Abstract
Purpose
To analyze the intraoperative challenges of cataract surgery in children, following glaucoma filtering surgery.
Methods
This was a retrospective study to analyze intra-op challenges and outcomes of pediatric cataract surgery in post-glaucoma filtration surgery eyes, between January 2007 and December 2019.
Results
We included 20 eyes of 16 children. The most common glaucoma surgery performed was trabeculectomy and trabeculotomy (14 eyes). The median age at the time of cataract surgery was 74.5 months. The most common cataract surgery performed was lens aspiration with posterior chamber intraocular lens implantation (LA + PCIOL) (9/20). The most common intraoperative challenge faced was difficulty in capsulorrhexis (ten eyes), followed by extension of primary posterior capsulotomy (six eyes). At the final follow up eight eyes had improvement in visual acuity, five eyes had stable visual acuity and five eyes had a drop in visual acuity. In 12/20 eyes IOL was implanted, nine eyes in-the-bag and three eyes had in ciliary sulcus. None of the IOLs in the bag had decentration of IOL. The median postoperative IOP (p = 0.12) and median number of postoperative AGM (p = 0.13) at 2 years remained stable compared to the preoperative values. The IOP remained well controlled in 4 eyes without anti-glaucoma medications and in 14 eyes with anti-glaucoma medications and none needed additional surgery for IOP control. Two eyes developed retinal detachment postoperatively.
Conclusion
Cataract surgery in pediatric eyes with prior glaucoma surgeries, have challenges with capsulorrhexis and IOL stability. The visual outcomes were reasonably good so was the IOP control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pediatric cataract surgery has certain unique challenges like a collapsible sclera due to low scleral rigidity, an elastic capsule making capsulorrexis challenging and the technical proficiency required in doing the posterior capsulotomy and anterior vitrectomy. The complexity increases in eyes following glaucoma filtration surgery. These eyes with hazy cornea, stretched limbus, deep anterior chamber along with lax capsular bag make it inherently challenging with a higher rate of intraoperative and postoperative complications. Besides, intraocular lens (IOL) stability during and after cataract surgery in buphthalmic eyes is a matter of concern [1, 2].
Although the precise reason for cataract development following glaucoma filtering surgery is not known, it does accelerate cataract formation [3]. Trabeculectomy is known to increase the risk of cataract formation by eightfold [4]. The shallow anterior chamber post glaucoma filtering surgery, topical steroid use, and inflammation in the post-operative period may contribute to early cataract formation [4, 5].
Sukhija and associates in a smaller series have reported IOL decentration during the post-operative period in two of the eight eyes with posterior capsulotomy [1]. However, in a larger series by the same group, they advise posterior capsulotomy to prevent visual axis opacification that would need additional surgery [6].
Our study delves the intraoperative challenges primarily and looks at visual outcomes and intraocular pressure (IOP control) as secondary outcome measures following pediatric cataract surgery in eyes with previous glaucoma filtration surgery.
Methods
This was a retrospective study to analyze intra-op challenges and outcomes of cataract surgery in eyes of children following glaucoma filtration surgery, between January 2007 and December 2019. As per Institute protocol all patients sign a consent form allowing access to clinical data and photographs if any for research and presentations. Ethics committee approval was taken. The demographic data collected included age at cataract surgery, gender, number of glaucoma surgeries, glaucoma diagnosis, and cataract surgery details. All children underwent a detailed ophthalmic examination. Corneal haze if present was graded as per the grading suggested by Tandon and associates as follows: grade 0- no corneal haze, 1- iris details seen, 2- pupil margin visible, iris details not seen, 3- pupil margin not visible, 4- total corneal opacity [7, 8]. If a dilated fundus exam was not possible due to hazy media then a B scan ultrasound was done.
The surgical steps included the following: side port incisions, anterior capsulorrexis after staining of capsule with tryphan blue, lens aspiration, main wound construction, IOL implantation, posterior capsulotomy and anterior vitrectomy and wound closure with non-absorbable 10-0 nylon suture.
The decision to perform a primary posterior capsulotomy (PPC) and anterior vitrectomy (AV) was based on age as well as visualization due to corneal haze. If the corneal haze did not allow adequate visualization of the posterior capsule, PPC and AV were avoided.
The decision to place an intraocular lens (IOL) was based on the corneal diameter, anterior segment findings like presence or absence of corneal haze which could preclude adequate visualization of anterior chamber structures, zonular laxity, phacodonesis, and surgeon discretion. If an IOL was placed in-the-bag, then a single piece IOL (Acrysof SA60AT, Alcon Laboratories, Inc., Fort Worth, TX, USA) was implanted; if an IOL could not be safely placed in-the-bag then a 3-piece IOL (Acrysof MN60AC, Alcon laboratories Inc., Fort Worth, TX, USA) was implanted in the sulcus. In all the patients axial length (Tomey AL-100, Germany), keratometry (Nidek HandyReF-K, Japan) and corneal diameter (Castroviejo calipers) were measured. In those eyes with scarred or irregular corneas, keratometry readings were taken from the fellow eye.
The glaucoma surgeries and the subsequent cataract surgery was performed by fellowship trained glaucoma specialists and pediatric ophthalmologists.
All patients were seen on day 1 in OPD and under anesthesia (EUA) at 1 week for refraction and suture removal. The children were followed up after 1 month, 3 months, and then yearly.
Statistical analysis- Statistical analysis was performed using Stata version 13.1 (StataCorp, College Station, Texas, USA) statistical software. The normality of data was checked using the Shapiro Wilk test. Mean with standard deviation and median with inter-quartile range were used to describe the parametric and non-parametric data respectively. Pre and post-surgery variables were compared using paired T-test.
Factors affecting the outcome of surgery were evaluated by assessing the relationship between the difference in visual acuity before and after cataract surgery and age, gender, number of glaucoma surgeries, horizontal corneal diameter, type of cataract, type of intraocular lens, and if IOL was placed in bag or sulcus and intraoperative challenges.
Results
Demographic and Clinical Features
We included 20 eyes of 16 children. There were 13 males and 7 females in the cohort. There were nine eyes diagnosed with primary congenital glaucoma, six eyes with developmental glaucoma, and 5 eyes with steroid-induced glaucoma. The median age of the patients at the time of cataract surgery was 74.5 months (IQR: 51,84, range 2–156). There were nine right eyes and 11 left eyes.
Before undergoing cataract surgery, six eyes had 1 glaucoma surgery, 13 eyes had 2 and 1 eye had 3 glaucoma surgeries. The median time interval between last glaucoma surgery and cataract surgery was 269.5 days (IQR 193, 416; range 77–998). Table 1 gives details of the types of prior glaucoma surgeries performed. Categorical variables are summarized with descriptive statistics as shown below in Table 2
Three eyes were noted to have synechiae (1 eye had anterior, 2 had posterior). The most common type of cataract noted was posterior subcapsular (9/20), the others being a total (7/20), and nuclear cataracts (4/20). Before cataract surgery 11 eyes had hazy corneas; 8 of them had grade 2 corneal haze and 4 eyes had grade 3 corneal haze.
The most common type of cataract surgery performed was Lens aspiration with posterior chamber intraocular lens implantation (LA + PCIOL) (9/20) followed by lensectomy (4/20), lens aspiration (4/20), and lens aspiration with primary posterior capsulotomy with anterior vitrectomy and posterior chamber intraocular lens implantation (LA + PPC + AV + PCIOL) (3/20). Of the 12 eyes in which intraocular lenses were implanted 11 were implanted with a hydrophobic IOL and 1 with a hydrophilic lens. In 9 eyes the IOL was placed in-the-bag and in 3 eyes it was placed in the ciliary sulcus.
Intraoperative Challenges
Intraoperatively 3 eyes had pre-existing zonular dialysis. The intra-operative difficulties faced by the surgeon are described in Table 3.
Outcomes of Cataract Surgery
The mean duration of follow up was 948 days (range 106–2282 days, SD 749.4).
The visual acuity remained stable post-cataract surgery (Table 4). At the final follow up 8 eyes had improvement in visual acuity, 5 eyes had stable visual acuity and 5 eyes had a drop in visual acuity. In 2 eyes, the children could not cooperate for visual acuity testing and were documented as fixing and following light.
In the 8 eyes that did have improvement in visual acuity, the median visual acuity pre-surgery was 1.34 Log Mar (range 1.09–2.17, Log Mar) and post- surgery was 0.79 Log Mar (range- 0.69–1.2).
The IOP was measured at all follow-up visits. On day 1, month 1, and last follow up the IOP remained stable (p = 0.12). The mean IOP was 16.5 (± 11.7) mm Hg pre-cataract surgery and post-surgery was 16.3 (± 3.6) mm Hg, the data was non-parametric hence the median was calculated, median IOP was 13.5 mm Hg pre cataract surgery and 17.5 mm Hg post cataract surgery at 1 year.
The IOP remained well controlled in 4 eyes without anti-glaucoma medications and in 14 eyes with anti-glaucoma medications. Two eyes developed late postoperative hypotony due to retinal detachment (RD). One developed RD 3 years following cataract surgery and one eye developed RD 1 year following cataract surgery. None of the eyes required any additional glaucoma surgery for IOP control following cataract surgery. The median number of antiglaucoma medications pre- and post-operatively was 1.35 and 1.8 respectively (p = 0.13).
Two eyes (2 children) who were steroid responders had an IOP of > 21 but < 30 mm Hg as noted at postoperative 1 week review. This was controlled by switching to low potency steroids and stepping up topical anti-glaucoma medications.
Postoperatively, three eyes with IOL placed in the sulcus had superior decentration and one of these eyes developed retinal detachment.
A paired t-test was conducted to compare the pre-operative visual acuity and the postoperative visual acuity. The difference between the visual acuity before cataract surgery (1.3 ± 0.55 log mar) and visual acuity post-cataract surgery (1.33 ± 0.67 log mar) was − 0.03 log MAR (95% CI, − 0.41–0.35), t (13) = − 0.18, p = 0.57.
The Intraocular pressure also remained stable in the post-operative period (Fig. 1).
Discussion
We highlight intraoperative challenges faced during cataract surgery in eyes of children following glaucoma filtration surgery. The most common intraoperative challenge faced by surgeons in our series of cases was in performing an anterior capsulorrhexis (10/20 eyes), along with an extension of posterior capsulotomy (6/20 eyes) and intraocular lens stability (3/20 eyes).
Certain unique challenges like corneal haze/scarring (impeding visualization during surgery), stretched limbus, a deep anterior chamber with intraoperative fluctuations and a poorly dilating pupil contribute to the difficult surgery [1, 9]. An enlarged ciliary ring, stretched and lax zonules and capsular bag makes IOL placement and stability very challenging [1, 9].
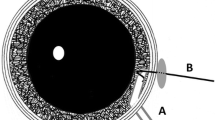
In our series, difficulty in the visualization of the anterior capsule despite using trypan blue was noted in 4 eyes. Although the capsulorrhexis was completed eventually, we recommend using a light pipe in the presence of corneal haze to initiate the capsulorrhexis. Difficulty to initiate the capsulorrhexis due to calcification of anterior capsule was noted in two eyes. In these two eyes, an automated vitrector was used to make the capsulotomy. In one of these eyes, a multipiece IOL was placed in the sulcus, and in the other eye, the initial plan was a lensectomy and hence an IOL was not placed. Maintaining the integrity of the capsulorrhexis was a challenge as well. In four eyes an extension of the CCC was noted. In glaucomatous eyes, this is an expected challenge. In children, the anterior capsule is extremely elastic and prone to extension. Inadequate visualization, a fluctuating anterior chamber, and zonular laxity make these eyes prone to extension. In three eyes the CCC could be salvaged, in one eye it could not be retrieved and an automated vitrector was used to complete the capsulorrhexis (Table 3, Fig. 2).
The other challenge noted in our series was difficulty in primary posterior capsulotomy. Similar to CCC, poor visualization, instability of the capsular bag makes this step a challenge. In 4 eyes PPC was attempted with an automated vitrector and after making an initial opening in the posterior capsule, a sudden enlargement of the PC opening was noted. In two other cases, a manual PPC was initiated which was noted to be extending, and hence PPC was completed with an automated vitrector.
In three eyes, there was superior decentration of the IOL. In both cases, the surgery performed was lens aspiration with IOL implantation with anterior vitrectomy and posterior capsulotomy and IOL was placed in the sulcus. These children were 4 and 6 years old respectively. This is similar to what is reported by Sukhijia and associates. The aforementioned intra-operative factors can challenge the stability of the IOL. We propose performing a PPC or perform an optic capture in all cases aged less than 8 years. This would reduce the need for a membranectomy as additional procedures can increase the risk of IOP elevation. However, if there are challenges in visualization due to corneal haze probably one may have to defer this step. The choice of IOL we prefer is a 3-piece IOL since the thinner haptic does not rub and irritate the iris like a single piece IOL. Studies have shown that an IOL placed in-the-bag does reduce this risk of iris chafing and chronic inflammation. Kiarudi and associates have used pre-operative ultrasound biomicroscopy to calculate the size of the capsular bag before planning an IOL placement [10]
The integrity of the paracentesis is yet another aspect that needs keen attention. Buphthalmic eyes often have a stretched limbus which can lead to fish mouthing of the paracentesis. We noted this in four eyes. Gentle handling of the instruments through the paracenteses and suturing these incisions is recommended.
Although there are no studies recommending measures to be taken during pediatric cataract surgery following GFS, Dada and associates recommend the following measures in adults during phacoemulsification [9]. These are careful paracentesis, gentle iris handling to prevent inflammation postoperatively, using a dispersive viscoelastic and chilled balanced salt solution to protect the corneal endothelium, a thorough cortical cleanup, and an in the bag IOL placement.
The choice of cataract surgery depends upon the anterior segment findings. Cataract in presence of a hazy cornea, poorly controlled IOP, stretched and lax capsular bag and zonules would mandate utmost care in decision making with lensectomy being a better option.
Cataract surgery in the presence of a filtering bleb can increase the chances of failure of bleb function. Studies done in adults who had phacoemulsification following trabeculectomy have shown through slit-lamp examination, ultrasound biomicroscopy (UBM), and anterior segment optical coherence tomography (AS-OCT) that there is a decrease in bleb height and a progressive flattening of bleb after cataract surgery. This is hypothesized due to the inflammation following cataract surgery, fibrosis under and around the bleb, and injury to the angle following trabeculectomy [11].
Bayoumi and associates have reported lenticular changes and surgeries performed in children with primary congenital glaucoma after glaucoma filtering surgery [12]
54 out of 422 children (8.8%) had lens pathologies. Cataract was noted to be the most common lens pathology, seen in 61% of the eyes (31/54). In this study 26 eyes underwent surgery for lens pathologies, with an almost equal number of eyes undergoing lensectomy and cataract surgery with intraocular lens implantation (14 vs 12). While 11 eyes underwent in the bag lens placement, interestingly in one eye an iris fixated anterior chamber intra ocular lens was placed. While they do not mention visual outcomes at final follow up, the mean IOP post-surgery was reported to be 8.8 ± 7.6, ranging from 0 to 26 mm of Hg.
Our visual outcomes, as well as IOP, remained stable through the period of follow up.
Amongst the five eyes where there was worsening of visual acuity, two eyes developed retinal detachment in the late postoperative period and two eyes had the development of band-shaped keratopathy in the late postoperative period. Besides, one eye developed amblyopia due to poor compliance with glasses. In all the five eyes where vision remained stable pre and post cataract surgery, there was pre-existing corneal haze which possibly led to a non-improvement in visual acuity.
To summarize, the use of light pipe in hazy corneas, use of automated vitrector for anterior and posterior capsulorrhexis, a slightly smaller than usual anterior capsulorrhexis, or optic capture, use of 3-piece IOL and suturing of paracentesis help in minimizing complications and achieving good outcome. Post-operative complications like retinal detachment and band keratopathy are possibilities that need to be monitored and parents need to be counseled as well.
We accept the limitations of our study. It is retrospective and has a small sample size. But what this study adds are the possible intra-op difficulties that need to be anticipated in eyes of children following glaucoma filtration surgery and possible complications.
To conclude, cataract surgery in pediatric eyes is challenging and if you were to consider that these eyes had prior glaucoma surgeries, it has its own set of challenges. Visual outcomes can be reasonably good and require adequate pre-op and Intra op awareness of difficulties and counseling of parents to discuss prognosis.
References
Sukhija J, Kaur S, Pandav SS, Kaushik S, Raj S, Ram J (2015) Pediatric cataract surgery in buphthalmos. Eur J Ophthalmol 25(3):260–262. https://doi.org/10.5301/ejo.5000533
Galvis V, Tello A, Rangel CM (2015) Cataract surgery in anterior megalophthalmos: a review. Med Hypothesis Discov Innov Ophthalmol 4(3):101–108
Morales-Fernández L, Martínez-de-la-Casa JM, Benito-Pascual B et al (2020) Cataract extraction in patients with primary congenital glaucoma. Eur J Ophthalmol 30(3):525–532. https://doi.org/10.1177/1120672119841190
Group CNTGS (1998) Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 126(4):487–497. https://doi.org/10.1016/S0002-9394(98)00223-2
Agis Investigators (2001) The advanced glaucoma intervention study, 8: risk of cataract formation after trabeculectomy. Arch Ophthalmol 119(12):1771. doi:https://doi.org/10.1001/archopht.119.12.1771
Sukhija J, Kaur S, Pandav SS, Kaushik S, Raj S, Ram J (2016) Cataract surgery in children with buphthalmos: cataract surgery in buphthalmos. Clin Exp Ophthalmol 44(8):739–740. https://doi.org/10.1111/ceo.12747
Gupta N, Kalaivani M, Tandon R (2011) Comparison of prognostic value of roper hall and Dua classification systems in acute ocular burns. Br J Ophthalmol 95:194–198
Choi H, Phillips C, Oh JY et al (2017) Comprehensive modeling of corneal alkali injury in the rat eye. Curr Eye Res 42:1348–1357
Bhartiya S, Dada T, Begum BN (2013) Cataract surgery in eyes with previous glaucoma surgery: pearls and pitfalls. J Curr Glaucoma Pract 7(3):99–105. https://doi.org/10.5005/jp-journals-10008-1145
Kiarudi M, Zare M, Eshraghi B, Masoule E (2011) Application of ultrasound biomicro-scopy in the planning of cataract surgery in anterior megalophthalmos. Indian J Ophthalmol 59(5):400. https://doi.org/10.4103/0301-4738.83625
Narita A, Morizane Y, Miyake T et al (2019) Impact of cataract surgery on filtering bleb morphology identified via swept-source 3-dimensional anterior segment optical coherence tomography. J Glaucoma 28(5):433–439. https://doi.org/10.1097/IJG.0000000000001204
Bayoumi N, El Shakankiry N, Fouad M, Elsayed EN (2021) Lens in primary congenital glaucoma eyes treated by combined angle and filtering surgery. Eye Contact Lens 47(11):611–616
Funding
Hyderabad Eye Research Institute.
Author information
Authors and Affiliations
Contributions
AB and SV wrote the manuscript, SV helped with statistics. SS came up with the idea and was involved in extensive review of the manuscript. KR, SKA and NG helped with review. NG and SKA were involved in data collection
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any potential conflict of interest to disclose.
Ethical Approval
L V Prasad Eye Institute, Hyderabad, LEC-BHR-R-03–24-1198.
Informed Consent
Consent was provided by the parents of the children.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Badakere, A., Badakere, S.V., Aijaz, S.K. et al. Intraoperative challenges and outcomes of pediatric cataract surgery following glaucoma filtering surgery. Int Ophthalmol 44, 231 (2024). https://doi.org/10.1007/s10792-024-03116-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03116-7