Abstract
Advancement of the effective, stable, earth-rich, low-cost, and highly efficient electro-catalysts commonly demanded large-scale commercial applications toward the low-carbon economy. Here our group demonstrated the synthesis, characterization and electrochemical performance of highly effective and versatile cobalt oxide nanoparticles (Co3O4 NP’s) layered on fluorinated tin oxide (FTO) for efficient water splitting strategy. The synthesized Co3O4 NP’s were analyzed by Scanning Electron Microscopy, Particle Size Analyzer, Zeta Potential, X-ray Diffraction, Ultraviolet–Visible Absorption Spectroscopy, Fourier Transform Infrared Spectroscopy (FTIR) and Raman spectroscopy. The Co3O4 NP’s were deposited on FTO using inexpensive as well as leading electro-deposition method and by simple spray method. Hence, prepared Co3O4 electrocatalysts is found to be highly efficient for water oxidation without any pre-conditioning. This Co3O4 electrocatalyst, layered at FTO through electro-deposition method, is first used in Oxygen evolution reactions (OER). Electrochemically deposited Co3O4 NP’s at FTO proved itself a better electrode than simple spray-coated Co3O4 NP’s at FTO electrode and initiated the oxygen onset potential around 1.49 V versus reversible hydrogen electrode (RHE) (ŋ = 260 mV). The observed Tafel slope was 53 mV dec−1 during the oxygen evolution reaction and the peak of the current density J/ 68 mA cm−2 was observed at 1.78 V versus RHE, which is novel for cobalt-based electrocatalyst system. The conclusions drawn were comparable with both the pricey state of the art IrO2 and RuO2 electrocatalyst systems. In the long term water electrolysis experiment, the electrocatalyst also offered durability and enduring stability. This innovative approach offers a simplest technique to prepare cost-effective and super effectual nanoscale electrocatalyst for water electrolysis.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The successive use of depleting conventional fossil fuels is causing adverse health and environmental problems that have directed modern scientists to develop alternative ways to produce energy [1, 2]. In this context, electrochemical H2O oxidation can be a promising way to generate CO2 free energy [3]. Water splitting requires a potential of 1.23 V vs. RHE thermodynamically, but it is the ideal case, rather it is a hectic task to split water at this potential [4]. It is well known that hydrogen evolution reaction (HER) is kinetically practicable in acidic electrolytes due to a plethora of protons but, on the other hand, one major critical step is oxygen evolution reaction (OER) in basic electrolyte expressed in Eq. (1) [5]. This half reaction is gridlock because of four-electrons transmit mechanisms and its lethargic kinetics [6]. Unfortunately, in an actual scenario, additional potential is required, called over potential, which restricts the validity of the process [7]. To accelerate this reaction as well as to reduce the overpotential, an efficient electrocatalyst is required.
Extensive research has been done over the last years to develop new catalysis for OER. That’s because the primary energy loss in water electrolysis occurs at the anode due to OER’s slow reaction kinetics [8]. Under acidic and basic conditions, the OER cannot prevent the use of metals such as iridium (Ir) because Ir is the only metal with comparatively higher probability of survival under the severe acidic and basic circumstances. On the other hand, it has been stated recently that the usage of metals can be minimized by using metal oxides [9], which is confirmed by using stability number analysis [10]. In this regard, a number of metal oxide catalysts have been reported for this purpose with low overpotential OER’s [11,12,13,14]. IrO2 and RuO2 are the most active metal oxide OER catalysis but the rarity and increased price of these valuable metals restrict their large scale implementation [15, 16]. The study of previous research shows that there are hundreds of attempts that were made to find out ways for controlled and efficient water splitting at lower potential with enormous elemental compositions and nanostructures. In the past, a vast number of metal oxides were investigated for the sake of lowering the onset potential including NiOx, MnOx, RuO2, spinal type Co3O4, NiCo2O4, LaCoO3 and LaNiO3. Pyrochlore type oxide catalyst has also been extensively used as an electrocatalyst and imparts significant reduction in the potential requirement [17,18,19,20,21,22].
Furthermore, the electrolyte has major dependence on the degree of electrolysis. Water catalysis with acidic and basic electrolytes has already been done by several researchers [23, 24]. The use of the acidic environment is troublesome and practically unfavourable due to its highly corrosive nature especially in the case of OER [25, 26]. Consequently, the operational cost of the process increases. Due to the fact that that most of the materials can survive in strong alkaline conditions, studies on designing OER catalysis in alkaline conditions is far more successful than that under the acidic environment [26].
Because of strong OER performance, easy availability and abundance, cobalt (Co) based materials (e.g. cobalt oxide, cobalt containing perovskites and cobalt phosphate) have been known to be effective catalyst to precious metal oxides [26,27,28]. Cobalt oxides are among the most researched metal oxides, mainly due to their theoretical specific capacitance (~ 3560 F/g) [29]. Also, theoretical models and experimental studies have already shown that OER behaviour is closely linked to the Co atom’s electronic structure including oxidation state, eg filling and O p-band centre [27, 30,31,32].
To the best of our understanding, there have been no studies on electro-deposited Co3O4@FTO for OER before. Our results demonstrate that electro-deposited Co3O4@FTO electrocatalyst is an advanced material that exhibits outstanding OER activity (lower onset potential, greater anodic current, smaller Tafel slope and stronger durability) in the 0.1 M KOH electrolyte solution for screening under constant voltage than most other cobalt based and spray coated Co3O4@FTO presented in this research. Furthermore, the electro-deposited Co3O4@FTO prepared in this research has an output comparable to the state of the art electrocatalyst based on Ru.
2 Experimental section
2.1 Materials and methods
Co3O4NP’s for electrochemical water splitting were prepared using co-precipitating agent without any capping agent. Cobalt Nitrate hexahydrate Co(NO3)2.6H2O and NaOH purchased from sigma Aldrich were dissolved in a ratio of 2:4 M in 40 mL of de-ionized water. 2 M cobalt nitrate solution was mixed with 4 M NaOH solution with constant magnetic stirring of 1000 rpm for 4 h at ambient temperature. The resulting solution was allowed to stay overnight. After 16–17 h, the cobalt hydroxide precipitates settled down and the liquor above precipitates was discarded carefully. The precipitates were centrifuged at 6000 rpm resulting in the formation of pink-colored Co3O4 NP’s. After repeatedly washing with ethanol, the Co3O4 NP’s were annealed at 500 °C for 6 h before coating [33]. Figure 1 describes the schematic illustration of OER using Co3O4 electrocatalyst coated FTO as an anode.
2.2 Synthesis of Co3O4 based electrode
Two general coating methods were employed for the preparation of cobalt oxide-based electrodes; the simple spray coating method, and the electro-deposition method. In the electro-deposition method, Co3O4 NP’s were deposited at FTO using cyclic voltammetry technique. The three-electrode glass cell was filled with 1 M Co3O4 solution with suspended FTO as a working electrode. The number of step crossings was taken 200 at the scan rate 20 mV s−1 between the limits − 1 to + 1 V vs. standard saturated Ag/AgCl electrode in 3.5 M KCl. A uniform layer on FTO was deposited as shown in Fig. 2b. Co3O4 coated FTO was calcined at 200 °C before electrochemical analysis. In a simple spray coating method involved the spray of 1 M Co3O4 NP’s solution in ethanol at FTO using a nozzle spray bottle (Fig. 2c). 1 M Co3O4 NP’s solution was sprayed at FTO 15 to 20 times with an interval of 60 min between each spray to explicate uniform growth of catalytic layers. Liquid molecules evaporated from the FTO surface leaving catalytic layers behind. Resulting FTO was calcined at 200 °C to increase the catalytic layer's strength [34,35,36,37]. Figure 2a is presenting bare FTO substrate without any catalyst coating.
3 Results and discussions
UV–Visible absorption studies were carried out using Agilent UV–Visible Spectrophotometer (Carry 60 UV–VIS). Co3O4 NP’s imparted many excellent features like intense absorption peaks at 463 nm and 520 nm (Fig. 3a). These were the characteristic absorption peaks for Co3O4 NP’s and already have been reported as Plasmon resonance absorption for Co3O4 NP’s [38]. The intense surface Plasmon could be responsible for the formation of non-oxidized nanostructures in Cobalt. A band from 500 to 600 nm is also reported in the literature and can be assigned to O2− → Co3+ charge transfer process [37].
Physicochemical analysis: a VU-Visible spectrum of Co3O4 NP’s: inset photograph shows the actual Co3O4 NP’s solution dispersed in ethanol b FT-IR graph presenting major functional groups with their peak positions c Zeta potential curve, showing negative value of ZP = − 26 mV in ethanol solution d Plot of Co3O4 NP’s particle size as a function of number in ethanol solution
FTIR investigations were executed using Agilent Carry 360 FTIR equipped with a DTGS detector (Fig. 3b). The existence of the hydroxyl group due to NaOH or by the absorption of water molecules during formation has been proved by stretching frequency at 3399 cm−1 and fragile asymmetric band at 2952 cm−1 [39]. C-N vibrations were ascribed by the band at 1180 cm−1 [40]. The Phase purity of the mono-disperse structure was shown by two strong stretching and bending frequencies at 1423 cm−1 and 812 cm−1 [41]. Peaks around 1500 cm−1 and 1640 cm−1 correspond to cobalt oxide asymmetric stretching vibrations while peaks at and 1450 cm−1and 1260 cm−1 represent the vibrations due to NH3 and NO3 groups as these functional groups were involved in the starting material [42, 43].
The storage and shelf stability of freshly prepared Co3O4 NP’s were predicted by the zeta potential parameter (Fig. 3c) using Anton paarLitesizer 500, using DLS technology λ = 658 nm. The zeta potential value we got was − 26 mV. The negative value of zeta potential exhibited long term stability and excellent colloidal nature due to negative-negative repulsions [44]. The particle size of Co3O4 NP’s was measured using the same instrument used for zeta potential measurement. The Particle diameter distribution curve discloses that Co3O4 NP’s obtained have poly-dispersed nature with average particle diameter 45–50 nm (Fig. 3d).
For morphological studies, SEM (TESCAN Vega LMU-Variable pressure Scanning Electron Microscope) images for different magnifications of electro-deposited Co3O4 layer on FTO are shown in Fig. 4. It is shown in Fig. 4 that a uniform thin layer of Co3O4NP’s with an average particle size of 40 nm is deposited on the FTO electrode. It is observed from Fig. 4a that small particles range from 40 to 50 nm while large agglomerated particles have an average size in the range of 100 nm. High resolution image of electro-deposited Co3O4 at FTO is given in Fig. S1. On the other hand, the SEM image of Co3O4 electrocatalyst coated on the FTO electrode using a spray coating technique shows a less homogeneous distribution of Co3O4 NP’s on FTO anode shown in Fig. 4b. This non-uniform coating could be due to the non-selective and non-specific nature of the spray coating strategy. Fig. S2 is showing the SEM image of bare FTO without any coating.
The crystallinity and the active phases of the Co3O4 nano-sized electrocatalyst were investigated by powder X-ray diffraction method using Philips PANalyticalX’Pert Powder with Cu kα radiation. The Co3O4 possesses spinal order with the Fd3m space group [45]. Figure 5a shows the intense diffraction peaks for the powder Co3O4 electrocatalyst. Peak positions and relative intensities of peaks at 32.8°, 38.8°,43.1°,68.5° and 79.8°correspond to (111), (222), (400), (531) and (622) planes respectively and are exactly matching to the values from JCPDS 76–1802. Moreover, the 2θ values at 46.8°, 54.6°, 66.6°, 69.5° and 76.3° are reflected to (400), (422), (531), (533) and (440) planes respectively and are consistent and similar for Co3O4 with the standard JCPDS 01–080-1533. Some other peaks were also raised due to the presence of impurities in the electrocatalyst like the peak at 25.2° showing CoOx and is indexed to the plane (222) and similar to JCPDS 47–1738. Likewise, peak at 51.7°was related to the plane (422) and presenting Co2O4 (PDF 80–1534) in the electrocatalyst.
Raman scatterings are also used here to determine the structure of nano-sized Co3O4 electrocatalyst using Avantes Compact Raman spectrometer. Figure 5b presents the Raman spectrum in the range of 0 to 1200 cm−1. Four major bands at 197 cm−1, 469 cm−1, 508 cm−1 and 678 cm−1 communicate to four active Raman modes of the Co3O4 electrocatalyst. The intense peak at 197 cm−1 has been reported earlier for the Co3O4 system. A medium intense peak at 469 cm−1 and a less intense peak at 508 cm−1 have Eg and E2g symmetry respectively. An Intense band at 678 cm−1 is allocated to A1g variety in the octahedral spectroscopic symmetry and is accredited to the octahedral sites of Co3+ [46].
4 Electrochemical characterizations
Electrochemical characterizations of Co3O4 coated on FTO electrodes was carried using cyclic voltammetry, controlled water pyrolysis, and chronoamperometry techniques using standard three-electrode cells provided by Metrohm auto lab. Spiral shaped platinum wire was used as a counter or auxiliary electrode while Co3O4 coated on FTO was employed as a working electrode for electrochemical OER and a reference electrode in this study was standard saturated Ag/AgCl electrode dipped in 3.5 M KCl solution. All electrochemical approaches cited in this paper were commenced in a low alkaline medium using a 0.1 M KOH solution (pH = 12 ± 0.1), ammonium buffer (pH = 9.25 ± 0.1) and phosphate buffer (pH = 7.4 ± 0.1). For all electrochemical data IR compensations have been rendered using Metrohm Autolab Nova 2 software and, apart from that, can also be calculated using Eq. 2.
In Eq. (3), R is the resistance of uncompensated solution determined from impedance spectroscopy. The potentials given in all this work were converted to a standard reversible hydrogen electrode (RHE) utilizing Nernst formula [47] i.e.
Here, EAg/AgCl is working electrode potential, EoAg/AgCl is + 0.205 V for Ag/AgCl electrode in 3.5 M KCl solution [48]. The pH of 0.1 M KOH solution was measured by MColorpHast pH paper and GroLine Hydroponics pH tester and was observed 12 ± 0.1 throughout the experiment. The onset potential was calculated by extrapolating the fast rising current portion of the peak linearly to the baseline current linear extrapolation, and the intercept potential was the onset potential. A diagram presenting the calculation of onset potential is given in Fig. S11. The electrochemical overpotential was determined by subtracting the thermodynamically calculated onset potential (1.23 V) from the actual onset potential. The Tafel slope was computed by overpotential vs. logarithm of current density plot by examining the rectilinear division of linear sweep voltammetry curve using equation [49].
In Eq. (4), ŋ is vivid overpotential, b is tafel slope and α represents charge transfer coefficient. To investigate the longevity of electrocatalyst, controlled potential water electrolysis (CPE), and controlled current electrolysis (CCE) curves were presented by plotting time vs. current density and time vs. standard potential respectively. Furthermore, to examine the stability of the electrocatalyst, multi-step chronoamperometry and multistep chronopotentiometry experiments were held for an extended period and were presented by plotting time vs. current density and time vs. Potential (RHE) respectively.
5 Electrochemical water splitting
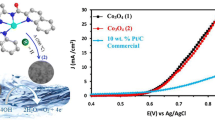
OER performance of prepared electrodes was assessed by the cyclic voltammetry technique. Figure 6 and Table S1 compare the CV curves of Co3O4-electrodeposited, and Co3O4-spray-coated FTO cycled in 0.1 M KOH alkaline electrolyte of pH = 12 ± 0.1. A pre-oxidation peak occurred before catalytic peak during forwarding sweep is attributed to Co+2/+3 oxidation [50]. Onset potential for Co3O4-electrodeposited on FTO electrode was achieved at the low potential of 1.49 V versus RHE with overpotential ŋ = 260 mV (Fig. 6a, Table S1). Amazingly, the current density of 10 mA cm−2 was secured at just 1.54 V vs. RHE with ŋ = 310 mV advocating that electro-deposited Co3O4 catalyst on FTO presented finer catalytic activity towards OER and profitable than benchmark IrOx and RuOx catalyst under similar test conditions [51,52,53]. The extreme current density of 68 mA cm−2was obtained just at 1.78 V vs. RHE with bubbles of oxygen at the electrode exterior that can be seen by the naked eye easily. Fig. S3-S4 and Table S1 compares the CV curves of electro-deposited Co3O4 on FTO in ammonium buffer and phosphate buffer and are presenting high onset potential with lifted overpotential due to reduced pH conditions. On the other hand, the spray-coated Co3O4 on FTO electrode (Fig. 6b, Table S1) exhibited less performance in OER as compared to electro-deposited Co3O4 on FTO electrode showing a decline in current initially at ≈1.40 V vs. RHE and then a sudden rise in current with final onset potential at 1.54 V vs. RHE (ŋ = 310 mV). Current density reaches maximum to 47 mA cm−2 at 1.95 V vs. RHE potential. Fig. S5 and S6 showing the CV curves for spray coated FTO at lower pH conditions.
Electrocatalysis: a Cyclic voltammetry (CV) curve for Co3O4 electrocatalyst on FTO anode prepared by electro-deposition technique in 0.1 M KOH electrolyte (pH = 12 ± 0.1) at scan rate of 5 mV s−1 .b Cyclic voltammetry (CV) curve for Co3O4 electrocatalyst on FTO anode prepared by spray coating technique in 0.1 M KOH electrolyte (pH = 12 ± 0.1) at a scan rate of 5 mV s−1 .c Concurrent 1st, 100th and 200th CV curve for electro-deosited Co3O4 electrocatalyst on FTO anode coated in 0.1 M KOH electrolyte (pH = 12 ± 0.1) at a scan rate of 10 mV s−1 showing long term stability of electrocatalyst in an alkaline environment .d Concurrent 1st, 100th and 200th CV curve for Co3O4electrocatalyst on FTO anode coated by simple spray coating strategy in 0.1 M KOH electrolyte (pH = 12 ± 0.1) at a scan rate of 10 mV s−1
From comparison of electro-deposited and spray coated Co3O4 electrocatalyst on FTO electrodes, it is evident that enhanced electrocatalytic performance is observed in case of electro-deposited electrodes which may be attributed due to the homogeneous fine layer and better catalytic layers strength.
The longevity of the catalyst has been tested via accelerated degradation through multi-scan CV at scan rate of 10 mV s−1. In this pursuit, 200 regular CV’s were plotted under identical conditions. Results show that both 100th and 200th CV scan overlap the 1st CV scan for both spray-coated Co3O4 on FTO and electro-deposited Co3O4 on FTO electrodes (Fig. 6, Table S1). For three scans shown in (Fig. 6c), the maxima current is observed at 68 mA cm−2 at the potential of just 1.78 V vs. RHE for electro-deposited Co3O4 on FTO electrode. On the other hand, the current density of 47 mA cm−2 is gained at just 1.95 V vs. RHE in three scans for spray coated Co3O4 on FTO electrode (Fig. 6d, Table S1).
As the majority of the stimulation barricade in H2O oxidation and overpotential arises from the tired dynamics of oxygen evolution reactions [54, 55]. Therefore we have also studied Tafel slope both for spray coated Co3O4 on FTO and electro-deposited Co3O4 on FTO considering the linear regime of linear sweep voltammetry curve shown in Fig. 7Fig. 7, Table S1. Tafel slope of 67 mV dec−1and 53 mV dec−1 was obtained for spray coated Co3O4 on FTO and electro-deposited Co3O4 on FTO respectively. These values are much better than 85 mV dec−1 and 141 mV dec−1 which have been reported for the state of the art IrO2 and RuO2 respectively under similar conditions [53, 56, 57]. The lower value of the Tafel slope suggests the well-balanced kinetics rate in the entire conversion chain [58]. Tafel slopes of electro-deposited and spray-coated FTO in ammonium buffer and in phosphate buffer are given in supplementary file as Fig. S7 to Fig. S10 and values are given in Table S1.
To evaluate the stability of the electrocatalyst, an extended period controlled current electrolysis (CCE) and controlled potential electrolysis (CPE) were performed under alkaline conditions. Current densities of 10 mA cm−2 and 20 mA cm−2 were chosen and employed during the experiment. Incredibly, the potential during CCE remained almost constant throughout the 45 h water oxidation test. There was an invariable potential of 1.53 V vs. RHE and 1.59 V vs. RHE for electro-deposited and spray-coated Co3O4 on FTO electrodes respectively at 10 mA cm−2 (Fig. 8a, Table S1). The CCE potential we gained in this experiment using the Co3O4 on FTO electrode was relatively low as compared to other cobalt-based electrocatalyst systems ( 1.61 mA cm−2 and 1.55 mA cm−2) [59, 60] and benchmark IrO2 based systems ( 1.6 V vs. Hg/HgO at 10 mA cm−2 and 1.55 V vs. Ag/AgCl at 10 mA cm−2) [61, 62]. Furthermore, we increased the current density to 20 mA cm−2 during the experiment and noticed an increment of almost 20 mV and 30 mV in the potential for electro-deposited and spray-coated Co3O4 on the FTO electrodes respectively. There was no remarkable degradation of electrocatalyst from the electrode surface and little observable change in the potential over the 45 h test. A polarization curve before and after 45 h CCE experiment using electro-deposited Co3O4@FTO is given in the supplementary information as Fig. S12 to further ensure the durability and long term operational ability of prepared electrocatalyst.
Electrocatalysis: a Prolonged anodic studies of water oxidation during the constant-current electrolysis (CCE) of water for long term for the Co3O4 electrocatalyst at stable current densities of J = 10 mA cm–2 and 20 mA cm–2 for Co3O4 electrocatalyst on FTO electrode coated by electrochemical means and J = 10 mA cm–2 and 20 mA cm–2 for Co3O4 electrocatalyst on FTO electrode coated by spray coating technique .b controlled potential electrolysis (CPE) for the Co3O4 coated on FTO by electro-deposited method and Co3O4 electrocatalyst coated on FTO anode by a spray coating method and CPE for bare FTO
The stability and effectiveness of the electrocatalyst can also be judged by controlled potential electrolysis (CPE) experiments in a strong alkaline electrolyte solution. Electro-deposited Co3O4 on FTO generated an elevated current density of 14.2 mAcm−2 at 1.86 V vs. RHE in the long-term CPE experiment (Fig. 8b, Table S1) which is better relative to benchmark RuO2 systems (< 10 mA cm−2) [63, 64]. At the start of the experiment, there was a small reduction in the current density that can also be attributed to the oxygen bubbles accumulated at the electrolyte interface. After that, the current density remained sustained throughout the experiment. A wealthy flow of oxygen bubbles emerging from the electrode interface through the experiment indicated the superior quality and stability of the electrocatalyst. Whereas, the CPE analysis of spray-coated Co3O4 on FTO produced an electron density of 7.9 mA cm−2 initially and current became stable after some time with a stream of oxygen bubbles from the electrode surface. The electrochemical response of electro-deposited Co3O4 on FTO in CPE and other experiments depicts the durability and extra mechanical stability of the electrode.
Furthermore, the catalytic stability and consistency in performance of the electrochemically coated Co3O4 on FTO and spray-coated Co3O4 on FTO during water electrolysis was also assessed by multistep chronoamperometry and multistep potentiometry. Figure 9a evaluates the multistep chronoamperometry graph for electro-deposited Co3O4 on FTO and broadcasting lofty current density under taper potential regime of 1.42 V vs. RHE to 1.79 V vs. RHE. It initiates from 1.42 V versus RHE by a stable current density of 2.9 mA cm−2 and persists for the coming 200 s until potential jumps to a next higher value. Similarly, in chronopotentiometry experiment, the prepared electrodes were made to work at fixed potentials for 200 s and change in J is scrutinized as an objective of the clock for both electro-deposited Co3O4 on FTO and results are shown in Fig. 9b, Table S1. Figure 9b expresses the multistep chronopotentiometry experiment in the current density range of 3 mA cm−2 to 67 mA cm−2. At 3 mA cm−2 the potential is stabilized and stays stable for the coming 200 s. Similarly, at higher densities, the same stable attitude of potential can be observed.
Electrocatalysis: a Chronoamperometry graph for electro-deposited Co3O4 on FTO anode presenting potential increment from 1.42 to 1.79 V vs. RHE as a function of current density b Multistep chronopotentiometry curve for Co3O4 electro-deposited on FTO electrode presenting current density regime from 3 to 67 mA cm−2
From these electrochemical characterization experiments, we suggest that these simply prepared electrodeposited and spray coated Co3O4 on FTO electrodes exhibit excellent mass transport efficiency, high catalytic performance, and enhanced mechanical stability.
We assume that the electro-deposited Co3O4@FTO’s remarkable behaviour could be correlated with its special electro-coating and this statement needs to be extensively explored in the immediate future with far more theoretical and experimental research.
6 Conclusion
Thin nanoscale coatings, via electro-deposition and simple but cheap spray methods, have tempted concentration due to their long term catalytic stability and durability in the long term OER catalytic systems. We have synthesized very first time a thin layer of Co3O4 electrocatalyst on FTO glass using the electro-deposition method and is compared with simple spray coating strategy. They are then further utilized for water electrolysis under alkaline conditions without pre-conditioning. Co3O4 coated FTO by electro-deposition technique proved itself very effective in water splitting cell by showing oxygen evolution onset potential at just 1.49 V vs. RHE and Tafel slope 53 mV dec−1. On the other hand, FTO coated with Co3O4 using a spray coating approach shows comparatively high onset oxygen evolution potential of 1.54 V vs. RHE and tafel slope 67 mV dec−1. There is no need to put metallic ions in the electrolyte solution with two approaches discussed for long-term stability testing. The maximum oxygen evolution current density approaches 68 mA cm−2 and 47 mA cm−2 for electro-deposited and spray-coated Co3O4 at FTO electrodes respectively. The current density of electro-deposited Co3O4 at FTO is the highest for any other cobalt-based electrocatalyst systems so far published and all electrochemical results are relatively better and contrastable to both of the costly state of the art RuO2and IrO2 research published before. Extended period controlled potential electrolysis presented brilliant current density of 14.2 mA cm−2 and 7.9mAcm−2 for electrodeposited and spray-coated Co3O4 at FTO electrodes respectively. Results given are highly spectacular for OER electrocatalysis based on metal oxide. We have made a major leap forward with designing inexpensive, fast and convenient water oxidation electrocatalysts. This investigation is expected to further enhance knowledge and skills for the preparation of strong and high activity catalytic materials for the anodic splitting of the water.
References
Bonchio M et al (2019) Hierarchical organization of perylene bisimides and polyoxometalates for photo-assisted water oxidation. Nat Chem 11(2):146–153
Joya KS, de Groot HJ (2016) Controlled surface-assembly of nanoscale leaf-type Cu-oxide electrocatalyst for high activity water oxidation. ACS Catalysis 6(3):1768–1771
Xu Y, Kraft M, Xu R (2016) Metal-free carbonaceous electrocatalysts and photocatalysts for water splitting. Chem Soc Rev 45(11):3039–3052
Joya KS et al (2013) Molecular catalytic assemblies for electrodriven water splitting. ChemPlusChem 78(1):35
Huh T, Savaskan G, Evans J (1992) Further studies of a zinc-air cell employing a packed bed anode part II: Regeneration of zinc particles and electrolyte by fluidized bed electrodeposition. J Appl Electrochem 22(10):916–921
Koper MTM (2011) Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis. J Electroanalytic Chem 660(2):254–260
Bandal HA et al (2017) Bimetallic iron cobalt oxide self-supported on Ni-Foam: An efficient bifunctional electrocatalyst for oxygen and hydrogen evolution reaction 249:253–262
Zhu J et al (2019) Back cover iridium-based cubic nanocages with 11-nm-thick walls a highly efficient and durable electrocatalyst for water oxidation in an acidic medium angew. Chem. Int. 58(22):7496–7496
Chen Y et al (2019) Exceptionally active iridium evolved from a pseudo-cubic perovskite for oxygen evolution in acid 10(1):1–10
Geiger S et al (2018) The stability number as a metric for electrocatalyst stability benchmarking 1(7):508–515
Gong M et al (2013) An advanced Ni–Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc 135(23):8452–8455
Yeo BSAT (2011) Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 133(14):5587–5593
Pintado S et al (2013) Fast and persistent electrocatalytic water oxidation by Co–Fe Prussian blue coordination polymers. J. Am. Chem. Soc 135(36):13270–13273
Louie MW (2013) An investigation of thin-film Ni–Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc 135(33):12329–12337
Tsuji E et al (2011) Electrocatalytic activity of amorphous RuO2 electrode for oxygen evolution in an aqueous solution. Electrochim Acta 56(5):2009–2016
Hu J-M, Zhang J-Q (2004) Oxygen evolution reaction on IrO2-based DSA® type electrodes: kinetics analysis of Tafel lines and EIS. Int J Hydro Ener 29(8):791–797
Sayed DM et al (2018) Activation/deactivation behavior of nano-NiOx based anodes towards the OER: influence of temperature 276:176–183
Pickrahn KL et al (2012) Active MnOx electrocatalysts prepared by atomic layer deposition for oxygen evolution and oxygen reduction reactions 2(10):1269–1277
Xu L et al (2016) Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem 128(17):5363–5367
Gao X et al (2016) Hierarchical NiCo2O4 hollow microcuboids as bifunctional electrocatalysts for overall water-splitting. Angew. Chem. Int. Ed 55(21):6290–6294
Lu Y et al (2018) Engineering oxygen vacancies into LaCoO3 perovskite for efficient electrocatalytic oxygen evolution. ACS Sustainable Chem. Eng 7(3):2906–2910
Shimizu Y et al (2000) Solid-state NOx sensor combined with NASICON and Pb–Ru-based pyrochlore-type oxide electrode. Sensors and Actuators B Chemical 65(1–3):141–143
Feng J et al (2015) Nickel-coated silicon photocathode for water splitting in alkaline electrolytes 8(5):1577–1583
Xue ZH et al (2017) Janus Co/CoP nanoparticles as efficient Mott-Schottky electrocatalysts for overall water splitting in wide pH range 7(12):1602355
Cherevko S et al (2014) Stability of nanostructured iridium oxide electrocatalysts during oxygen evolution reaction in acidic environment 48:81–85
Pawar SM et al (2017) Self-assembled two-dimensional copper oxide nanosheet bundles as an efficient oxygen evolution reaction (OER) electrocatalyst for water splitting applications. J. Mater. Chem. A. 5(25):12747–12751
Suntivich J et al (2011) A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles 334(6061):1383–1385
Kanan, M.W., D.G.J.S. Nocera, In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. 2008. 321(5892): p. 1072–1075.
Cheng H et al (2010) A facile method to improve the high rate capability of Co 3 O 4 nanowire array electrodes. Nano Res. 3(12):895–901
Kanan MW et al (2010) Structure and valency of a cobalt− phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J. Am. Chem. Soc. 132(39):13692–13701
McAlpin JG et al (2010) EPR evidence for Co (IV) species produced during water oxidation at neutral pH. J. Am. Chem. Soc. 132(20):6882–6883
Grimaud A et al (2013) Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution 4(1):1–7
Liu Z et al (2017) Promising cobalt oxide and cobalt oxide/silver photocathodes for photoelectrochemical water splitting. Sol Energy Mater Sol Cells 161:46–51
Babar N-U-A et al (2020) Spray-assembled nanoscale cobalt-oxide as highly efficient and durable bifunctional electrocatalyst for overall water splitting 17:100434
Islavath N et al (2015) Spray coated seed layer for scalable synthesis of aligned ZnO nanowire arrays on FTO substrate and their photovoltaic properties 41(3):4118–4122
Alver Ü et al (2011) Spray pyrolysis deposition of ZnO thin films on FTO coated substrates from zinc acetate and zinc chloride precursor solution at different growth temperatures 72(6):701–704
Sima M et al (2019) Graphitic carbon nitride based photoanodes prepared by spray coating method 44(45):24430–24440
Trotochaud L et al (2012) Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J Am Chem Soc 134(41):17253–17261
Sebastian M et al (2010) Synthesis, structural characterization and catalytic activity study of Mn (II), Fe (III), Ni (II), Cu (II) and Zn (II) complexes of quinoxaline-2-carboxalidine-2-amino-5-methylphenol: Crystal structure of the nickel (II) complex. Polyhedron 29(15):3014–3020
Xaba T et al (2018) synthesis and characterization of cubic structured cobalt oxide nanoparticles capped with topo through the decomposition of bis (n-cyclohexyl-1-naphthaldehydato) cobalt (ii) complex as a single source precursor. Digest journal of nanomaterials and Biostructures 13(4):1141–1147
Pal J, Chauhan P (2010) Study of physical properties of cobalt oxide (Co3O4) nanocrystals. Mater Charact 61(5):575–579
Allaedini G, Muhammad A (2013) Study of influential factors in synthesis and characterization of cobalt oxide nanoparticles. Journal of Nanostructure in Chemistry 3(1):77
Khan S et al (2011) Sci Total Envi-ron 409:2987
Riddick TM (1968) Control of colloid stability through zeta potential. Blood 10(1):52–68
Bulavchenko O et al (2009) In situ XRD study of nanocrystalline cobalt oxide reduction. Kinet Catal 50(2):192–198
Farhadi S, Javanmard M (2016) Characterization of cobalt oxide nanoparticles prepared by the thermal decomposition. Acta Chim Slov 63(2):335–343
Cai YAB (2004) The reversible hydrogen electrode: potential-dependent activation energies over platinum from quantum theory. J. Phys. Chem. B 108(28):9829–9833
Sawyer D, Sobkowiak A, Roberts J (1995) Electrochemistry for Chemists, Chaps 1–3. Wiley, New York
Saha S et al (2016) Probing synergetic effects between platinum nanoparticles deposited via atomic layer deposition and a molybdenum carbide nanotube support through surface characterization and device performance 4(23):9253–9265
Babar N-U-A et al (2020) Spray-assembled nanoscale cobalt-oxide as highly efficient and durable bifunctional electrocatalyst for overall water splitting. Materials Today Energy 17:100434
Cherevko S et al (2016) Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal Today 262:170–180
Lee Y et al (2012) Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. The journal of physical chemistry letters 3(3):399–404
Xu, Z.J., T.D. Nguyen, and G.G. Scherer, A Facile Synthesis of Size-Controllable IrO2 and RuO2 Nanoparticles for the Oxygen Evolution Reaction. 2016.
Joya KS et al (2016) Atomically monodisperse nickel nanoclusters as highly active electrocatalysts for water oxidation 8(18):9695–9703
Suryanto BH et al (2013) Controlled electrodeposition of cobalt oxides from protic ionic liquids for electrocatalytic water oxidation 3(43):20936–20942
Kuo D-Y et al (2018) Measurements of oxygen electroadsorption energies and oxygen evolution reaction on ruo2 (110): a discussion of the sabatier principle and its role in electrocatalysis. J Am Chem Soc 140(50):17597–17605
Stoerzinger KA et al (2014) Orientation-dependent oxygen evolution activities of rutile IrO2 and RuO2. The journal of physical chemistry letters 5(10):1636–1641
Jahan M, Liu Z, Loh KP (2013) A Graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for HER, OER, and ORR. Adv Func Mater 23(43):5363–5372
Xu W et al (2018) Porous cobalt oxide nanoplates enriched with oxygen vacancies for oxygen evolution reaction. Nano Energy 43:110–116
Gong L et al (2018) Enhanced catalysis of the electrochemical oxygen evolution reaction by iron (III) ions adsorbed on amorphous cobalt oxide. ACS Catalysis 8(2):807–814
Morimitsu M, Oshiumi N (2009) Accelerated oxygen evolution and suppressed MnOOH deposition on amorphous IrO2–Ta2O5 coatings. Chem Lett 38(8):822–823
Reyter D, Bélanger D, Roué L (2010) Nitrate removal by a paired electrolysis on copper and Ti/IrO2 coupled electrodes–Influence of the anode/cathode surface area ratio. Water Res 44(6):1918–1926
Cruz J et al (2011) Preparation and characterization of RuO2 catalysts for oxygen evolution in a solid polymer electrolyte. Int J Electrochem Sci 6(12):6607–6619
Sugimoto W et al (2004) Evaluation of the pseudocapacitance in RuO2 with a RuO2/GC thin film electrode. Electrochim Acta 49(2):313–320
Funding
This research received no specific grant from any funding agency in the public, commercial or non-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in preparing this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehboob, A., Gilani, S.R., Anwar, A. et al. Nanoscale cobalt-oxide electrocatalyst for efficient oxygen evolution reactions in alkaline electrolyte. J Appl Electrochem 51, 691–702 (2021). https://doi.org/10.1007/s10800-021-01529-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01529-1