowiak, P., Walker, C. K., Bremer, A. A., Baker, A. S., Ozonoff, S., Hansen, R. L., & Hertz-Picciotto, I. (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics, 129(5), e1121–e1128.
Kyrou, I., & Tsigos, C. (2009). Stress hormones: Physiological stress and regulation of metabolism. Current Opinion in Pharmacology, 9(6), 787–793.
Man, K. K., Tong, H. H., Wong, L. Y., Chan, E. W., Simonoff, E., & Wong, I. C. (2015). Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: A systematic review and meta-analysis of observational studies. Neuroscience and Biobehavioral Reviews, 49C, 82–89.
Miles, J. H. (2011). Autism spectrum disorders—a genetics review. Genetics in Medicine, 13(4), 278–294.
Moss, B. G., & Chugani, D. C. (2014). Increased risk of very low birth weight, rapid postnatal growth, and autism in underweight and obese mothers. American Journal of Health Promotion, 28(3), 181–188.
Newschaffer, C. J., Croen, L. A., Daniels, J., Giarelli, E., Grether, J. K., Levy, S. E., et al. (2007). The epidemiology of autism spectrum disorders. Annual Reviews in Public Health, 28, 235–258.
Olefsky, J. M., & Glass, C. K. (2010). Macrophages, inflammation, and insulin resistance. Annual Review of Physiology, 72, 219–246.
Pardo, C. A., Vargas, D. L., & Zimmerman, A. W. (2005). Immunity, neuroglia and neuroinflammation in autism. International Review of Psychiatry, 17(6), 485–495.
Ramsay, J. E., Ferrell, W. R., Crawford, L., Wallace, A. M., Greer, I. A., & Sattar, N. (2002). Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. Journal of Clinical Endocrinology and Metabolism, 87(9), 4231–4237.
Reynolds, L. C., Inder, T. E., Neil, J. J., Pineda, R. G., & Rogers, C. E. (2014). Maternal obesity and increased risk for autism and developmental delay among very preterm infants. Journal of Perinatology, 34(9), 688–692.
Rosen, B. N., Lee, B. K., Lee, N. L., Yang, Y., & Burstyn, I. (2014). Maternal smoking and autism spectrum disorder: A meta-analysis. Journal of autism and developmental disorders, 45(6), 1689–1698.
Sandin, S., Lichtenstein, P., Kuja-Halkola, R., Larsson, H., Hultman, C. M., & Reichenberg, A. (2014). The familial risk of autism. JAMA, 311(17), 1770–1777.
Simonoff, E., Pickles, A., Charman, T., Chandler, S., Loucas, T., & Baird, G. (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47(8), 921–929.
Suren, P., Gunnes, N., Roth, C., Bresnahan, M., Hornig, M., Hirtz, D., et al. (2014). Parental obesity and risk of autism spectrum disorder. Pediatrics, 133(5), e1128–e1138.
Wells, G., Shea, B., O’Connell, D., Peterson, J., Welch, V., & Losos, M. (2013) The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses 2009.
Xu, G., Jing, J., Bowers, K., Liu, B., & Bao, W. (2014). Maternal diabetes and the risk of autism spectrum disorders in the offspring: A systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 44(4), 766–775.
Zavalza-Gómez, A. B., Anaya-Prado, R., Rincón-Sánchez, A. R., & Mora-Martínez, J. M. (2008). Adipokines and insulin resistance during pregnancy. Diabetes Research and Clinical Practice, 80(1), 8–15.
Zumoff, B., & Strain, G. W. (1994). A perspective on the hormonal abnormalities of obesity: Are they cause or effect? Obesity Research, 2(1), 56–67.
Author Contributions
Ya-Min Li and Si-Yuan Tang conceived and designed the experiments. Jian-Jun Ou, Li Liu and Dan Zhang performed the experiments. Ya-Min Li, Jing-Ping Zhao and Si-Yuan Tang analyzed the data. Ya-Min Li and Jian-Jun Ou contributed software, hardware and analysis tools. Ya-Min Li, Jing-Ping Zhao and Si-Yuan Tang wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Li, YM., Ou, JJ., Liu, L. et al. Association Between Maternal Obesity and Autism Spectrum Disorder in Offspring: A Meta-analysis. J Autism Dev Disord 46, 95–102 (2016). https://doi.org/10.1007/s10803-015-2549-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-015-2549-8