Abstract
Purpose
To clarify the characteristics of intraocular lens (IOL) dislocation requiring IOL suture or intraocular scleral fixation.
Methods
This retrospective consecutive case series included 21 eyes (21 patients) who required sutured or sutureless intrascleral IOL fixation following IOL extraction owing to IOL dislocation at the outpatient clinic in the Department of Ophthalmology, Saitama Red Cross Hospital, Japan, between January and December 2019. Medical records were retrospectively reviewed for background diseases, location of the dislocated IOL (intracapsular/extracapsular), insertion of a capsular tension ring (CTR), and the period from IOL insertion to dislocation.
Results
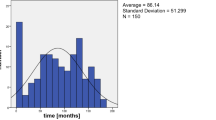
We included 21 eyes of 21 patients who required IOL suture or intrascleral fixation for IOL dislocation at our clinic from January to December 2019 were included. The most common background disease was pseudoexfoliation syndrome (four cases), followed by atopic dermatitis, dysplasia/dehiscence of the zonule, post-retinal detachment surgery, high myopia, and uveitis (three cases each). At the time of dislocation, the IOLs were either intracapsular (16 cases, including 3 cases with CTR insertion) or extracapsular (5 cases). The time from IOL insertion to IOL dislocation was 13.7 ± 8.1 years (maximum: 31.3 years, minimum: 1.7 years).
Conclusions
In this study, all 21 cases represented late IOL dislocations occurring after 3 months postoperatively. Among these late IOL dislocation cases, IOL dislocation occurred in a short-medium period of time, especially in those with CTR insertion and weakness/dehiscence of the zonule, with an average of 3 to 5 years postoperatively. We propose referring to these cases as intermediate-term IOL dislocation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over 50% of the population experiences cataracts by 75 years of age; in many countries, cataract surgery is the most frequently performed surgery in the health care system. [1] Among adverse events following cataract surgery, intraocular lens (IOL) dislocation after IOL implantation is the most serious with regards to visual loss and requires surgical intervention. Its frequency is rare; the probabilities of postoperative IOL dislocation are 0.1% at 10 years, 0.7% at 20 years, [2] 1% at 10 years,[3] and 3% at 20 years.[4] As the number of cataract surgeries is increasing with increasing longevity, the number of IOL dislocation cases will likely increase. Risk factors for IOL dislocation include pseudoexfoliation syndrome (PE), connective tissue disorders, uveitis, retinitis pigmentosa, high myopia, and patients who have undergone vitreoretinal surgery. [5,6,7,8,9,10]
This study reviewed cases of IOL dislocations that required IOL suture or intrascleral fixation at our institution. The purpose of this retrospective case study was to evaluate the risk factors for IOL dislocation and the typical latency period before dislocation.
Methods
This retrospective consecutive case series included 21 eyes of 21 patients who required sutured or sutureless intrascleral IOL fixation following IOL extraction owing to IOL dislocation at our outpatient clinic from January to December 2019 (Table 1). Medical records were retrospectively reviewed to determine background diseases, surgical findings at the time of initial cataract surgery, location of the dislocated IOL (intraocular/extraocular), whether a capsular tension ring (CTR) was inserted, IOL shape (one-piece/three-piece), time from the initial IOL insertion to the IOL dislocation. Additionally, pre- and postoperative corrected distance visual acuity (CDVA) at 3 months (± 4 weeks) and objective refractive values by autorefractometry were recorded. Refractive error was determined by the difference between the predicted spherical equivalent calculated using the Barrett Universal II formula and the actual subjective postoperative refraction equivalent.
Surgery was performed under 0.75% anapain sub-Tenon's or post-bulbar anesthesia using a 25G Constellation vitrectomy system (Alcon Laboratories, TX), and a RESIGHT wide-angle viewing system (Carl Zeiss Meditec AG, Jena, Germany). Viscoelastic materials were injected into the anterior chamber to safeguard the corneal endothelium. In cases where IOLs dislocated into the anterior vitreous cavity, they were pulled up into the anterior chamber using hooks or IOL forceps. IOLs dislocated into the posterior vitreous cavity were pulled up into the anterior chamber with a vitreous cutter or forceps through pars plana vitrectomy ports. If the dislocated IOL was of soft material, it was either cut into two pieces, spiral-shaped, [11] or folded and explanted through an upper 3.5–4 mm sclerocorneal incision, a 6 mm sclerocorneal incision, or a temporal 3–4 mm sclerocorneal incision. However, rigid lenses that could not be cut or folded were explanted directly through an upper 3 × 3 mm L-shaped scleral pocket insicion [12] or a 6 mm sclerocorneal incision on the upper or temporal side. Subsequently, a pars plana vitrectomy was performed, including core vitrectomy and peripheral fundus confirmation of the fundus in the periphery. Patients underwent intrascleral fixation for IOLs using the double-needle and flange methods of Yamane et al., [13] with a 3-piece IOL of 7 mm diameter placed 2 mm from the corneal limbus at 2 and 8 o'clock, typically NX70/NX70S (Santen, Osaka, Japan), with low power or high power outside the NX70 range, or a 3-piece AN6MA/AN6KA (KOWA, Japan). Three-piece IOLs were inserted into the anterior chamber with an injector, and an angled scleral incision was made at the conjunctiva using a 30-gauge thin-walled needle (TSK super thin-walled needle, Tochigi Seiko, Japan). The scleral penetration point was located 2 mm posterior to the limbus at the 2 o'clock position, directed 20° medially to the posterior chamber. Using forceps, the leading haptic was pushed into the needle lumen. Subsequently, a second sclerotomy was made at the 8 o'clock position using a 30-gauge thin-walled needle, and the same procedure was repeated for the second haptic. Following extraction of both haptics, the flange was created at the top of each haptic using ophthalmic cautery (Accu-Temp Cautery; Beaver-Visitec International, Inc.). The haptics were then pushed back to secure them in the scleral tunnel, and the IOL was centered. To prevent iris capture of the IOL, a peripheral iridotomy was performed at the surgeon's discretion using a vitrectomy cutter after miosis. In cases where IOLs were sutured, an improved method was employed. [14, 15] The IOLs used for scleral sutures were VA70AD (HOYA, Japan) and MA30BA (Alcon, USA) (Table 1). For scleral sutures, a straight Pair-Pak needle (Pair-Pak, Alcon Surgical, Fort Worth, TX) was inserted under the scleral semilaminar flap at the 2 o'clock position, 1.5 mm from the corneal limbus.
Similarly, a 27G needle was inserted under the scleral semilaminar flap at the 8 o'clock position, 1.5 mm from the corneal limbus. A straight pair-pak needle was threaded through the 27G needle and out of the eye. A 9–0 polypropylene suture was then passed through the upper scleral incision wound using a hook and fastened externally to the haptics of the IOL. After the IOL was inserted into the anterior chamber with an injector, the 9–0 polypropylene suture was sutured under the scleral semilaminar flap. Finally, the 3 × 3 mm L-shaped and 3 mm temporal sclerocorneal incisions were sutureless. The 6 mm sclerocorneal incision was sutured with multiple single ligatures or shoelace sutures using 10–0 nylon sutures as appropriate. The 3.5–4 mm sclerocorneal incision was typically sutureless, with single ligatures or shoelace sutures using 10–0 nylon sutures added as needed.
Paired t-tests were conducted to compare preoperative and postoperative data. In contrast, t-tests and multiple regression analysis on time to IOL dislocation were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), [16] a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were reported as mean ± SD; a P value less than 0.05 was considered statistically significant.
The Saitama Red Cross Hospital ethics committee approved this retrospective consecutive case series. All procedures conducted in this study adhered to the tenets of the Declaration of Helsinki. Our institution used an opt-out consent process.
Results
A total of 21 cases were included, comprising 12 male and 9 female patients (Table 1). The time from the initial cataract surgery to IOL dislocation was 14.8 ± 8.4 years (mean ± standard deviation) for male patients and 12.2 ± 8.0 years for female patients, with no significant difference between the sexes (P = 0.489, t-test). The time from the initial cataract surgery to IOL dislocation was 13.7 ± 8.1 years, with a minimum of 1.7 years and a maximum of 31.3 years in all 21 patients. No early IOL dislocation occurred less than 3 months after the initial cataract surgery (Table 1). Concerning IOL extraction and re-implantation procedures, two patients underwent sutured intrascleral IOL fixation, 18 underwent sutureless intrascleral IOL fixation, and one underwent IOL extraction (Table 1).
If the dislocated IOL was of soft material, it was either cut into two pieces (9 cases), shaped into a spiral [11] (1 case, 4.8%), or folded (3 cases, 14%), and then explanted through various types of incisions: an upper 3.5–4 mm sclerocorneal, a 6 mm sclerocorneal incision, or a temporal 3–4 mm sclerocorneal incision (Table 1). However, for rigid lenses that could not be cut or folded, they were directly explanted through either an upper 3 × 3 mm L-shaped scleral pocket insicion [12] (2 cases, 9.5%) or a 6 mm sclerocorneal incision keratotomy on the upper or temporal side (5 cases, 24%) (Table 1). In one case, an MA30BA + 20.0D (Alcon) IOL had been sewn in during previous left cataract surgery, with the nasal suture of the IOL haptics remaining intact and the IOL undamaged. Therefore, an upper 2.4-mm corneal incision was made, the temporal free IOL haptics were explanted from the eye and sutured externally, and the IOL was reused without removal (Table 1). Subsequently, 18 patients (86%) who underwent intrascleral fixation for IOLs were treated using the double-needle and flange methods described by Yamane et al., [13] with a 3-piece IOL of 7 mm diameter placed at 2 mm from the corneal limbus at 2 and 8 o'clock, mainly NX70/NX70S (Santen, Osaka, Japan) (15 cases, 71%). In contrast, those outside the NX70 range were a 3-piece AN6MA/AN6KA (KOWA, Japan) (3 cases, 14%; Table 1). A new suturing method was employed in the two cases where IOLs were sutured. [14, 15] The IOLs used for scleral sutures were VA70AD (HOYA, Japan) and MA30BA (Alcon, USA; Table 1).
The preoperative astigmatism was 1.92 ± 1.62, and the increase in astigmatism after surgery was 0.21 ± 1.69 D. The postoperative astigmatism (2.13 ± 1.26) was not statistically significantly different from the preoperative astigmatism (paired t-test, P = 0.568). The mean preoperative CDVA was 0.76 ± 0.41. Postoperative CDVA (0.76 ± 0.42) was not statistically significantly different from that preoperatively (paired t-test, P = 0.971). The refractive error was − 0.57 ± 0.90D (range, − 2.15 to + 1.51D). The distribution of postoperative refractive error is shown in Fig. 1.
Intracapsular fixation was the most common IOL position, accounting for approximately ¾ of the cases (Table 2). There was no difference in time to IOL dislocation between intracapsular and extracapsular fixation (P = 0.462, t-test). Three patients with intracapsular IOL fixation with CTR insertion had a shorter time to dislocation (4.9 years) than the other 18 patients (P = 0.039, t-test). One-piece and three-piece IOLs each were used in half of the cases, and there was no difference in the time to IOL dislocation between one-piece and three-piece IOLs (P = 0.65, Tukey's honestly significant difference [HSD] test).
PE was the most common background disease for IOL dislocation (4 cases (19%); Table 3), followed by atopic dermatitis, weakness/dehiscence of the zonule, post-retinal detachment surgery, high myopia, and uveitis (3 cases (14%), each). The time to dislocation was shortest for those with weakness/dehiscence of the zonule (average 3.4 years), while the time to dislocation tended to be shorter for those with PE, atopic dermatitis, and traumatic cataracts (average 10 years (10.9 ± 3.3, 11.4 ± 8.7, and 10.9 ± 4.5 years, respectively)). Patients with PE were oldest at the time of dislocation (mean age 86.2 ± 1.4 years), while patients with atopic dermatitis were youngest (46.6 ± 7.0 years). Patients with weakness/dehiscence of the zonule, post-retinal detachment surgery, uveitis, and traumatic cataracts tended to be in their 50 s to 60 s (64.8 ± 10.8, 56.6 ± 17.6, 62.6 ± 14.7, and 57.7 ± 1.6, respectively) (Table 3).
Multiple regression analysis of the time to IOL dislocation in these 21 cases revealed that the time to dislocation was significantly shorter in patients with weakness/dehiscence of the zonule (P = 0.04, Table 4). Although the differences were insignificant, PE, atopy, and traumatic cataracts tended to shorten the time to dislocation (Table 4).
Discussion
We reviewed the characteristics of 21 cases of IOL dislocation at our hospital, including background diseases and time to dislocation. Diseases and conditions associated with IOL dislocation include PE, previous trauma, previous vitrectomy, uveitis, high myopia, and retinitis pigmentosa. [5,6,7,8,9,10] Out of the 21 patients in this study, four (19%) had PE; three each (14%) had atopy, post-retinal detachment surgery, high myopia, and uveitis; and two (10%) had trauma or posterior capsule rupture.
IOL dislocation is classified into early IOL dislocation (occurring within 3 months after surgery) and late IOL dislocation (occurring > 3 months after surgery), depending on the time of onset. [5, 7,8,9, 17] In the present study, all 21 cases involved late IOL dislocations occurring after 3 months. The average time to dislocation in these 21 cases was 13.7 years. Among the 21 cases in this study, the average time to dislocation was 3.4 years in cases of weakness/dehiscence of the zonule and 4.9 years in the cases of CTR insertion; these estimates are considered to be relatively early among cases of late IOL dislocations, and we propose the term "intermediate-term IOL dislocation." IOL dislocation in the relatively early stage (3 to 5 years) of late IOL dislocation (intermediate-term IOL dislocation) may replace early-stage IOL dislocation with advances in technology for cataract surgery, such as CTR insertion and expansion of the indications for IOL insertion. Ucar et al. also reported a mean time from primary surgery to IOL removal of 81.7 ± 37.1 months in cases of IOL dislocation. Similar to the cases in our study, their cases were late IOL dislocations occurring after 3 months postoperatively [10].
The material of the dislocated IOLs was not included in the study because the surgical records were unavailable for some cases, such as those in which the initial surgery was performed at another hospital (13 of the 21 cases were unknown). However, many of the one-piece IOLs in the 21 cases required a wider wound opening to extract the IOL because it could not be cut in half or folded; they were likely made of silicone or polymethyl methacrylate (PMMA). As surgeons, we should remember that performing a hemisection or folding when removing a dislocated IOL may not be possible. On the other hand, for lenses made of soft material, Ucar et al. [10] reported re-folding within the main incision, bisecting the IOL, and enlarging the main incision; we did the same with bisecting, spiral cutting, [11] and folding. In addition, IOL insertion for IOL dislocation was performed by sutureless intrascleral IOL fixation (flange method13) in 18 of the 21 cases (86%), which requires less time than sutured intrascleral IOL fixation and results in good IOL fixation. Ucar et al. [10] reported improved CDVA in all groups: refolding within the main incision, bisection of the IOL, and removal with an enlarged main incision. They also reported that the refolding and bisection of the IOL resulted in little or no increase in astigmatism. In the present study, the postoperative CDVA (0.76 ± 0.42) showed no statistically significant improvement compared with preoperative CDVA; however, it is possible that our patient had better preoperative visual acuity, which may have prevented further improvement. The postoperative increase in astigmatism was 0.21 ± 1.69 D, which was mild and comparable to that reported by Ucar et al. [10] Regarding the postoperative refractive error, Tokuhisa et al. [18]. reported -0.86 ± 1.52 D (range, − 10.58 to + 0.47 D) after intrascleral fixation, and our results were comparable.
Limitations of the study include the low evidence level due to the limited number of cases (21) and the study design (retrospective case series). However, all 21 cases of IOL dislocation referred to Saitama Red Cross Hospital, a local flagship hospital in Saitama Prefecture, 2019 were included in the study without excluding a single case, and we believe this can provide a certain level of reference. In conclusion, all 21 cases involved late IOL dislocations after 3 months postoperatively. Background diseases in these cases included pseudoexfoliation syndrome, atopic dermatitis, and weakness/dehiscence of the zonule. IOL dislocation occurred in a short-medium period of time, especially in cases of CTR insertion and weakness/ dehiscence of the zonule. Specifically, they occurred within an average span of 3–5 years postoperatively. Thus, we propose referring to these cases as those involving intermediate-term IOL dislocation. This study provides insight into the effects of underlying conditions on the time to IOL dislocation, providing clinicians with a reference to guide the follow-up time in an era where IOL dislocation is expected to increase because of the aging population (the era of a 100-years life expectancy). Moreover, this insight will prove helpful due to the expansion of indications for IOL insertion in high-risk cases following advances in cataract surgery technology.
References
Klein R, Klein BE (2013) The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.13-12789
Pueringer SL, Hodge DO, Erie JC (2011) Risk of late intraocular lens dislocation after cataract surgery, 1980–2009: a population-based study. Am J Ophthalmol 152:618–623
Monestam EI (2009) Incidence of dislocation of intraocular lenses and pseudophakodonesis 10 years after cataract surgery. Ophthalmology 116:2315–2320
Monestam E (2019) Frequency of intraocular lens dislocation and pseudophacodonesis, 20 years after cataract surgery–a prospective study. Am J Ophthalmol 198:215–222
Kristianslund O, Dalby M, Drolsum L (2021) Late in-the-bag intraocular lens dislocation. J Cataract Refract Surg 47:942–954
Lee GI, Lim DH, Chi SA, Kim SW, Shin DW, Chung TY (2020) Risk factors for intraocular lens dislocation after phacoemulsification: a nationwide population-based cohort study. Am J Ophthalmol 214:86–96
Ascaso FJ, Huerva V, Grzybowski A (2015) Epidemiology, etiology, and prevention of late IOL-capsular bag complex dislocation: review of the literature. J Ophthalmol 2015:805706
Davis D, Brubaker J, Espandar L, Stringham J, Crandall A, Werner L, Mamalis N (2009) Late in-the-bag spontaneous intraocular lens dislocation: evaluation of 86 consecutive cases. Ophthalmology 116:664–670
Hayashi K, Hirata A, Hayashi H (2007) Possible predisposing factors for in-the-bag and out-of-the-bag intraocular lens dislocation and outcomes of intraocular lens exchange surgery. Ophthalmology 114:969–975
Ucar F, Cetinkaya S, Kahraman H, Yener HI (2024) Changes in intraocular lens explantation indications and comparison of various explantation techniques. Am J Ophthalmol 257:84–90
Hisato Gunji KA, Itou Y, Gekka T, Tsuneoka H (2010) New surgical technique for cutting foldable intraocular lens, enabling removal through very small incision (in Japanese). Japan J Ophthalmic Surg 23:603–607
Hennekes RL, Van den Dooren KA (1999) Asymmetric L-shaped corneal no-stitchtunnel incisions for cataract surgery. J Cataract Refract Surg 25:550–555
Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K (2017) Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology 124:1136–1142
Lewis JS (1991) Ab externo sulcus fixation: SLACK incorporated thorofare. NJ 22:692–695
Por Y, Lavin M (2005) Techniques of intraocular lens suspension in the absence of capsular/zonular support. Surv Ophthalmol 50:429–462
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl 48:452–458
Riedl JC, Rings S, Schuster AK, Vossmerbaeumer U (2023) Intraocular lens dislocation: manifestation, ocular and systemic risk factors. Int Ophthalmol 43:1317–1324
Tokuhisa T, Watanabe T, Watanabe A, Nakano T (2022) Refractive error induced by intraocular lens tilt after intrascleral intraocular lens fixation. Int Ophthalmol 42:1213–1220
Acknowledgements
We thank Editage (www.editage.com) for English language editing. The Japan Society supported this study for the Promotion of Science (JSPS) KAKENHI, Grant Number 19 K18837 (SN). We sincerely appreciate Yoshihito Kato orthoptist for his assistance in data entry and Editage (www.editage.com) for English language editing.
Funding
The Japan Society supported this work for the Promotion of Science (JSPS) KAKENHI, Grant Number 19 K18837 (SN). The funding body played no role in the study's design, data collection, analysis, interpretation, or manuscript writing.
Author information
Authors and Affiliations
Contributions
SN collected, analyzed, and interpreted the clinical data and wrote the manuscript. SN and KI contributed to the design and concept of this report. SN, KT, KO, MT, and KI contributed substantially to the acquisition of clinical data. All authors commented on previous versions of the manuscript and reviewed and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in accordance with the principles of the Declaration of Helsinki. The Ethics Committee of Saitama Red Cross Hospital granted approval.
Consent to participate
An opt-out consent process was used at our institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakagawa, S., Totsuka, K., Okinaga, K. et al. Background factors determining the time to intraocular lens dislocation. Int Ophthalmol 44, 240 (2024). https://doi.org/10.1007/s10792-024-03166-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03166-x