Abstract
Polyaniline (PANI) salt was prepared with 3-(Cyclohexylamino)-1-propanesulfonic acid (CAPS) as a novel dopant by aqueous polymerization pathway. Effects of sodium lauryl sulfate surfactant, mineral acid (H2SO4), and a combination of surfactant with mineral acid during the polymerization reaction were also determined. PANI-CAPS showed semicrystalline with flake-like morphology. The use of the sodium lauryl sulfate along with CAPS resulted in the formation of highly crystalline nanospheres with flake-like morphology. In order to find out the effect of surfactant, sodium lauryl sulfate was used in the reaction. The combination of sodium lauryl sulfate, CAPS, and H2SO4 brings about an extended nanosphere morphology. These polyaniline salts were used as electrode materials in the supercapacitor application, in a symmetric two-electrode cell configuration. The values of specific capacitance, energy, and power densities of PANI-CAPS-DHS-H2SO4 material at 2 mA cm−2 were 495 F g−1, 90 kJ kg−1, and 120 J Kg−1 s−1, respectively. Moreover, 85 % of the original capacitance was retained after 3,000 galvanostatic charge–discharge cycles with a coulombic efficiency of 96–99 %. The value of phase angle is close to 90 at low frequencies, indicating a good capacitive behavior.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Supercapacitors, which are also termed as electrochemical capacitors or ultracapacitors, have been studied for application in digital communication devices, digital cameras, mobile phone, power supplies, and hybrid electric vehicles. Supercapacitors have higher power density and longer cycle life compared to secondary batteries and higher energy density compared to conventional electrochemical double-layer capacitors [1–3]. The performances of supercapacitors are primarily determined by the electrode materials [4]. Capacitance performance of supercapacitors depends on active electrode materials based on carbon materials, metal oxides, and conducting polymers, which are having their own advantages and disadvantages. Carbon-based materials can provide high power density and long cycle life, but its low specific capacitance limits its application for high energy density devices [5, 6]. Metal oxides/hydroxides possess pseudocapacitance in addition to double layer capacitance and have wide charge/discharge potential range, higher energy density, and better cycling stability, but they have a key weakness of poor conductivity and high cost [7, 8]. On the other hand, conducting polymers have been intensively studied as electrodes in supercapacitors due to their high electrical conductivity, electrochemical reversibility, larger pseudo-capacitance, and faster doping/dedoping rate during charge/discharge process, but they have low mechanical stability and cycle life [9]. Among the conducting polymers, polyaniline (PANI) has been regarded as one of the most promising conductive polymers due to its low cost, easy synthesis, controllable electrical conductivity, and good environmental stability [10]. We have written a chapter on “Recent advances in the approach of polyaniline as electrode for supercapacitor,” wherein we have covered the polyaniline materials for supercapacitor application [11]. Very recently, inkjet-printed polyaniline was used as electrode in supercapacitor application [12, 13].
Herein, we report (i) a facile synthesis of highly crystalline nanostructured polyaniline via an aqueous polymerization pathway using 3-(Cyclohexylamino)-1-propanesulfonic acid (CAPS) as a novel dopant; (ii) effects of surfactant and mineral acid in the preparation of polyaniline salt; and (iii) the use of these polyaniline salts as electrodes in supercapacitor cell and their performances.
2 Experimental
2.1 Instruments and characterization
Powder of polyaniline was pressed into a disk of 13-mm diameter and about 1.5-mm thickness under a pressure of 120 kg cm−2. The resistance of the pellet was measured by four-probe method using Keithley constant source (Model-6220) and nanovoltmeter (Model-2182A) (Keithley, Cleveland, Ohio, USA). Pellet density was measured from mass per unit volume of the pressed pellet. FT-IR spectra of polymer samples were registered on a FT-IR spectrometer (Thermo Nicolet Nexus 670, USA) using KBr-pressed pellet technique. X-ray diffraction profiles for polymer powders were obtained on a Siemens/D-500 X-ray diffractometer, USA, using Cu Kα radiation and the scan speed of 0.045° min−1. Morphology studies (microstructural and elemental analysis) of the polymer samples were carried out using a Hitachi S-4300 FE-SEM (Tokyo, Japan). The sample was mounted on a carbon disk with the help of double-sided adhesive tape and sputter-coated with a thin layer of gold to prevent sample-charging problems.
The electrode was made by pressing 5 mg of polyaniline sample on stainless steel mesh by the application of 100 kg cm−2 pressure. Supercapacitor cell (Swagelok-type cell) was constructed using two polyaniline electrodes in 1 M aqueous H2SO4 electrolyte solution without a reference electrode, and a cotton cloth was utilized as a separator. Cyclic voltammetry and galvanostatic charge–discharge experiments were carried on supercapacitor cell using a WonATech multichannel potentiostat/galvanostat (WMPG1000, GyeongGi-do, Korea) equipment. Cyclic voltammograms were recorded from −0.2 to 0.6 V at various sweep rates. Galvanostatic charge–discharge experiments were carried out from 0 to 0.6 V at various current densities. Electrochemical impedance spectroscopy measurements were carried out using IM6ex zahner-Elektrik (Germany) equipment in the frequency range of 40 kHz–10 mHz at various voltages using three-electrode configuration, i.e., polyaniline salt as working electrode, platinum as counter electrode, saturated calomel electrode (SCE) as reference electrode, and 1 M aqueous H2SO4 electrolyte solution. All the electrochemical measurements were performed at the ambient temperature.
2.2 Preparation of PANI-CAPS salt
Aniline (0.93 g, 0.1 M) and 3-(Cyclohexylamino)-1-propanesulfonic acid (2.21 g, 0.1 M) were dissolved in 50 ml of distilled water. To this solution, 50 mL distilled water containing ammonium persulfate (2.28 g, 0.1 M) was added as a whole. The mixture was stirred constantly for 4 h at the ambient temperature. The green precipitate was filtered and washed several times with distilled water followed by acetone. The powder sample was dried at 50 °C in oven.
2.3 Preparation of PANI-CAPS-DHS salt
In the above reaction, sodium lauryl sulfate (1 g, 0.035 M) was taken along with aniline and 3-(Cyclohexylamino)-1-propanesulfonic acid in 50 ml water, and further process was carried out by the above procedure.
2.4 Preparation of PANI-CAPS-H2SO4 salt
In the above reaction (PANI-CAPS), aqueous sulfuric acid (1 M) was taken along with aniline and 3-(Cyclohexylamino)-1-propanesulfonic acid in 50 ml water, and further process was carried out by the above procedure.
2.5 Preparation of PANI-CAPS-DHS-H2SO4 salt
In the above reaction (PANI-CAPS), sodium lauryl sulfate (1 g, 0.035 M) and aqueous sulfuric acid (1 M) were taken along with aniline and 3-(Cyclohexylamino)-1-propanesulfonic acid in 50 ml water, and further process was carried out by the above procedure.
3 Results and discussion
In this work, an organic acid, 3-(Cyclohexylamino)-1-propanesulfonic acid (CAPS), was used as a novel dopant for the preparation of polyaniline salt (PANI-CAPS) via an aqueous polymerization process by oxidizing aniline using ammonium persulfate (Scheme 1). In order to find out the effect of surfactant, sodium lauryl sulfate was used in the reaction. In the course of polymerization, sodium lauryl sulfate got converted into dodecyl hydrogen sulfate (DHS) under the acidic condition and incorporated into polyaniline system along with CAPS (PANI-CAPS-DHS). Mineral acid (H2SO4) was also tried out along with an organic acid, wherein, polyaniline got doped with both CAPS and H2SO4 (PANI-CAPS-H2SO4). The use of mixture of surfactant and mineral acid along with CAPS led to the formation of PANI-CAPS-DHS-H2SO4. The values of yield and conductivity for the polyaniline salts are included in Scheme 1.
The values of yield and conductivity of sample PANI-CAPS were 0.5 g and 0.03 S cm−1, respectively. The values of yield and conductivity increased with the use of surfactant and then increased further with the use of mineral acid and still further increased with the use of surfactant mineral acid mixture. This result indicates that the oxidizing and doping power is higher with the use of surfactant and mineral acid. However, pellet density (1.33 g cm−3) calculated from mass per unit volume of the pellet was found to be independent of the use of surfactant and mineral acid.
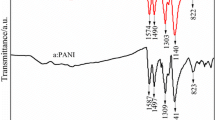
3.1 Infrared spectra of polyaniline salts
The FT-IR spectra of PANI-CAPS, PANI-CAPS-DHS, PANI-CAPS-H2SO4, and PANI-CAPS-DHS-H2SO4 are shown in Fig. 1, and their corresponding peak positions are reported in Table 1 along with the reported “conventional” polyaniline SALT and BASE [14]. IR spectra of PANI-CAPS-H2SO4, PANI-CAPS-DHS, and PANI-CAPS-DHS-H2SO4 are very nearly the same, which result is in turn similar to that of the reported PANI SALT. Moreover, IR spectrum of PANI-CAPS-DHS-H2SO4 shows two additional peaks 1,635 and 1,010 cm−1 which could be attributed to SO3H groups of H2SO4 and/or CAPS. However, IR spectrum of PANI-CAPS shows three peaks at 1,585, 1,500, and 820 cm−1 which are assigned to PANI BASE, and the remaining peaks are assigned to PANI SALT. A peak at 1,040 cm−1 originates from SO3H group of CAPS. The IR spectrum of PANI-CAPS signposts that the doping efficiency is less with the use of only CAPS as dopant.
3.2 XRD patterns of polyaniline salts
The X-ray diffraction profile registered for PANI-CAPS-H2SO4 (Fig. 2c) shows four clear peaks around 2θ = 15, 20, 25, and 27° which corresponds to semicrystalline Polyaniline [15]. However, the X-ray diffraction pattern of PANI-CAPS (Fig. 2a) shows only two peaks at 25 and 20°, which indicates less crystallinity than that of PANI-CAPS-H2SO4. The X-ray diffraction pattern of PANI-CAPS-DHS-H2SO4 (Fig. 2d) shows five peaks at 6.4, 15, 20, 25, and 27°; the last four peaks indicate the semicrystalline nature of polyaniline, and the first peak at 6.4° can be assigned to the long-range ordering of polyaniline chains via the doping of the surfactant molecules [16]. The X-ray diffraction pattern of PANI-CAPS-DHS (Fig. 2b) displays many peaks around 2θ = 14–44°; the main peaks at 17, 20, 22–24, and 29° are due to polyaniline, and the remaining higher angle peaks are owing to the aromatic chain–chain interaction in the polyaniline chain.
3.3 FE-SEM images of polyaniline salts
FE-SEM images of PANI-CAPS samples were recorded at 20 kV × 80 k × 500-nm resolution and are shown in Fig. 3. In the oxidative polymerization of aniline to polyaniline salt, the use of a weak acid (CAPS) results in the formation of flake-like morphology (Fig. 3a); the use of sodium lauryl sulfate surfactant along with CAPS results in nanospheres with flakes (Fig. 3b); strong mineral acid and CAPS upshot the formation of flakes (Fig. 3c); and the combined use of sodium lauryl sulfate, mineral acid and CAPS lead to the formation of nanospheres (Fig. 3d). These results indicate that surfactant induces the formation of nanospheres in the aniline polymerization.
3.4 Charge–discharge study of polyaniline salts
CD experiments were performed for the PANI-CAPS samples in cell configuration at 1 mA cm−2, and the values of specific capacitance (CD-C s), energy (E d), and power densities (P d ) by considering the weight of one electrode were calculated. The values are reported in Table 2. The result shows that the values of specific capacitance and energy densities increase with the increasing value of conductivity of PANI-CAPS salts. In order to find out the stability of the electrode, galvanostatic CD measurements were carried out for PANI-CAPS-DHS-H2SO4 from lower to higher scan rates (Fig. 4). Ragone plot of energy density versus power density is shown as an inset in Fig. 4.
The CD-Cs values calculated from the CD tests with respect to the mass of one electrode are 530, 495, 515, 400, and 365 F g−1 at current densities of 1, 2, 3, 5, and 10 mA cm−2, respectively. Capacitance retention over prolonged charge–discharge cycles is essential for practical supercapacitor materials. Hence, CD experiments were performed up to 3,000 cycles at 2 mA cm−2 for PANI-CAPS-DHS-H2SO4 sample. CD-Cs, coulombic efficiency (CE), and equivalent series resistance (ESR) with cycles are shown in Fig. 5. The retention in the value of specific capacitance at 3,000 cycles is 85 % with its initial capacitance value of 495 F g−1. CE values are almost constant with cycle numbers (96–99 %). ESR value increases from 4 to 40 Ω at the end of 3,000 cycles.
3.5 Electrochemical impedance spectroscopy study of polyaniline salts
EIS is an important analytical technique used to gain information about the characteristic frequency responses of supercapacitors and the capacitive phenomena occurring at the electrodes. EIS experiments were carried out for the PANI-CAPS systems in the frequency range from 40 kHz to 10 mHz at an applied voltage of 0.7 V (Fig. 6), and the EIS parameters are reported in Table 3. Solution resistance of the four samples is nearly the same (0.5–0.7 Ω), indicating the good conductivity of the electrolyte and very low internal resistance of the electrode. The time constant value is in the range of 0.1–0.4 ms.
The value of charge-transfer resistance is the main part of the resistance of the supercapacitor. If the materials have low charge-transfer resistance, then it has high electrical conductivity and fast response ability of the electrode. The value of charge-transfer resistance is in the range of 0.4–1.1 Ω, which indicates high electrical conductivity and the fast response ability of the electrode. The value of capacitance obtained at 10-mHz frequency for an applied voltage of 0.7 V follows the order: PANI-CAPS-DHS-H2SO4 > PANI-CAPS-H2SO4 > PANI-CAPS-DHS > PANI-CAPS (Table 3). Bode plots of frequency versus phase angle for PANI-CAPS samples carried out at 0.7 V applied voltage are shown in Fig. 7. The values of phase angles at 10 mHz obtained for PANI-CAPS, PANI-CAPS-DHS, PANI-CAPS-H2SO4, and PANI-CAPS-DHS-H2SO4 are 82, 85, 72, and 78, respectively. Ideal supercapacitor gives a phase angle value of 90, and its value less than 90 shows deviation from the ideal capacitor behavior. The value of phase angle is close to 90 at low frequencies, indicating a good capacitive behavior. Low values of phase angle obtained in the case of PANI-CAPS-H2SO4 and PANI-CAPS-DHS-H2SO4 salts indicate that the use of mineral acid decreases the capacitive behavior.
4 Conclusions
Polyaniline salts containing organic acid (PANI-CAPS), organic acid-surfactant (PANI-CAPS-DHS), organic and mineral acids (PANI-CAPS-H2SO4), or organic and mineral acids and surfactant (PANI-CAPS-DHS-H2SO4) were prepared. These polyaniline salts were explored as electrode material for electrochemical supercapacitor. Among the PANI salts, PANI-CAPS-DHS-H2SO4 material showed higher capacitance (530 F g−1 at 1 mA cm−2 current density). Highly crystalline form with nanosphere morphology was obtained for PANI-CAPS-DHS salt.
References
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854. doi:10.1038/nmat2297
Conway BE (1999) Electrochemical supercapacitors: scientific fundamentals and technological applications. Kluwer-Plenum, New York
Miller JR, Simon P (2008) Electrochemical capacitors for energy management. Science 321:651–652. doi:10.1126/science.1158736
Zheng JP, Cygan PJ, Jow TR (1995) Hydrous ruthenium oxide as an electrode material for electrochemical capacitors. J Electrochem Soc 142:2699–2703. doi:10.1149/1.2050077
Frackowiak E, Be´guin F (2001) Carbon materials for the electrochemical storage of energy in capacitors. Carbon 39:937–950. doi:10.1016/S0008-6223(00)00183-4
Zhang LL, Zhao XS (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 38:2520–2521. doi:10.1039/B813846J
Lokhande CD, Dubal DP, Joo OS (2011) Metal oxide thin film based supercapacitors. Curr Appl Phys 11:255–270. doi:10.1016/j.cap.2010.12.001
Deng W, Ji X, Chen Q, Banks CE (2011) Electrochemical capacitors utilising transition metal oxides: an update of recent developments. RSC Adv 1:1171–1178. doi:10.1039/C1RA00664A
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources 196:1–12. doi:10.1016/j.jpowsour.2010.06.084
Bhadra S, Khastgir D, Singha NK, Lee JH (2009) Progress in preparation, processing and applications of Polyaniline. Prog Polym Sci 34:783–810. doi:10.1016/j.progpolymsci.2009.04.003
Sydulu S, Palaniappan S (2013) Recent advances in the approach of polyaniline as electrode for supercapacitor. Nova, New York
Chiolerio A, Bocchini S, Porro S (2014) Inkjet printed negative supercapacitors: Synthesis of -polyaniline-based inks, doping agent effect, and advanced electronic devices applications. Adv Funct Mater 24:3375–3383. doi:10.1002/adfm.201303371
Bocchini S, Chiolerio A, Porro S, Accardo D, Garino N, Bejtka K, Perrone D, Pirri CF (2013) Synthesis of polyaniline-based inks, doping thereof and test device printing towards electronic applications. J Mater Chem C 1:5101–5109. doi:10.1039/c3tc30764f
Rajender B, Palaniappan S (2014) Emeraldine base form of polyaniline nanofibers as new, economical, green and efficient catalyst for synthesis of Z-aldoximes. J Catalysts 2014:1–6. doi:10.1155/2014/515428
Gupta B, Prakash R (2009) Synthesis of processible doped polyaniline-polyacrylic acid composites. J Appl Polym Sci 114:874–882. doi:10.1002/app.3055410.1155/2014/515428
Jinish Antony M, Jayakannan M (2010) Molecular template approach for evolution of conducting polymer nanostructures: Tracing the role of morphology on conductivity and solid state ordering. J Phys Chem B 114:1314–1324. doi:10.1021/jp910636s
Acknowledgments
The authors thank the Department of Science and Technology, New Delhi, India for funding under the project DST/TSG/PT/2011/179-G. The authors thank Dr. Lakshmi Kantam, Director, CSIR-IICT for her support and encouragement. The authors also thank Dr. Vijayamohanan K. Pillai, Director, CSIR—CECRI, Karaikudi for his valuable suggestion. Authors BR and BR are thankful to UGC, India for providing research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bolagam, R., Boddula, R. & Srinivasan, P. Synthesis of highly crystalline polyaniline with the use of (Cyclohexylamino)-1-propanesulfonic acid for supercapacitor. J Appl Electrochem 45, 51–56 (2015). https://doi.org/10.1007/s10800-014-0753-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-014-0753-4