Abstract
Adolescence is a period of heightened emotional reactivity, which is reflected in greater activation in emotion-processing regions of the brain in adolescents relative to children and adults. While elevated emotional reactivity and poor emotion regulation are thought to contribute to the rise in rates of internalizing psychopathology, including anxiety, during adolescence, little research has examined factors predicting individual differences in the neural regulation of emotion that can explain why only some adolescents experience anxiety. To address this gap, the present study examined the contribution of childhood negative emotionality (NE) and cognitive control (CC) to neural processing of emotion in adolescence. A sample of 44 girls (M age = 15.5, SD = 0.35) was selected from a longitudinal study that included self, parent, and teacher report of NE and CC between 2nd and 7th grades. Following 9th grade, girls completed an emotion regulation task during a functional magnetic resonance imaging scan. Neural regulation of emotion was indexed by functional connectivity between the amygdala and right ventrolateral prefrontal cortex (rVLPFC) during emotion regulation. Analyses revealed that NE predicted a less mature pattern of amygdala-rVLPFC connectivity while CC predicted a more mature pattern of amygdala-rVLPFC connectivity. Additionally, we found an interaction between NE and CC, such that NE predicted emotion dysregulation at low but not high levels of CC. Neural dysregulation of emotion was associated with anxiety symptoms across the following nine months. These findings identify important individual differences in the development of emotion dysregulation that contribute to risk for anxiety in adolescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adolescence is a period of rapid emotional and cognitive development during which youth experience increasing autonomy and exposure to novel social and emotional stressors (Spear 2000). Failure to develop effective emotion regulation strategies puts youth at risk for emotional difficulties, such as anxiety, that increase in prevalence and stability across adolescence (Pine et al. 1998). Contemporary theory and research on adolescent development implicate this stage as a time of increased neural and behavioral sensitivity to emotion (Casey et al. 2008; Ernst et al. 2006). However, most youth develop effective ways of regulating emotions, while only a subset goes on to develop anxiety. It is therefore important to consider pre-existing individual differences that may lead to variability in neural signatures of emotion regulation during adolescence. Addressing this gap, the present study explored the contribution of two childhood traits, negative emotionality and cognitive control, to neural regulation of emotion in adolescence. In particular, to overcome limitations created by the independent investigation of individual brain regions (Pfeifer and Allen 2016), functional connectivity analysis was used to examine coordination across regions involved in emotional reactivity (i.e., amygdala) and top-down control of emotion (i.e., prefrontal cortex). Moreover, to enhance understanding of the psychological implications of neural processing of emotion in adolescence, this study also examined the association between functional connectivity during emotion regulation and subsequent anxiety symptoms.
Emotion Processing in Adolescence
Several lines of research support the idea that adolescence is a period of heightened emotional sensitivity. Adolescents, especially mid-adolescents, report more negative emotions and greater day-to-day variability in emotional states than younger children, later adolescents, or adults (Larson et al. 2002; Silk et al. 2009; for reviews, see Ernst et al. 2006; Somerville et al. 2010). Adolescents also show greater physiological reactivity to emotion words than do children (Silk et al. 2009). Despite this adolescent-specific peak in emotional reactivity (Casey 2015), age-related increases in emotion regulation abilities allow adolescents to begin adapting to their changing environment and developing strategies to effectively regulate their emotions. For example, research reveals age-related increases in the ability to successfully down-regulate emotional reactivity to negatively valenced pictures (Silvers et al. 2012). Unfortunately, the growth of regulatory skills occurs at a slower rate than the peak in emotional reactivity, resulting in more difficulty regulating emotions during mid-adolescence than during childhood or adulthood (McRae et al. 2012).
Neural Correlates of Emotion Processing in Adolescence
One proposed explanation for the maturational gap between emotional reactivity and emotion regulation during mid-adolescence is that the neural systems involved in emotional reactivity develop earlier than those involved in cognitive control (Casey et al. 2008; Ernst et al. 2006; Nelson et al. 2016; Steinberg 2008; Tillfors and Van Zalk 2015). Some evidence supports heightened amygdala reactivity to emotion in adolescents relative to children and adults (e.g., Hare et al. 2008), but evidence regarding the development of regulatory regions is less consistent. Many studies have found that compared to activity in emotion-processing regions, activity in the prefrontal cortex (PFC) reflects slower maturation, with adults showing greater PFC activation than adolescents during emotion regulation tasks (Casey 2015; Shulman et al. 2016). However, others have found that adolescents show greater PFC activation than children or adults during emotion regulation tasks (Crone and Dahl 2012; Pfeifer and Allen 2012).
This inconsistency highlights the need to move beyond studying differences in activation within specific regions to consider patterns of connectivity between regions, such as the amygdala and PFC, that interact to support effective emotion regulation. Although few studies compare connectivity patterns at different developmental stages, some research reveals that, relative to children, adolescents and adults show more negative functional connectivity between the amygdala and PFC while passively viewing (Gee et al. 2014; Guyer et al. 2008) and labeling (Monk et al. 2008) emotional faces. This pattern is thought to reflect improved top-down regulation of amygdala hyperactivity, as evidenced by the findings that PFC activation is negatively correlated with amygdala activation during emotion regulation tasks (Silvers et al. 2016), and more negative amygdala-PFC functional connectivity predicts faster amygdala habituation to fearful faces (Hare et al. 2008). More negative amygdala-PFC connectivity is also associated with other indicators of effective emotion regulation, including less pupil dilation and better facial muscle control (Lee et al. 2012). Maintaining more positive functional coupling throughout adolescence may thus reflect a more immature pattern of connectivity and a failure to effectively down-regulate emotional reactivity.
Individual Differences in Neural Processing of Emotion
Despite this heightened reactivity to emotion in adolescents, many develop effective emotion regulation strategies, whereas a smaller proportion develop maladaptive strategies that put them at risk for adverse emotional outcomes. An important next step for research on adolescent neural development is to identify earlier individual differences that promote or prevent the development of adaptive emotion regulation strategies during adolescence (Pfeifer and Allen 2012; Somerville et al. 2010). To address this critical question, the present study examined whether negative emotionality and cognitive control, two temperamental traits measured in childhood, predict individual differences in emotion regulation in adolescents.
Negative Emotionality as a Predictor of Emotion Regulation
Negative emotionality (NE) is a dimension of temperament that may predict less effective emotion regulation in adolescence. NE is characterized by susceptibility to experiencing negative emotions, difficulty being soothed once aroused, and heightened sensitivity to negative social cues (Rothbart et al. 2001). Trait differences in NE are associated with ruminative responses to negative emotions (Verstraeten et al. 2009) and maladaptive emotion regulation strategies in children (Santucci et al. 2008) and adults (Gross and John 2003). Little research has examined the impact of NE on neural systems involved in emotion regulation, although the related temperamental dimension of childhood behavioral inhibition (characterized by a tendency to show fear or reticence in novel social situations) predicts greater amygdala activation to emotional faces in early adolescence (Perez-Edgar et al. 2007). Additionally, NE is associated with greater amygdala activation during emotion maintenance in adults (Schaefer et al. 2002). High trait NE may sensitize individuals to emotionally salient information by biasing their attention towards negatively valenced emotion cues (Schultz et al. 2004), increasing their emotional reactivity to these cues (Calkins et al. 1999), and making it more difficult to effectively regulate this response (Gross and John 2003). Accordingly, we hypothesized that childhood NE would predict a less mature pattern of amygdala-PFC functional coupling (i.e., more positive functional connectivity) during emotion regulation in adolescence.
Cognitive Control as a Predictor of Emotion Regulation
Cognitive control (CC) develops across childhood and adolescence and plays an important role in emotion regulation. CC is characterized by the ability to maintain directed attention toward goals and to disengage from task-irrelevant information (Miller and Cohen 2001). While CC increases into adulthood, there are stable individual differences in CC that distinguish individuals throughout development (Ochsner and Gross 2005; Zhou et al. 2012). Neuroscience research supports the role of trait CC in emotion regulation. One longitudinal study found that self-regulation measured during childhood predicted greater PFC activation during an emotion regulation task completed in adulthood (Casey et al. 2011), suggesting that individuals with higher CC may more effectively recruit regions of the PFC to down-regulate activity in emotion-processing regions of the brain. Therefore, we hypothesized that childhood CC would predict a more mature pattern of amygdala-PFC functional coupling (i.e., more negative functional connectivity) during emotion regulation in adolescence.
Interactive Contribution of NE and CC to Emotion Regulation
Beyond the independent effects of NE and CC, there may be important interactive effects of these traits on emotion regulation. While heightened emotional sensitivity (i.e., high NE) may predict less effective emotion regulation, this effect may be weaker in youth who have better self-regulation abilities (i.e., high CC). Past research suggests that CC may moderate the effect of NE on coping styles (Lengua and Long 2002), responses to parent-child stress (Yap et al. 2011), rumination (Verstraeten et al. 2009), and emotional distress (Gulley et al. 2016), such that NE leads to worse outcomes at low, but not high, levels of CC. Yet research has not yet addressed the interactive effects of NE and CC on neural regulation of emotion. Past models of adolescent neural development have suggested a pattern of heightened activation in regions involved in emotional reactivity relative to regions involved in cognitive control (Casey et al. 2008; Ernst et al. 2006; Steinberg 2008). However, these studies have not examined how this general pattern is impacted by trait differences in reactivity and regulation. Accordingly, it is important to understand how individual differences in these traits interact to predict functional coupling of limbic and frontal regions during emotion regulation. It is hypothesized that NE will predict more positive (i.e., maladaptive) functional coupling at low, but not high, levels of CC, suggesting that CC may serve as a buffer against the negative effects of NE on emotion regulation during adolescence.
Neural Processing of Emotion and Anxiety
In addition to identifying the childhood antecedents of individual differences in adolescent neural regulation of emotions, it is important to understand the psychological implications of these patterns for adolescent adjustment. Previous research suggests that age-related changes in neural responses to emotion may contribute to risk for anxiety in adolescence (Casey et al. 2008; Ernst et al. 2006). By mid-adolescence, most youth show a pattern of negative amygdala-PFC connectivity during emotion regulation (Gee et al. 2014). Maintaining a less mature pattern of amygdala-PFC coupling into mid-adolescence may be maladaptive, preventing adolescents from effectively regulating reactivity to emotional stimuli. As a result, adolescents may experience more emotional distress such as anxiety. Indeed, previous research examining resting state amygdala-PFC connectivity has found that non-anxious adults show more negative coupling between the amygdala and dorsal medial PFC than do anxious adults (Kim et al. 2010). Task-based connectivity research with adolescents has found that those adolescents who show a less mature (i.e., more positive) pattern of amygdala-PFC connectivity during emotion processing (Monk et al. 2008) and social evaluation (Guyer et al. 2008) are more likely to experience anxiety. Expanding on prior research, we used a longitudinal design to examine whether a less mature pattern of amygdala-PFC coupling (i.e., more positive functional connectivity) would predict more future anxiety symptoms in adolescents.
Emotion Processing in Adolescent Girls
Adolescence also is a crucial period in the development of gender differences in emotional sensitivity. Compared to adolescent boys, girls are exposed to higher levels of social stressors (Hankin et al. 2007; Rose and Rudolph 2006) and experience more emotional reactivity and emotional distress following exposure to stressors (Charbonneau et al. 2009; Rudolph et al. 2009). There is also some evidence of gender differences in neural systems involved in emotion processing. Early adolescent girls show less amygdala habituation following repeated exposure to fearful faces than do boys (Thomas et al. 2001). Additionally, there is some evidence that age-related shifts in amygdala and PFC activity during emotion processing are more pronounced in girls than in boys (Killgore et al. 2001). Given girls’ heightened exposure and sensitivity to emotion-inducing contexts during adolescence (Charbonneau et al. 2009; Hankin et al. 2007), only girls were recruited for the present study.
Study Overview
This study had two main goals. The first goal was to build upon past models of normative adolescent development by examining how individual differences in childhood NE and CC confer risk for, or resilience against, neural dysregulation of emotion in adolescent girls. The second goal was to explore whether differences in the neural processing of emotion contribute to anxiety. We hypothesized that (a) NE would predict a less mature pattern of amygdala-PFC connectivity; (b) CC would predict a more mature pattern of amygdala-PFC connectivity; (c) the association between NE and less mature patterns of amygdala-PFC connectivity would be stronger at low than high levels of CC (i.e., NE x CC interaction); and (d) a less mature pattern of connectivity would predict more future anxiety symptoms.
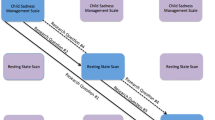
We used a longitudinal, multi-method, multi-informant design to examine the role of childhood temperament as a predictor of neural processing of emotion in adolescence. NE and CC were assessed annually via parent-, teacher-, and child-report questionnaires when girls were in 2nd through 7th grade, allowing us to create indices of NE and CC that reflect stable traits across childhood. Neural processing of emotion was assessed following 9th grade using functional magnetic resonance imaging (fMRI) while participants completed an emotion labeling task. The comparison of interest in this task was the difference in brain connectivity when adolescents labeled facial expressions of negative emotions compared to passively viewing them. This task is thought to measure implicit emotion regulation engaged as a result of labeling emotions (Hariri et al. 2000; Lieberman et al. 2007). Several past studies have suggested that emotion labeling is a form of implicit emotion regulation, and is associated with decreases in amygdala activity and less emotional reactivity (Brooks et al. 2016; Gyurak et al. 2011). To examine the link between emotion dysregulation and subsequent anxiety, girls reported on anxiety symptoms across the nine months following the scan.
Methods
Participants and Procedures
Participants were 44 adolescent girls (mean age = 15.5 years, SD = 0.35; 68.2% White, 27.3% African American, 2.3% Asian, and 2.3% Latina) recruited from a larger longitudinal study (e.g., Rudolph et al. 2014; Sugimura and Rudolph 2012) that was conducted in elementary schools beginning when youth were in second grade (mean age in second grade = 7.97, SD = 0.37). This larger longitudinal study included 636 youth (338 girls) who, along with their parents and teachers, completed questionnaires annually between second and seventh grades. Youth completed questionnaires in small groups during elementary school and by classroom in middle school. Parents and teachers completed questionnaires independently and received monetary compensation. During the summer following 9th grade,Footnote 1 a subset of 99 girls was contacted about potential participation in an fMRI study.Footnote 2 Of the 99 girls who were contacted, seven declined to participate, eight met exclusion criteria for fMRI (e.g., metal braces, claustrophobia), and 34 were contacted but did not participate before the target sample of 50 was collected. This target sample size was selected based on previous studies that have found individual differences in neural activity using similarly sized samples (e.g., Gee et al. 2014) as well as logistical limitations of scanning additional participants. Of the 50 girls who completed the scan, six were excluded from analyses due to an initial error in the design of the emotion regulation task, leaving a final sample of 44 girls. Girls who participated in the study did not differ from those who were contacted but did not participate in NE (t(97) = 0.789, ns), or CC (t(98) = −0.854, ns). Participants completed the emotion regulation task while undergoing an fMRI scan, and completed questionnaires three, six, and nine months after the scan. Participants received $50 for completion of the fMRI scan and gift cards equaling $5 for completion of each set of follow-up questionnaires. Youth provided written assent and parents provided written consent. All procedures were approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign.
Measures
Table 1 provides descriptive and psychometric information on the measures.
Negative Emotionality
Trait negative emotionality (NE) was assessed using the Temperament in Middle Childhood Questionnaire (TMCQ; Simonds et al. 2007; Simonds and Rothbart 2004), which includes 25 items reflecting the tendency to show intense negative emotions, including anger (e.g., “Gets irritated when she has to stop doing something she is enjoying.”) and sadness (e.g., “Becomes sad when told to do something she doesn’t want to do.”) as well as low soothability (e.g., “Is very difficult to soothe when she has become upset.”). The NE subscale shows strong internal consistency and inter-rater reliability (Rothbart et al. 2001), including between parents and children (Lengua 2003). Construct validity has been supported through associations with behavioral (Wilson 2006) and clinical (Sugimura and Rudolph 2012) outcomes. Parents reported on youths’ NE in second through fifth grade. Youth and teachers reported on youths’ NE in fifth through seventh grade. Composite scores of trait NE were created by standardizing ratings within informant and averaging across all informants (i.e., youth, parent, and teacher) and waves. Creation of a composite NE score allowed us to examine stable, trait-like aspects of NE that emerged in a number of settings across childhood. Additionally, as at least one time point of data was missing for 19 participants, the creation of composite scores allowed us to calculate NE for each participant. Participants who had complete data (i.e., reports from each informant at each time point) did not differ from those who did not on NE, t(42) = −1.96, ns. Higher scores on this composite measure reflect higher levels of childhood negative emotionality.
Cognitive Control
Cognitive control (CC) was assessed using the effortful control scale from the TMCQ (Simonds et al. 2007; Simonds and Rothbart 2004) and an overall score of executive function from the Brief Rating Inventory of Executive Function (BRIEF; Gioia et al. 2000). The effortful control scale includes 15 items assessing attentional control (e.g., “When working on an activity, has a hard time keeping her mind on it.”) and inhibitory control (e.g., “Can stop herself from doing things too quickly.”). This scale shows strong internal consistency as well as test-retest and inter-rater reliability (Simonds et al. 2007). Construct validity is supported in community child samples (Kochanska et al. 2000; Muris et al. 2008). The BRIEF includes 86 items assessing different deficits in executive function. For this study, a subset of 40 items was selected to assess working memory (e.g., “Has trouble with chores or tasks that have more than one step.”), planning (e.g., “Does not plan ahead for school assignments.”), shifting (e.g., “Tries the same approach to a problem over and over, even when it does not work.”), and inhibition (e.g., “Has trouble putting the brakes on her actions.”). The BRIEF shows strong internal consistency and inter-rater reliability, including between parents and teachers (Agoston and Rudolph 2016; Gioia and Isquith 2004). Construct validity of the BRIEF has been supported in both clinical and community samples (Gioia et al. 2000). Parents reported on youths’ effortful control in second through fifth grade. Youth and teachers reported on youths’ effortful control in fifth through seventh grade. Teachers reported on youths’ executive function in sixth and seventh grade. Because effortful control and executive function overlap conceptually and are correlated in child samples (Zhou et al. 2012), including our sample (rs = 0.51–0.87, ps < 0.001), composite scores of CC were created by reverse scoring the BRIEF and then standardizing ratings within informant and averaging across all measures, informants (i.e., youth, parent, and teacher), and waves. All participants had a composite score of CC based on available data, although 19 participants were missing at least one time point of data. Participants who had complete data (i.e., reports from each informant at each time point) did not differ from those who did not on CC, t(42) = 1.68, ns. High scores on this composite measure reflect higher levels of cognitive control.
Anxiety Symptoms
Youth completed the Revised Child Manifest Anxiety Scale (RCMAS; Reynolds and Richmond 1985). The RCMAS includes 28 items assessing anxiety symptoms (e.g., “I often worry about something bad happening to me.”). The RCMAS shows strong internal consistency (Reynolds and Richmond 1978) and test-retest reliability (Wisniewski et al. 1987). Construct validity, including convergent and divergent validity (Reynolds 1982), has been supported through comparisons of youth with and without anxiety disorders (Seligman et al. 2004). Youth reported their anxiety symptoms at three, six, and nine months following the scan session. Completion rate across the follow-up period was 98%, 89%, and 93% at three, six, and nine months respectively. An overall follow-up anxiety score was calculated by averaging across the nine months following the scan session (n = 43).
Emotion Regulation fMRI Task
The fMRI scan included an emotion regulation task modified from Lieberman and colleagues (Lieberman et al. 2007). During one functional run, participants were presented with faces displaying negative emotions (anger, sadness, and fear). Participants completed two blocks during which they were instructed to observe the emotional faces (observe; Fig. 1), and two blocks during which they were instructed to match the emotional face to one of two emotion word labels presented below the image (label; Fig. 1b). Each block consisted of six trials, each of which lasted six seconds. Block order was randomized across participants, and a 10-s rest period occurred between blocks. The race of models (half African American and half European American) and emotion types were randomized within the blocks; all photos were of women taken from the NimStim (Tottenham et al. 2009).
Data Acquisition and Analysis
fMRI Data Acquisition
The fMRI data were collected using a 3 Tesla Siemens Trio MRI scanner. The task included T2*-weighted echoplanar images (EPI) [slice thickness = 3 mm; 38 slices; TR = 2 s; TE = 25 msec; matrix = 92 × 92; FOV = 230 mm; voxel size 2.5 × 2.5x3mm3]. Structural scans consisted of a T2*weighted, matched-bandwidth (MBW), high-resolution, anatomical scan (TR = 4 s; TE = 64 msec; matrix = 192 × 192; FOV = 230; slice thickness = 3 mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 1.9 s; TE = 2.3 msec; matrix = 256 × 256; FOV = 230; sagittal plane; slice thickness = 1 mm; 192 slices).
fMRI Data Analysis
The fMRI data were preprocessed using statistical parametric mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Images were spatially realigned to correct for head movement. Functional data were coregistered to the structural MPRAGE, which was then segmented into cerebrospinal fluid, gray matter, and white matter. Structural and functional images were then transformed into standardized stereotactic space as defined by the Montreal Neurological Institute. The normalized functional data were smoothed using an 8 mm Guassian kernel, full-width-at-half-maximum, to increase signal-to-noise ratio.
For each participant’s data, a general linear model (GLM) was created using regressors that corresponded to the two task conditions. High-pass temporal filtering with a cutoff of 128 s was applied to remove low-frequency drift in the data. Parameter estimates resulting from the GLM were then used to create linear contrasts. Because we were interested in emotion regulation, we focused on the contrast between the label and observe conditions (i.e. label > observe). We chose the label > observe contrast because it is thought that putting an emotion into words via labeling helps down-regulate emotional reactivity, whereas passively observing an emotion may elicit more emotional reactivity (Lieberman et al. 2007).
To assess emotion regulation, we focused on connectivity between the amygdala and PFC. We used psychophysiological interactions (PPI) to examine neural connectivity, using the bilateral amygdala as the seed region. The amygdala region of interest (ROI) was defined by combining the left and right anatomically-defined amygdala. The automated gPPI toolbox in SPM (gPPI; McLaren et al. 2012) was used (1) to extract the deconvolved times series from the bilateral amygdala ROI for each participant to create the physiological variables, (2) to convolve each trial type with the canonical HRF to create the psychological regressor, and (3) to multiply the time series from the psychological regressors with the physiological variable to create the PPI interaction. Given our a priori hypotheses, we restricted our PPI analyses to the right ventrolateral PFC (rVLPFC). We focused on the rVLPFC because past research has found this region to be more active during emotion labeling than passive viewing, and activation in this region is positively correlated with reduced amygdala activation in the label condition (Lieberman et al. 2007; Torrisi et al. 2013). The rVLPFC was defined using the inferior frontal orbital gyrus in the AAL atlas in the WFU PickAtlas (Maldjian et al. 2003, 2004; Tzourio-Mazoyer et al. 2002). Parameter estimates of signal intensity were extracted from rVLPFC following the PPI analysis and represent connectivity between the amygdala and rVLPFC. We used a linear regression model in SPSS with NE, CC, and their interaction as predictors of functional connectivity between the amygdala and rVLPFC during the label > observe contrast.
Results
Bivariate Correlations
Table 2 presents bivariate correlations among the variables. NE and CC were negatively correlated. As predicted, amygdala-rVLPFC functional connectivity during emotion regulation (label > observe) was positively correlated with NE (Fig. 2a) and negatively correlated with CC (Fig. 2b).
Negative Emotionality x Cognitive Control Predicting Amygdala-VLPFC Functional Connectivity
A hierarchical linear regression analysis was conducted to examine the independent and interactive contribution of NE and CC to amygdala-rVLPFC functional connectivity during the emotion regulation task (Table 3). NE and CC were standardized, and their interaction was calculated as the product of the standardized variables. The main effects of NE and CC were entered in the first step of the regression, and their interaction was entered in the second step. The NE x CC interaction was decomposed using simple slopes analysis at low (−1 SD), moderate (mean), and high (+1 SD) levels of CC (Aiken and West 1991).
We examined several assumptions of hierarchical linear regression, including ensuring low multicollinearity of predictor variables, homoscedasticity, and multivariate normality of the model. The tolerance of the model was 0.301, suggesting that NE and CC were not highly muticollinear. Additionally, the spread of the residuals was approximately normally distributed and the Shapiro-Wilk statistic was non-significant (W(44) = 0.98, p = 0.74), providing no evidence for heteroscedasticity or non-normality. The regression analysis revealed a significant main effect of NE and a nonsignificant main effect of CC in Step 1 (when only the main effects were entered). As hypothesized, the interaction of NE and CC significantly predicted functional connectivity during emotion regulation (Table 3). As shown in Fig. 2c, decomposition of the interaction revealed that NE significantly predicted more positive functional connectivity at low (b = 0.38, t = 2.40, p = 0.02) but not moderate (b = 0.19, t = 1.04, p = 0.31) or high (b = −0.002, t = −0.008, p = 0.99) levels of CC.
Functional Connectivity Predicting Anxiety
To explore behavioral correlates of functional connectivity during the emotion regulation task, we examined the association between amygdala-rVLPFC functional connectivity and subsequent anxiety (Table 2). As expected, functional connectivity was positively associated with anxiety across the following nine months (Fig. 3).
Discussion
Past theory and research have identified adolescence as a period of sensitivity in emotion processing regions coupled with less effective down-regulation of this reactivity by regulatory regions (Casey et al. 2008; Crone and Dahl 2012). However, not all adolescents exhibit neural dysregulation of emotion, highlighting the importance of identifying factors that predict which youth may show this pattern due to individual differences earlier in development (Pfeifer and Allen 2012). To address the scarcity of research in this area, the present study examined how individual differences in two childhood temperamental traits, NE and CC, predict variability in functional connectivity between the amygdala and PFC during emotion regulation. In line with our hypotheses, we found that childhood NE predicted less mature amygdala-PFC connectivity during adolescence, but this effect was specific to adolescent girls with low levels of childhood CC. Additionally, an immature pattern of amygdala-PFC connectivity during emotion regulation predicted future anxiety symptoms.
Temperamental Traits and Neural Dysregulation
The observed link between temperament and neural regulation of emotion suggests a mechanism through which individual differences in childhood traits may confer risk for, or resilience against, emotional difficulties during adolescence. While adolescence has been implicated as a time of heightened emotional reactivity combined with compromised emotion regulation, the present study suggests that this disjuncture may be especially relevant for youth with high levels of NE. NE has previously been associated with attentional biases towards negative emotional information (Schultz et al. 2004) and less effective emotion regulation strategies in children (Santucci et al. 2008) and adults (Gross and John 2003). Additionally, youth with high NE are more reactive to negative emotion cues (Calkins et al. 1999), which may make it more difficult for them to down-regulate amygdala reactivity during an emotion regulation task. Building on prior research identifying adverse psychological consequences of high NE, the present study identifies a neural correlate (immature amygdala-PFC connectivity) of NE that might explain its association with less effective emotion regulation.
Importantly, however, in this study we found that the link between NE and emotion dysregulation was specific to girls with lower levels of CC. This pattern builds on past research revealing that CC moderates the effect of NE on responses to stress (Lengua and Long 2002), ruminative emotion regulation strategies (Verstraeten et al. 2009), and symptoms of emotional distress (Gulley et al. 2016). In our study, NE predicted a less mature pattern of amygdala-PFC connectivity only in adolescent girls with poor CC, suggesting that CC may mitigate against the effects of NE on neural dysregulation of emotions, allowing youth to more effectively recover from negative information and redirect their attention towards task-relevant goals.
Neural Dysregulation and Anxiety
This study also found that neural dysregulation of emotion predicts prospective anxiety symptoms during adolescence. Past studies have found that anxious adolescents exhibit more positive amygdala-PFC connectivity while viewing angry faces (Monk et al. 2008) and anticipating social feedback (Guyer et al. 2008). The present study builds upon this research, finding that less mature amygdala-PFC connectivity during emotion regulation predicted higher levels of anxiety over the nine months following the scan. This research adds to existing knowledge by examining connectivity during emotion regulation, when PFC down-regulation of amygdala is especially salient, and by examining the prospective association between neural dysregulation of emotion and anxiety symptoms. Support for this prospective association indicates that this pattern of connectivity predicts ongoing rather than merely current anxiety symptoms, suggesting its utility as a marker of enduring anxiety.
While adolescence has long been of interest as a period of increasing risk for the development of anxiety, these findings help explain why only certain adolescent girls show enduring anxiety during this time (Pfeifer and Allen 2012). Most youth show a pattern of more negative amygdala-PFC connectivity by mid-adolescence (Gee et al. 2014). Our results suggest that girls who retain a less mature pattern of connectivity may be especially susceptible to anxiety. Ineffective regulation of amygdala activity may lead to hypervigilance for negative emotional cues and inability to direct attention away from emotionally distracting information, setting the stage for sustained worry and anxiety. In support of this proposed pathway, prior research has found that adolescents higher in trait anxiety show less amygdala habituation following repeated exposure to emotional faces (Hare et al. 2008).
Contributions and Limitations
This study has many methodological strengths, including the use of a multi-informant assessment of NE and CC across childhood to predict adolescent neural regulation as well as a comprehensive assessment of anxiety symptoms across a nine-month period. Overall, this research highlights potential strategies for improving emotion regulation and preventing anxiety in girls during adolescence. Specifically, interventions that increase CC may promote the development of more effective emotion regulation in adolescence and may be especially beneficial to girls with high levels of NE. In support of this idea, research on mindfulness, a therapeutic approach that increases CC (Chambers et al. 2008), has found that mindfulness improves emotion regulation (Hilt and Pollak 2004), alters connections between brain regions involved in emotion reactivity and regulation (Lutz et al. 2014), and decreases symptoms of anxiety (Hofmann et al. 2010).
Despite the novel contributions of this study, it has noteworthy limitations. First, although previous research suggests emotion labeling is an effective implicit emotion regulation strategy (Brooks et al. 2016; Gyurak et al. 2011; Hariri et al. 2000; Lieberman et al. 2007), we did not explicitly instruct participants to regulate their emotions during the task. As we cannot be certain that labeling emotions engaged emotion regulation strategies to a similar extent in all of our participants, future studies examining patterns of functional connectivity during explicit emotion regulation tasks are warranted. Second, this study only examined neural regulation of emotion in adolescent girls. While girls tend to show heightened emotional reactivity during adolescence (Charbonneau et al. 2009), it is not clear whether these findings generalize to boys. Third, given our limited sample size, our analyses used composite scores of NE and CC aimed to capture stability in these traits rather than tracking changes over time; likewise, our anxiety score captured symptoms across a nine-month period during which symptoms were highly stable, and we measured neural processing of emotion at a single point in time. However, it is likely that temperament, neural systems supporting emotion regulation, and anxiety symptoms have complex and bidirectional effects across development. These interdependent effects could be probed more deeply using larger samples that would allow for more sophisticated analyses examining how changing temperamental and neural factors contribute to the onset and exacerbation of anxiety across adolescence.
While the primary goal of our study was to examine the effect of childhood temperament on adolescent neural regulation of emotion, it is also important to consider whether neural processes explain the link between temperament and later anxiety. Past research has found that different dimensions of CC can serve as risk or protective factors against the development of anxiety (e.g., White et al. 2011), and that NE and CC interact during childhood to predict later risk for anxiety (Gulley et al. 2016). As our limited sample size prevented us from examining how neural processing of emotion might account for the link between childhood NE and adolescent anxiety at different levels of CC, future research could build upon this work by examining whether neural dysregulation of emotion mediates the link between childhood temperament and the development of anxiety across adolescence.
Conclusion
The present study examined whether individual differences in childhood temperament predict variability in neural regulation of emotion during adolescence. Findings revealed that childhood NE predicted less mature amygdala-PFC connectivity during emotion regulation in adolescence, an effect that held only in girls with poor childhood CC. Furthermore, less mature connectivity predicted higher levels of anxiety across the following nine months, identifying an important psychological impact of neural dysregulation of emotion and highlighting the importance of considering individual differences in emotion regulation during adolescence.
Notes
Two adolescents completed the laboratory visit during the summer following 10th grade due to prior ineligibility for the fMRI scan (i.e., metal braces).
This subset of youth was selected from 252 girls still participating in the longitudinal study. For the purpose of another task not discussed in this manuscript, girls were selected based on their history of peer victimization; both those with high and low peer victimization during childhood are included in analyses.
References
Agoston, A. M., & Rudolph, K. D. (2016). Interactive contributions of cumulative peer stress and executive function deficits to depression in early adolescence. The Journal of Early Adolescence, 36(8), 1070–1094. https://doi.org/10.1177/0272431615593176.
Aiken, L. S., & West, S. G. (1991). Multiple regression: Testing and interpreting interactions. Thousand Oaks: Sage.
Brooks, J. A., Shablack, H., Gendron, M., Satpute, A. B., Parrish, M. H., & Lindquist, K. A. (2016). The role of language in the experience and perception of emotion: A neuroimaging meta-analysis. Social Cognitive and Affective Neuroscience, 12(2), 169–183. https://doi.org/10.1093/scan/nsw121.
Calkins, S. D., Gill, K. L., Johnson, M. C., & Smith, C. L. (1999). Emotional reactivity and emotional regulation strategies as predictors of social behavior with peers during toddlerhood. Social Development, 8(3), 310–334. https://doi.org/10.1111/1467-9507.00098.
Casey, B. J., Somerville, L. H., Gotlib, I. H., Ayduk, O., Franklin, N. T., Askren, M. K., et al. (2011). Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences, 108(36), 14998–15003. https://doi.org/10.1073/pnas.1108561108.
Casey, B. J. (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66, 295–319. https://doi.org/10.1146/annurev-psych-010814-015156.
Casey, B. J., Jones, R. M., & Hare, T. A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124(1), 111–126. https://doi.org/10.1196/annals.1440.010.
Chambers, R., Lo, B. C. Y., & Allen, N. B. (2008). The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cognitive Therapy and Research, 32(3), 303–322. https://doi.org/10.1007/s10608-007-9119-0.
Charbonneau, A. M., Mezulis, A. H., & Hyde, J. S. (2009). Stress and emotional reactivity as explanations for gender differences in adolescents’ depressive symptoms. Journal of Youth and Adolescence, 38(8), 1050–1058. https://doi.org/10.1007/s10964-009-9398-8.
Crone, E. A., & Dahl, R. E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. https://doi.org/10.1038/nrn3313.
Ernst, M., Pine, D. S., & Hardin, M. (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36(03), 299–312. https://doi.org/10.1017/S0033291705005891.
Gee, D. G., Gabard-Durnam, L., Telzer, E. H., Humphreys, K. L., Goff, B., Shapiro, M., et al. (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25(11), 2067–2078. https://doi.org/10.1177/0956797614550878.
Gioia, G. A., & Isquith, P. K. (2004). Ecological assessment of executive function in traumatic brain injury. Developmental Neuropsychology, 25, 135–158. https://doi.org/10.1080/87565641.2004.9651925.
Gioia, G. A., Isquith, P. K., Guy, S. C., & Kenworthy, L. (2000). Behavior rating inventory of executive function: BRIEF. Odessa: Psychological Assessment Resources.
Gross, J. J., & John, O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and wellbeing. Journal of Personality and Social Psychology, 85, 348–362. https://doi.org/10.1037/0022-3514.85.2.348.
Gulley, L. D., Hankin, B. L., & Young, J. F. (2016). Risk for depression and anxiety in youth: The interaction between negative affectivity, effortful control, and stressors. Journal of Abnormal Child Psychology, 44, 207–218. https://doi.org/10.1007/s10802-015-9997-7.
Guyer, A. E., Lau, J. Y., McClure-Tone, E. B., Parrish, J., Shiffrin, N. D., Reynolds, R. C., et al. (2008). Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry, 65(11), 1303–1312.
Gyurak, A., Gross, J. J., & Etkin, A. (2011). Explicit and implicit emotion regulation: A dual-process framework. Cognition and Emotion, 25(3), 400–412. https://doi.org/10.1080/02699931.2010.544160.
Hankin, B. L., Mermelstein, R., & Roesch, L. (2007). Sex differences in adolescent depression: Stress exposure and reactivity models. Child Development, 78(1), 279–295. https://doi.org/10.1111/j.1467-8624.2007.00997.x.
Hare, T. A., Tottenham, N., Galvan, A., Voss, H. U., Glover, G. H., & Casey, B. J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–934. https://doi.org/10.1016/j.biopsych.2008.03.015.
Hariri, A. R., Bookheimer, S. Y., & Mazziotta, J. C. (2000). Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport, 11(1), 43–48.
Hilt, L. M., & Pollak, S. D. (2004). Getting out of rumination: Comparison of three brief interventions in a sample of youth. Journal of Abnormal Child Psychology, 40(7), 1157–1165. https://doi.org/10.1007/s10802-012-9638-3.
Hofmann, S. G., Sawyer, A. T., Witt, A. A., & Oh, D. (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169–183.
Killgore, W. D., Oki, M., & Yurgelun-Todd, D. A. (2001). Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport, 12(2), 427–433.
Kim, M. J., Gee, D. G., Loucks, R. A., Davis, F. C., & Whalen, P. J. (2010). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex, 21(7), 1667–1673.
Kochanska, G., Murray, K. T., & Harlan, E. T. (2000). Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology, 36(2), 220–232. https://doi.org/10.1037/0012-1649.36.2.220.
Larson, R. W., Moneta, G., Richards, M. H., & Wilson, S. (2002). Continuity, stability, and change in daily emotional experience across adolescence. Child Development, 73(4), 1151–1165. https://doi.org/10.1111/1467-8624.00464.
Lee, H., Heller, A. S., van Reekum, C. M., Nelson, B., & Davidson, R. J. (2012). Amygdala-prefrontal coupling underlies individual differences in emotion regulation. NeuroImage, 62(3), 1578–1581. https://doi.org/10.1016/j.neuroimage.2012.05.044.
Lengua, L. J. (2003). Associations among emotionality, self-regulation, adjustment problems, and positive adjustment in middle childhood. Journal of Applied Developmental Psychology, 24(5), 595–618. https://doi.org/10.1016/j.appdev.2003.08.002.
Lengua, L. J., & Long, A. C. (2002). The role of emotionality and self-regulation in the appraisal–coping process: Tests of direct and moderating effects. Journal of Applied Developmental Psychology, 23(4), 471–493. https://doi.org/10.1016/S0193-3973(02)00129-6.
Lieberman, M. D., Eisenberger, N. I., Crockett, M. J., Tom, S. M., Pfeifer, J. H., & Way, B. M. (2007). Putting feelings into words affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science, 18(5), 421–428. https://doi.org/10.1111/j.1467-9280.2007.01916.x.
Lutz, J., Herwig, U., Opialla, S., Hittmeyer, A., Jäncke, L., Rufer, M., et al. (2014). Mindfulness and emotion regulation—An fMRI study. Social Cognitive and Affective Neuroscience, 9(6), 776–785. https://doi.org/10.1093/scan/nst043.
Maldjian, J. A., Laurienti, P. J., & Burdette, J. H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage, 21(1), 450–455. https://doi.org/10.1016/j.neuroimage.2003.09.032.
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–1239. https://doi.org/10.1016/S1053-8119(03)00169-1.
McLaren, D. G., Ries, M. L., Xu, G., & Johnson, S. C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. https://doi.org/10.1016/j.neuroimage.2012.03.068.
McRae, K., Gross, J. J., Weber, J., Robertson, E. R., Sokol-Hessner, P., Ray, R. D., et al. (2012). The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience, 7(1), 11–22. https://doi.org/10.1093/scan/nsr093.
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. https://doi.org/10.1146/annurev.neuro.24.1.167.
Monk, C. S., Telzer, E. H., Mogg, K., Bradley, B. P., Mai, X., Louro, H. M., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65(5), 568–576. https://doi.org/10.1001/archpsyc.65.5.568.
Muris, P., van der Pennen, E., Sigmond, R., & Mayer, B. (2008). Symptoms of anxiety, depression, and aggression in non-clinical children: Relationships with self-reports and performance based measures of attention and effortful control. Child Psychiatry and Human Development, 39, 455–467. https://doi.org/10.1007/s10578-008-0101-1.
Nelson, E. E., Jarcho, J. M., & Guyer, A. E. (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. https://doi.org/10.1016/j.dcn.2015.12.008.
Ochsner, K. N., & Gross, J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. https://doi.org/10.1016/j.tics.2005.03.010.
Perez-Edgar, K., Roberson-Nay, R., Hardin, M. G., Poeth, K., Guyer, A. E., Nelson, E. E., et al. (2007). Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage, 35(4), 1538–1546. https://doi.org/10.1016/j.neuroimage.2007.02.006.
Pfeifer, J. H., & Allen, N. B. (2012). Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences, 16(6), 322–329. https://doi.org/10.1016/j.tics.2012.04.011.
Pfeifer, J. H., & Allen, N. B. (2016). The audacity of specificity: Moving adolescent developmental neuroscience towards more powerful scientific paradigms and translatable models. Developmental Cognitive Neuroscience, 17, 131–137. https://doi.org/10.1016/j.dcn.2015.12.012.
Pine, D. S., Cohen, P., Gurley, D., Brook, J., & Ma, Y. (1998). The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry, 55(1), 56–64. https://doi.org/10.1001/archpsyc.55.1.56.
Reynolds, C. R. (1982). Convergent and divergent validity of the revised Children's manifest anxiety scale. Educational and Psychological Measurement, 42(4), 1205–1212. https://doi.org/10.1177/001316448204200429.
Reynolds, C. R., & Richmond, B. O. (1978). What I think and feel: A revised measure of children's manifest anxiety. Journal of Abnormal Child Psychology, 6(2), 271–280. https://doi.org/10.1007/BF00919131.
Reynolds, C. R., & Richmond, B. O. (1985). Revised Children's manifest anxiety scale. Los Angeles: Western Psychological Services.
Rose, A. J., & Rudolph, K. D. (2006). A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin, 132(1), 98–131. https://doi.org/10.1037/0033-2909.132.1.98.
Rothbart, M. K., Ahadi, S. A., Hershey, K. L., & Fisher, P. (2001). Investigations of temperament at three to seven years: The Children's behavior questionnaire. Child Development, 72(5), 1394–1408. https://doi.org/10.1111/1467-8624.00355.
Rudolph, K. D., Flynn, M., Abaied, J. L., Groot, A., & Thompson, R. (2009). Why is past depression the best predictor of future depression? Stress generation as a mechanism of depression continuity in girls. Journal of Clinical Child and Adolescent Psychology, 38(4), 473–485. https://doi.org/10.1080/15374410902976296.
Rudolph, K. D., Troop-Gordon, W., Monti, J. D., & Miernicki, M. E. (2014). Moving against and away from the world: The adolescent legacy of peer victimization. Development and Psychopathology, 26, 721–734. https://doi.org/10.1017/S0954579414000340.
Santucci, A. K., Silk, J. S., Shaw, D. S., Gentzler, A., Fox, N. A., & Kovacs, M. (2008). Vagal tone and temperament as predictors of emotion regulation strategies in young children. Developmental Psychobiology, 50, 205–216. https://doi.org/10.1002/dev.20283.
Schaefer, S. M., Jackson, D. C., Davidson, R. J., Aguirre, G. K., Kimberg, D. Y., & Thompson-Schill, S. L. (2002). Modulation of amygdalar activity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience, 14(6), 913–921. https://doi.org/10.1162/089892902760191135.
Schultz, D., Izard, C. E., & Bear, G. (2004). Children's emotion processing: Relations to emotionality and aggression. Development and Psychopathology, 16(02), 371–387. https://doi.org/10.1017/S0954579404044566.
Seligman, L. D., Ollendick, T. H., Langley, A. K., & Baldacci, H. B. (2004). The utility of measures of child and adolescent anxiety: A meta-analytic review of the revised Children's manifest anxiety scale, the state–trait anxiety inventory for children, and the child behavior checklist. Journal of Clinical Child and Adolescent Psychology, 33(3), 557–565. https://doi.org/10.1207/s15374424jccp3303_13.
Shulman, E. P., Smith, A. R., Silva, K., Icenogle, G., Duell, N., Chein, J., & Steinberg, L. (2016). The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience, 17, 103–117. https://doi.org/10.1016/j.dcn.2015.12.010.
Silk, J. S., Siegle, G. J., Whalen, D. J., Ostapenko, L. J., Ladouceur, C. D., & Dahl, R. E. (2009). Pubertal changes in emotional information processing: Pupillary, behavioral, and subjective evidence during emotional word identification. Development and Psychopathology, 21(01), 7–26. https://doi.org/10.1017/S0954579409000029.
Silvers, J. A., McRae, K., Gabrieli, J. D., Gross, J. J., Remy, K. A., & Ochsner, K. N. (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion, 12(6), 1235–1247. https://doi.org/10.1037/a0028297.
Silvers, J. A., Insel, C., Powers, A., Franz, P., Helion, C., Martin, R. E., et al. (2016). vlPFC–vmPFC–amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27(7), 3502–3514. https://doi.org/10.1093/cercor/bhw073.
Simonds, J., Kieras, J. E., Rueda, M. R., & Rothbart, M. K. (2007). Effortful control, executive attention, and emotional regulation in 7–10-year-old children. Cognitive Development, 22(4), 474–488. https://doi.org/10.1016/j.cogdev.2007.08.009.
Simonds, J., & Rothbart, M. K. (2004). The temperament in middle childhood questionnaire (TMCQ): A computerized self-report measure of temperament for ages 7–10. Athens: Poster presented at the Occasional Temperament Conference.
Somerville, L. H., Jones, R. M., & Casey, B. J. (2010). A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72(1), 124–133. https://doi.org/10.1016/j.bandc.2009.07.003.
Spear, L. P. (2000). Neurobehavioral changes in adolescence. Current Directions in Psychological Science, 9(4), 111–114. https://doi.org/10.1111/1467-8721.00072.
Steinberg, L. (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. https://doi.org/10.1016/j.dr.2007.08.002.
Sugimura, N., & Rudolph, K. D. (2012). Temperamental differences in children’s reactions to peer victimization. Journal of Clinical Child and Adolescent Psychology, 41, 314–328. https://doi.org/10.1080/15374416.2012.656555.
Thomas, K. M., Drevets, W. C., Dahl, R. E., Ryan, N. D., Birmaher, B., Eccard, C. H., et al. (2001). Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry, 58(11), 1057–1063. https://doi.org/10.1016/S0006-3223(00)01066-0.
Tillfors, M., & Van Zalk, N. (2015). Easier to accelerate than to slow down: Contributions of developmental neurobiology for the understanding of adolescent social anxiety. In K. Ranta, A. M. La Greca, L.-J. Garcia-Lopez, & M. Marttunen (Eds.), Social anxiety and phobia in adolescents (pp. 71–94). New York: Springer.
Tottenham, N., Tanaka, J. W., Leon, A. C., McCarry, T., Nurse, M., Hare, T. A., et al. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. https://doi.org/10.1016/j.psychres.2008.05.006.
Torrisi, S. J., Lieberman, M. D., Bookheimer, S. Y., & Altshuler, L. L. (2013). Advancing understanding of affect labeling with dynamic causal modeling. NeuroImage, 82, 481–488. https://doi.org/10.1016/j.neuroimage.2013.06.025.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289. https://doi.org/10.1006/nimg.2001.0978.
Verstraeten, K., Vasey, M. W., Raes, F., & Bijttebier, P. (2009). Temperament and risk for depressive symptoms in adolescence: Mediation by rumination and moderation by effortful control. Journal of Abnormal Child Psychology, 37(3), 349–361. https://doi.org/10.1007/s10802-008-9293-x.
White, L. K., McDermott, J. M., Degnan, K. A., Henderson, H. A., & Fox, N. A. (2011). Behavioral inhibition and anxiety: The moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology, 39(5), 735–747. https://doi.org/10.1007/s10802-011-9490-x.
Wilson, B. J. (2006). The entry behavior of aggressive/rejected children: The contributions of status and temperament. Social Development, 15, 463–479. https://doi.org/10.1111/j.1467-9507.2006.00351.x.
Wisniewski, J. J., Genshaft, J. L., Mulick, J. A., & Coury, D. L. (1987). Test-retest reliability of the revised Children's manifest anxiety scale. Perceptual and Motor Skills, 65(1), 67–70. https://doi.org/10.2466/pms.1987.65.1.67.
Yap, M. B., Allen, N. B., O'Shea, M., Di Parsia, P., Simmons, J. G., & Sheeber, L. (2011). Early adolescents' temperament, emotion regulation during mother-child interactions, and depressive symptomatology. Development and Psychopathology, 23(1), 267–282. https://doi.org/10.1017/S0954579410000787.
Zhou, Q., Chen, S. H., & Main, A. (2012). Commonalities and differences in the research on children’s effortful control and executive function: A call for an integrated model of self-regulation. Child Development Perspectives, 6(2), 112–121. https://doi.org/10.1111/j.1750-8606.2011.00176.x.
Acknowledgments
We would like to thank the families and schools who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a University of Illinois Research Board Award, National Institute of Mental Health Grant [MH68444] to KDR, and National Institute of Mental Health Grant [MH105655] to EHT and KDR.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethical approval was obtained from the InstitutionalReview Board of the University of Illinois at Urbana-Champaign.
Informed Consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Davis, M.M., Miernicki, M.E., Telzer, E.H. et al. The Contribution of Childhood Negative Emotionality and Cognitive Control to Anxiety-Linked Neural Dysregulation of Emotion in Adolescence. J Abnorm Child Psychol 47, 515–527 (2019). https://doi.org/10.1007/s10802-018-0456-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10802-018-0456-0