Abstract
Background
To investigate the long-term effectiveness and safety of XEN45 implant, either alone or in combination with phacoemulsification, in eyes with open-angle glaucoma (OAG).
Methods
Retrospective and single center study conducted on consecutive OAG patients who underwent a XEN45 implant between February-2017 and December-2021. The primary endpoint was the mean intraocular pressure (IOP) lowering from preoperative values. Surgical success was defined as an IOP-lowering from preoperative values ≥ 20% and an IOP absolute value between 6 and 13 mm Hg, without (Complete-success) or with (Qualified-success) antiglaucoma medications.
Results
A total of 158 eyes (34 (21.5%) eyes XEN-solo and 124 (78.5%) XEN + Phaco) were included. The median follow-up time was 28.5 months. In the overall study population, the mean preoperative IOP was significantly lowered from 19.4 ± 6.5 mm Hg to 12.4 ± 5.0 mm Hg. The mean preoperative (95% confidence interval) IOP was significantly lowered from 21.3 (19.3–23.2) mm Hg and 18.8 (17.7–20.0) mm Hg to 12.0 (10.4–13.6) mm Hg and 12.5 (11.6–13.5) mm Hg in the XEN-Solo and XEN + Phaco groups, respectively (p < 0.0001 each, respectively). The mean number of ocular-hypotensive medications was significantly reduced in the overall study sample (from 3.4 ± 0.9 to 0.9 ± 1.3, p < 0.0001), XEN-Solo (from 3.5 ± 1.1 to 0.6 ± 1.0, p < 0.0001, and XEN + Phaco (from 3.4 ± 1.1 to 0.9 ± 1.3, p < 0.0001) groups. Eighty-four (53.2%) eyes were categorized as success, with 49 (58.3%) classified as complete success. Eighty-one (51.3%) eyes underwent needling and 15 (9.5%) eyes required an additional surgical procedure. One (0.6%) eye had endophthalmitis.
Conclusion
XEN implant, either alone or in combination with phacoemulsification significantly lowered IOP and reduced the need of ocular-hypotensive medication, while maintaining a good safety profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past two decades, glaucoma surgery has experienced significant advances. Among them, minimally or microinvasive glaucoma surgery (MIGS) devices have been developed as safer and less traumatic means of lowering intraocular pressure (IOP) in patients with glaucoma [1].
The definition of the term MIGS has been evolving since its inception [2, 3], and the generally accepted definition of MIGS has been changing over the years [4].
The XEN45 gel stent may be defined as a minimally invasive or micro-incisional filtration surgery (MIFS) device that allows flow of aqueous humor from the anterior chamber to the subconjunctival space [2, 3, 5, 6].
Although many studies have been published evaluating the effectiveness and safety of XEN45 implant [7,8,9,10,11,12,13,14,15,16,17,18,19,20], data evaluating its long-term clinical outcomes are very limited [11, 17, 21,22,23,24].
The main purpose of the current study was to evaluate the long-term effectiveness and safety of XEN45 implant, either alone or in combination with cataract surgery, in patients with open-angle glaucoma (OAG).
Methods
Design
Retrospective and single center study conducted on consecutive OAG patients who underwent a XEN45 implant, either alone or in combination with cataract surgery, between February 2017 and December 2021.
The study adhered to the tenets of the Declaration of Helsinki and the Good Clinical Practice/International Council for Harmonization Guidelines.
Any information that could lead to an individual being identified has been encrypted or removed, as appropriate, to guarantee their anonymity.
Study participants
Patients aged ≥ 18 years old with insufficiently medically controlled early to advanced OAG, according to Hodapp et al. [25]; intolerance to topical hypotensive treatments; or poor treatment adherence, who underwent a XEN45 implant procedure, either alone or in combination with cataract surgery, were included in the study.
Patients with any form of glaucoma other than OAG (either primary or secondary); severe conjunctival problems; phacodonesis; progressive retinal or optic nerve disease of any cause; or history of major ocular surgery (except phacoemulsification) within the previous 6 months were excluded of the study.
Subjects with more than 8% of missing data were also excluded.
Surgical technique
All the surgical procedures were performed, under topical anesthesia.
All surgeries were performed with mitomycin-C (MMC) (dose 0.1 mg/ml), which was injected intra-tenon in the supero-nasal quadrant.
The device was placed in the superior nasal quadrant using a standard ab interno technique [12].
Study groups
The study sample was divided in two groups: XEN, eyes who underwent XEN implant alone; XEN + Phaco, eyes who underwent XEN gel stent implantation combined with phacoemulsification surgery.
Definitions
Surgical success was defined as an IOP lowering from preoperative values ≥ 20% and an IOP absolute value between 6 and 13 mm Hg, without (Complete success) or with (Qualified success) antiglaucoma medications.
Failure was defined as an IOP > 13 mm Hg or < 20% IOP reduction from preoperative values at the end of the follow-up period, need for additional glaucoma surgery, or those who needed more ocular hypotensive medications than preoperatively. Patients with an IOP < 6 mm Hg for more than two consecutive visits were also considered a failure.
Needling or surgical bleb revision, as needed, was indicated in those cases of failure of the procedure due to fibrosis or encapsulation of the bleb that did not respond to massage in the slit lamp and topical hypotensive medications.
Outcomes
The primary endpoint was the mean IOP lowering from preoperative values.
Secondary endpoints included the mean IOP at month-12, month-24, month-36, and last follow-up visit; reduction in number of ocular hypotensive medications from baseline; proportion of eyes achieving a final IOP ≤ 12 mm Hg, ≤ 14 mm Hg, ≤ 16 mm Hg, ≤ 18 mm Hg, or ≤ 20 mm Hg with and without medications irrespective of the preoperative IOP lowering; proportion of eyes achieving an IOP reduction ≥ 20% and ≥ 30% at month-12, month-24, month-36, and last follow-up visit; and incidence of adverse events.
Statistical analysis
Statistical analysis was performed with the MedCalc® Statistical Software version 20.216 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2023).
Mean and standard deviation (SD); mean and 95% confidence interval (95% CI); median and interquartile range (IqR), and number (percentage) were used as appropriated.
Data were tested for normal distribution using the Shapiro–Wilk test.
The last-observation-carried-forward method was used to impute missing data.
A repeated measures ANOVA or a Friedman’s two-way analysis test, as appropriate, were used to assess the changes in IOP and in number of antiglaucoma medications. Post hoc analysis for pairwise comparisons were done with the Scheffe’s method (ANOVA) or the Conover method (Friedman).
Repeated analysis of covariance (MANCOVA) was performed to assess the changes in IOP between study groups. The model included “type of surgery” (XEN alone or combined surgery) as a factor and age, preoperative IOP, number of preoperative ocular hypotensive medications, and diagnosis as covariates.
The Mann–Whitney U test was used for testing preoperative differences between study groups.
Success survival rates were plotted for XEN solo and XEN + Phaco groups using Kaplan–Meier analysis and were compared using a log-rank test.
Categorical variables were compared using a Chi-square test and a Fisher’s exact test, as needed. P value of less than 0.05 was considered significant.
Results
Among the 184 eyes who underwent a XEN45 implant during the recruitment period, 158 (131 patients) fulfilled the demands of the inclusion and exclusion criteria and were included in the analysis.
Preoperative demographic and clinical characteristics
The Table 1 shows the main demographic and clinical characteristics of the study sample.
In the overall study sample, the mean age was 69.8 ± 8.7 years, with no significant differences between study groups (p = 0.7109). Seventy-seven (48.7%) were women; 154 (100%) were Caucasian; and 131 (82.9%) eyes were diagnosed with primary-OAG (POAG).
The mean preoperative IOP was significantly higher in the XEN-solo (21.3 ± 5.6
mm Hg) than in the XEN + Phaco (18.8 ± 6.6 mm Hg) group (Hodges–Lehmann median differenced: 3.0 mm Hg; 95% CI 1.0 mm Hg to 5.0 mm Hg, p = 0.0137).
Except for the IOP, there were no significant differences in any of the preoperative variables between XEN-alone and XEN + Phaco groups.
Intraocular pressure
The median follow-up time was 28.5 (IqR: 12.0–36.0) months; with 92 (59.7%) eyes with a minimum follow-up of 24 months and 56 (36.4%) eyes with a minimum follow-up of 36 months.
In the overall study population, the mean preoperative IOP was significantly lowered from 19.4 ± 6.5 mm Hg to 12.4 ± 5.0 mm Hg (mean difference: − 6.9 mm Hg; 95% CI − 8.1 mmHg to − 5.8 mm Hg, p < 0.0001. Repeated ANOVA) (Fig. 1).
Mean intraocular pressure (IOP) over the course of follow-up in the overall study sample. The vertical bars represent the 95% confidence interval. *p < 0.0001 as compared to baseline (repeated measures ANOVA and the Greenhouse–Geisser correction). IOP: Intraocular pressure; Preop: Preoperative; D: Day; W: Week; M: Month; NA: Not applicable
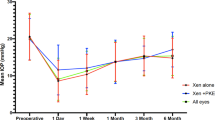
The mean preoperative (95% CI) IOP was significantly lowered from 21.3 (19.3–23.2) mm Hg and 18.8 (17.7–20.0) mm Hg to 12.0 (10.4–13.6) mm Hg and 12.5 (11.6–13.5) mm Hg in the XEN-alone and XEN + Phaco groups, respectively (p < 0.0001 each, Repeated ANOVA). No significant differences were observed at any of the time-points measured between the two study groups (Fig. 2).
Overview of the mean intraocular pressure (IOP) throughout the study in the overall, XEN-solo, and XEN + Phaco study population. The vertical bars represent the 95% confidence interval. No significant differences were observed at any of the time-points measured between the two study groups.*p < 0.0001 as compared to preoperative values (repeated measures ANOVA and the Greenhouse–Geisser correction). IOP: Intraocular pressure
Regarding IOP, no significant differences were observed between the XEN-Solo and the XEN + Phaco groups, apart from the preoperative IOP (which was significantly greater in the XEN-Solo group, p = 0.0080) and the IOP at Day-1 IOP (which was significantly lower in the XEN solo group, p = 0.0111) (Fig. S1).
At the last follow-up visit the mean unadjusted IOP lowering, in terms of percentage, from preoperative values was 30.0 ± 34.2%; 39.3 ± 30.0%; and 27.4 ± 35.0 in the overall, XEN-solo, and XEN + Phaco samples, respectively.
The Fig. 3 shows the proportion of eyes who achieved ≥ 20% (Fig. 3A) and ≥ 30% (Fig. 3B) IOP lowering from preoperative values at month-12, month-24, month-36, and last follow-up visit. In the overall study sample, the proportion of eyes achieving ≥ 20% and ≥ 30% IOP lowering from preoperative values was 80.4% and 67.1% at month-36, respectively.
After adjusting for age, preoperative IOP, number of preoperative ocular hypotensive medications, and diagnosis (POAG versus secondary OAG) mean IOP lowering were similar at any of the different time-point measured in both groups, except for the Day-1 and Week-1 IOP lowering that were significantly greater in the XEN-Solo group (p = 0.0003 and p = 0.0030, respectively) (Table 2).
As topical therapy is burdensome in many patients and represents an issue due to poor adherence or ocular surface toxicity, several patients underwent xen implantation to reduce or avoid drops. Preoperatively, 48 (30.4%) eyes had an IOP ≤ 15 mmHg, 6 (12.5%) eyes in the XEN-solo group and 42 (87.5%) eyes in the XEN + Phaco group. In these eyes, IOP did not significantly change from preoperative values (12.6 ± 1.7 mmHg) to the last follow-up visit (11.9 ± 5.3 mm Hg) (mean difference: − 0.7 ± 5.3 mmHg; 95% CI − 2.3 to 0.8 mm Hg; p = 0.3492) (Fig. S2A).
Success
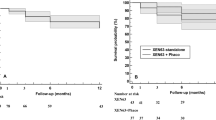
In the overall study sample, 84 (53.2%) eyes were categorized as success (IOP ≤ 13 mm Hg and IOP lowering ≥ 20% from preoperative values), and of those, 49 (58.3%) were classified as complete success. Regarding the eyes with a minimum follow-up of 36 months, 38 (67.9%) eyes were categorized as success (Fig. 4A), with 23 (41.1%) eyes classified as complete success. The Table 3 shows the proportion of eyes who achieved different IOP targets irrespective of the percentual reduction from baseline.
Kaplan–Meier survival curves for failure. A In the overall study sample. Failure occurred in 74 (46.8%) eyes. B In eyes treated with XEN-Solo (solid line) and combined surgery XEN + phacoemulsification (XEN + PHACO) (dotted line). Failure occurred in 13 (38.9%) XEN-Solo-treated eyes and 61 (49.2%) XEN + Phaco-treated eyes. Mean hazard ratio: 0.79, 95% confidence interval: 0.43–1.45; p = 0.4422
Kaplan–Meier survival analysis did not find any difference in the success rate between XEN-Solo and XEN + Phaco groups (mean hazard ratio: 0.79; 95% confidence interval 0.43–1.45; p = 0.4422). (Fig. 4B).
Ocular hypotensive medication
The mean number of ocular hypotensive medications was significantly reduced in the overall study sample (from 3.4 ± 0.9 to 0.9 ± 1.3, p < 0.0001), in the XEN-Solo (from 3.5 ± 1.1 to 0.6 ± 1.0, p < 0.0001, and in the XEN + Phaco (from 3.4 ± 1.1 to 0.9 ± 1.3, p < 0.0001) groups (Fig. 5).
There were no significant differences in the mean ocular hypotensive treatment reduction between XEN-Solo and the XEN + Phaco groups (mean difference: 0.4 medications; 95% CI −0.1 to 1.0; p = 0.1265).
In the eyes with a preoperative IOP ≤ 15 mmHg, the preoperative number of antiglaucoma medications was significantly reduced from 3.3 ± 1.0 to 0.9 ± 1.2 (mean difference: −2.4 ± 1.3; 95% CI −2.8 to −2.0; p < 0.0001) (Fig. S2B).
Safety
Among the total study sample, 81 (51.3%) eyes underwent needling, with 21 (13.3%) eyes undergoing an additional needling procedure, and 5 (3.2%) eyes requiring a third needling procedure. One (0.6) eye underwent a bleb revision after a failed needling procedure. Detailed information about the number of eyes who underwent needling in the XEN-Solo and XEN + Phaco groups is shown in fig. S3.
Fifteen (9.5%) eyes required an additional surgical procedure. MicroShunt implant was performed in 5 (3.2%) eyes (month-1 [1 eye], month-9 [1 eye], and month-27 [3 eyes]); Non-penetrating deep sclerectomy was performed in 5 (3.2%) eyes at month-6 (2 eyes), month-9, month-27, and month-48; Glaucoma drainage implant was performed in 2 (1.3%) eyes at month-15 and month-39; One (0.6%) underwent trabeculectomy at month-39; one (0.6%) eye underwent an Express(R) implant at month-15; and 0ne (0.6%) eye underwent a bleb revision (Table S1).
Sixty (38.0%) eyes reported any adverse event (AE), 11 (18.3%) in the XEN-solo group and 49 (81.7%) in the XEN + Phaco one (Table 4). The most frequently reported AE was hyphema (15/60), followed by blood traces in the anterior chamber (13/60); and Xen-iris contact (6/60). One (0.6%) eye had endophthalmitis at month 44, which required its enucleation.
Discussion
According to the results of the current study, XEN45 stent, either alone or in combination with phacoemulsification, significantly lowered the IOP and reduce the number of ocular-hypotensive medications in patients with OAG in a real clinical setting.
In addition, considering the primary endpoint, there was a significant IOP lowering at month-36 as compared to preoperative values. There were no differences in IOP at month 36 (p = 0.0978) or in the adjusted IOP lowering (p = 0.5581) between XEN-solo and XEN + Phaco groups. Furthermore, the number of ocular hypotensive drugs was significantly reduced, without differences between study groups.
Among the 158 eyes included in the study, 84 (53.2%) eyes were categorized as success (IOP ≤ 13 mm Hg and IOP lowering ≥ 20% from preoperative values), of those, 49 (58.3%) were classified as a complete success. Finally, it should be highlighted the high proportion of patients achieving low target IOPs, with 128 (81.0%%) eyes achieving an IOP ≤ 14 mm Hg and 102 (64.6%) eyes an IOP ≤ 12 mm Hg.
From a clinical point of view, few studies have reported the long-term efficacy, in terms of IOP-lowering and the amount of ocular-hypotensive medications reduction, and safety of XEN45 implant, either alone or in combination with phacoemulsification surgery, in OAG patients [11, 17, 21,22,23] (Table 5). Although the results of our study did not significantly differ in term of IOP lowering (%) from the currently available scientific evidence [11, 17, 21,22,23], it should be mentioned that the reduction of the number of ocular hypotensive medications seemed to be slightly greater in our study. Interestingly, it is worth mentioning that the preoperative IOP in our study are, in general terms, slightly lower than those reported by other studies.
Furthermore, mean IOP at month-36 have been ≤ 12 mm Hg, not only in the general population, but also in the group of eyes that underwent the implant alone and those that underwent combined surgery.
Although the success rates observed in our study may not seem particularly high, it is important to note that in this study success criteria have been very strict; therefore, it is difficult to compare them with those of other authors. For example, Lenzhofer et al. [11] defined surgical success as a postoperative IOP ≤ 18 mm Hg and ≥ 20% IOP reduction compared to baseline. According to the Lenzhofer et al. [11] criteria, the complete success rate of our study would be 46.4% (26/56 eyes).
Considering success criteria used by Gillmann et al. [17] [an IOP ≤ 15 mm Hg and a relative IOP reduction ≥ 20%], complete success was achieved by 26 (46.4%) eyes (as compared to 31.5% reported by Gillman et al.).
Since the publication of the results of the Advanced Glaucoma Intervention Study [26], the close relationship between a low IOP and the reduction of glaucomatous damage progression became clear. We must, therefore, be increasingly demanding with our success criteria and look for IOPs in the range of 12–13 mm Hg.
Regarding the effectiveness of the XEN-Solo versus XEN in combination with cataract surgery, after adjusting by different covariates (namely, age, preoperative IOP and number of ocular hypotensive medications, and diagnosis) there were no significant differences between groups with the exception of IOP lowering at Day-1 and Week-1, which were significantly greater in the XEN-Solo group (Fig. S1). This greater IOP lowering at postoperative day-1 and week-1 might be due either to the fact that the eyes who underwent XEN alone had a higher preoperative IOP, or to the fact that some viscoelastic could remain after the combined surgery.
These findings are in line with those reported by a meta-analysis published recently, which found a statistically significant difference in IOP reduction favoring Standalone XEN45 at post-operative day 1, week 1, months 1 [27]. Similarly, the results of other meta-analysis found that XEN-solo was more effective (in term of IOP lowering) than XEN + Phaco within 1 week after surgical procedures, although that difference was not observed beyond that [28]. However, Chen et al. [29], in a systematic-review and meta-analysis reported that both XEN-solo and XEN + Phaco significantly lowered the IOP, without significant differences between them.
An interesting aspect of our study to take into account is the fact that 63 (39.9%) eyes underwent surgery with a preoperative IOP ≤ 16 mm Hg, with 23 (14.6%) eyes with a preoperative IOP ≤ 12 mm Hg (Table S2).
Although it was not originally planned, we conducted a sub analysis of those eyes who underwent surgery with a preoperative IOP ≤ 15 mm Hg. In this group, there was a significant reduction in the number of ocular hypotensive drugs (mean reduction: 2.4 ± 1.3; p < 0.0001). However, the mean IOP was not significantly lowered from preoperative values (mean difference: −0.7 mm Hg; p = 0.3492).
These findings confirm that, in many patients, the device was implanted with the main purpose of reducing the number of ocular hypotensive medications rather than lowering IOP. Furthermore, in the combined surgery group, many eyes required only cataract surgery, but XEN was implanted for reducing the number of ocular hypotensive medications.
Although traditional glaucoma filtering surgery and drainage devices are very effective for lowering IOP and reducing the number antiglaucoma medications, they may be associated with severe complications that may lead to visual impairment [30, 31], which might make the surgeon much more reluctant when indicating surgery.
On the contrary, as the surgeons have been gaining experience with the device and knowing its effectiveness and safety profile, has allowed a greater number of patients to benefit from it.
As regards to the safety profile, the incidence and type of complications did not significantly differ from current evidence [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. The most frequently reported AE was hyphema (25.0%), followed by blood traces in the anterior chamber (21.7%); and iris contact (10.0%). Most of these adverse events were limited in time and resolved satisfactorily without treatment. Regarding serious adverse events, it should be noted that one eye had endophthalmitis at month 44 after surgery, which required its evisceration.
In our study, throughout the follow-up period, 15 (9.5%) eyes required an additional surgical procedure. The rate of needling was 51.3% (95% CI 40.7–63.7%), with 21 (13.3%; 95% CI 8.2–20.3%) eyes undergoing an additional needling procedure.
The main limitation of the current study is its retrospective design. Selection bias and potential confounders are inherent to retrospective studies. Nevertheless, the selection of strict inclusion / exclusion criteria, as well as the inclusion of a large number of eyes, may minimized these issues.
Conclusions
The results of this study showed that XEN implant, either alone or in combination with cataract surgery is an effective treatment for lowering IOP and reducing the need of ocular hypotensive medication, while maintaining a good safety profile.
Additionally, except for the day-1 and week-1, our study did not find significant differences in IOP lowering at any of the different time-point measured between XEN-solo and XEN + Phaco.
Data availability
Data not here published are obtainable on reasonable request from the corresponding author.
References
Bar-David L, Blumenthal EZ (2018) Evolution of glaucoma surgery in the last 25 years. Rambam Maimonides Med J 9(3):e0024. https://doi.org/10.5041/RMMJ.10345
Saheb H, Ahmed II (2012) Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol 23(2):96–104. https://doi.org/10.1097/ICU.0b013e32834ff1e7
Ahmed II (2015) MIGS and the FDA: What’s in a name? Ophthalmol 122(9):1737–1739. https://doi.org/10.1016/j.ophtha.2015.06.022
Caprioli J, Kim JH, Friedman DS et al (2015) Special commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery: summary of a joint meeting of the american glaucoma society and the food and drug administration. Ophthalmol 122(9):1795–1801. https://doi.org/10.1016/j.ophtha.2015.02.029
Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM (2017) Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS ONE 12(8):e0183142. https://doi.org/10.1371/journal.pone.0183142
Ansari E (2017) An update on implants for minimally invasive glaucoma surgery (MIGS). Ophthalmol Ther 6(2):233–241. https://doi.org/10.1007/s40123-017-0098-2
Hengerer FH, Kohnen T, Mueller M, Conrad-Hengerer I (2017) Ab Interno gel implant for the treatment of glaucoma patients with or without prior glaucoma surgery: 1-year results. J Glaucoma 26(12):1130–1136. https://doi.org/10.1097/IJG.0000000000000803
Mansouri K, Guidotti J, Rao HL, Ouabas A, D’Alessandro E, Roy S, Mermoud A (2018) Prospective evaluation of standalone XEN gel implant and combined phacoemulsification-XEN gel implant surgery: 1-year results. J Glaucoma 27(2):140–147. https://doi.org/10.1097/IJG.0000000000000858
Reitsamer H, Sng C, Vera V, Lenzhofer M, Barton K, Stalmans I, Apex Study Group (2019) Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 257(5):983–996. https://doi.org/10.1007/s00417-019-04251-z
Karimi A, Lindfield D, Turnbull A et al (2019) A multi-centre interventional case series of 259 ab-interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye (Lond) 33(3):469–477. https://doi.org/10.1038/s41433-018-0243-8
Lenzhofer M, Kersten-Gomez I, Sheybani A et al (2019) Four-year results of a minimally invasive transscleral glaucoma gel stent implantation in a prospective multi-centre study. Clin Exp Ophthalmol 47(5):581–587. https://doi.org/10.1111/ceo.13463
Ibáñez-Muñoz A, Soto-Biforcos VS, Rodríguez-Vicente L et al (2020) XEN implant in primary and secondary open-angle glaucoma: A12-month retrospective study. Eur J Ophthalmol 30(5):1034–1041. https://doi.org/10.1177/1120672119845226
Fea AM, Bron AM, Economou MA, Laffi G, Martini E, Figus M, Oddone F (2020) European study of the efficacy of a cross-linked gel stent for the treatment of glaucoma. J Cataract Refract Surg 46(3):441–450. https://doi.org/10.1097/j.jcrs.0000000000000065
Wagner FM, Schuster AK, Emmerich J, Chronopoulos P, Hoffmann EM (2020) Efficacy and safety of XEN®-implantation vs trabeculectomy: data of a “real-world” setting. PLoS ONE 15(4):e0231614. https://doi.org/10.1371/journal.pone.0231614
Gabbay IE, Allen F, Morley C, Pearsall T, Bowes OM, Ruben S (2020) Efficacy and safety data for the XEN45 implant at 2 years: a retrospective analysis. Br J Ophthalmol 104(8):1125–1130. https://doi.org/10.1136/bjophthalmol-2019-313870
Theilig T, Rehak M, Busch C, Bormann C, Schargus M, Unterlauft JD (2020) Comparing the efficacy of trabeculectomy and XEN gel microstent implantation for the treatment of primary open angle glaucoma: a retrospective monocentric comparative cohort study. Sci Rep 10(1):19337. https://doi.org/10.1038/s41598-020-76551-y
Gillmann K, Bravetti GE, Rao HL, Mermoud A, Mansouri K (2021) Combined and stand-alone XEN 45 gel stent implantation: 3-year outcomes and success predictors. Acta Ophthalmol 99(4):e531–e539. https://doi.org/10.1111/aos.14605
Wanichwecharungruang B, Ratprasatporn N (2021) 24-month outcomes of XEN45 gel implant versus trabeculectomy in primary glaucoma. PLoS ONE 16(8):e0256362. https://doi.org/10.1371/journal.pone.0256362
Subaşı S, Yüksel N, Özer F, Yılmaz Tugan B, Pirhan D (2021) A retrospective analysis of safety and efficacy of XEN 45 microstent combined cataract surgery in open-angle glaucoma over 24 months. Turk J Ophthalmol 51(3):139–145. https://doi.org/10.4274/tjo.galenos.2020.47629
Rauchegger T, Angermann R, Willeit P, Schmid E, Teuchner B (2021) Two-year outcomes of minimally invasive XEN gel stent implantation in primary open-angle and pseudoexfoliation glaucoma. Acta Ophthalmol 99(4):369–375. https://doi.org/10.1111/aos.14627
Nuzzi R, Gremmo G, Toja F, Marolo P (2021) A retrospective comparison of trabeculectomy, baerveldt glaucoma implant, and microinvasive glaucoma surgeries in a three-year follow-up. Semin Ophthalmol 36(8):839–849. https://doi.org/10.1080/08820538.2021.1931356
Reitsamer H, Vera V, Ruben S et al (2022) Three-year effectiveness and safety of the XEN gel stent as a solo procedure or in combination with phacoemulsification in open-angle glaucoma: a multicentre study. Acta Ophthalmol 100(1):e233–e245. https://doi.org/10.1111/aos.14886
Gabbay IE, Goldberg M, Allen F, Lin Z, Morley C, Pearsall T, Muraleedharan V, Ruben S (2021) Efficacy and safety data for the Ab interno XEN45 gel stent implant at 3 Years: a retrospective analysis. Eur J Ophthalmol. https://doi.org/10.1177/11206721211014381
Cappelli F, Cutolo CA, Olivari S et al (2022) Trabeculectomy versus Xen gel implant for the treatment of open-angle glaucoma: a 3-year retrospective analysis. BMJ Open Ophthalmol 7(1):e000830. https://doi.org/10.1136/bmjophth-2021-000830
Hodapp E, Parrish R, Anderson D (1993) Clinical decisions in glaucoma. Mosby-Year Book Inc, St. Louis
The AGIS Investigators (2000) The advanced glaucoma intervention study (AGIS): 7 the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 130(4):429–440. https://doi.org/10.1016/s0002-9394(00)00538-9
Lim SY, Betzler BK, Yip LWL, Dorairaj S, Ang BCH (2021) Standalone XEN45 gel Stent implantation versus combined XEN45-phacoemulsification in the treatment of open angle glaucoma-a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol 259(11):3209–3219. https://doi.org/10.1007/s00417-021-05189-x
Wang B, Leng X, An X, Zhang X, Liu X, Lu X (2020) XEN gel implant with or without phacoemulsification for glaucoma: a systematic review and meta-analysis. Ann Transl Med 8(20):1309. https://doi.org/10.21037/atm-20-6354
Chen XZ, Liang ZQ, Yang KY, Lv K, Ma Y, Li MY, Wu HJ (2022) The outcomes of XEN gel stent implantation: a systematic review and meta-analysis. Front Med (Lausanne) 9:804847. https://doi.org/10.3389/fmed.2022.804847
Jampel HD, Musch DC, Gillespie BW, Lichter PR, Wright MM, Guire KE, Collaborative Initial Glaucoma Treatment Study Group (2005) Perioperativecomplications of trabeculectomy in the collaborative initial glaucoma treatmentstudy (CIGTS). Am J Ophthalmol 140(1):16–22. https://doi.org/10.1016/j.ajo.2005.02.013
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, Tube versus Trabeculectomy Study Group (2012) Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol 153(5):789-803. https://doi.org/10.1016/j.ajo.2011.10.026
Acknowledgements
Medical writing and Editorial assistant services have been provided by Antonio Martínez (MD) of Ciencia y Deporte S.L.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Roberto G. Carassa], [Gabriele Corsini] and [Giacinto Triolo]. The first draft of the manuscript was written by [Gabriele Corsini] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethic Committee of the Centro Italiano Glaucoma Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Due to the characteristics of the study, the Ethic Committee of the Centro Italiano Glaucoma Hospital waived the need for written informed consent. Any information that could lead to an individual being identified has been encrypted or removed, as appropriate, to guarantee their anonymity.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carassa, R.G., Corsini, G. & Triolo, G. Long-term effectiveness and safety of XEN45 in open-angle glaucoma patients. Int Ophthalmol 44, 310 (2024). https://doi.org/10.1007/s10792-024-03234-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03234-2