Abstract
Background
To compare the effect of two different prostaglandin analogues (Travatan® vs. Xalatan®) on ocular surface parameters.
Methods
This study includes 44 eyes of 44 patients with newly diagnosed primary open-angle glaucoma (POAG) or ocular hypertension (OHT). Patients were randomly divided into two groups and treated with either benzalkonium chloride (BAK)-preserved latanoprost and polyquad-preserved travoprost. Changes in intraocular pressure (IOP) levels and ocular surface parameters including ocular surface disease index (OSDI) questionnaire, tear breakup time (TBUT), ocular surface staining scores, and Schirmer test scores of patients were performed at baseline, 1, 3, 6, and 12 months of treatment and compared.
Results
The age, sex ratio, visual acuity, central corneal thickness, and cup/disc ratio were similar between the groups. A decrease in IOP levels (23.3 ± 2.5 to 15.5 ± 2.3), TBUT (5.5 ± 2.3 to 4.1 ± 1.7 s), Schirmer test values (11.3 ± 5.9 to 8.6 ± 4.7 s), and a worsening in OSDI scores (44.6 ± 15.2 to 55.1 ± 13.1) and staining scores (1.7 ± 1.6 to 2.3 ± 1.8) were observed in all patients in the first month of treatment (p < 0.05, for all). No further worsening was detected during the 1-year follow-up. There was no difference between the groups in terms of alterations in IOP levels and ocular surface parameters.
Conclusion
Travatan® and Xalatan® have a similar effect on IOP levels and ocular surface parameters in patients with POAG and OHT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is an optic neuropathy, which is the leading cause of irreversible blindness around the world. The only proved treatment option of glaucoma is to decrease the intraocular pressure (IOP) [1]. Prostaglandin analogs are a group of topical glaucoma medications used for this purpose, which increase the outflow of the humor aqueous through the uveoscleral pathway [2]. They are usually preferred in the first choice treatment because of their fewer local and systemic adverse effects than the other glaucoma medications. In addition, their daily single dose improves patient adherence to treatment.
Dry eye disease (DED) affects millions of people throughout the world and is one of the most frequent causes of patient visits to ophthalmology clinics. One of the increasing risk factors of DED in recent years is iatrogenic DED, which occurs due to a variety of topical and systemic drugs, using contact lenses, surgical interventions, and cosmetic procedures. Topical medications may cause dry eye through their allergic, toxic, and immune-inflammatory effects on the ocular surface [3]. The high prevalence of glaucoma in the elderly population, such as DED, and the necessity of long-term use of topical medication in treatment glaucoma cause iatrogenic DED to be seen more commonly among patients with glaucoma and ocular hypertension [4]. Approximately 60% of patients with glaucoma have ocular surface disease and its related symptoms including dryness, stinging, itching, burning, foreign body sensation, and visual disturbance [5,6,7]. In the pathogenesis, both active ingredients and preservatives have toxic effects on the ocular surface; however, the direct relationship between the occurrence of iatrogenic dry eye and the number of drugs used in the therapy, regardless of the type of glaucoma medication, indicates that a common molecule found in eye drops, preservatives, is the main cause of the pathogenesis [3, 5, 8,9,10,11].
Benzalkonium chloride (BAK) is one of the most commonly used preservatives in topical ophthalmic medications [12]. It is a quaternary ammonium compound that acts as a detergent and kills microorganisms via cell membrane lysis [13]. It is highly effective as a preservative against Gram-positive bacteria, Gram-negative bacteria, and fungi. However, in addition to its bacteriostatic and bactericidal properties, its adverse effects on the ocular surface are frequently investigated and well-described in the literature [14, 15]. The adverse effects occur via mechanisms including cell membrane lysis, mucous and lipid layer damage, the release of inflammatory cytokines, and disruption of tight junctions. These act trigger inflammation, epithelial cytotoxicity, tear instability, and increased tear osmolarity. Also, inflammatory cell infiltration, increased apoptosis, squamous metaplasia, and decreased goblet cell number and corneal microvilli were pathologically observed in BAK-exposed ocular surfaces [16]. Due to the proved ocular surface adverse effects of BAK, less toxic preservatives, called BAK-free preservatives, have become manufactured as an alternative to BAK, including SofZia, Purite, and Polyquad (PQ). Fortunately, preservative-free glaucoma medications have been available in recent years. These are completely free of preservatives and manufactured in a single-dose container [17].
In comparison studies based on both symptoms and various diagnostic tests for dry eye, the prevalence of ocular surface disease significantly increased in patients who used glaucoma medications containing preservatives [18]. In addition, an improvement in symptoms and signs was also reported by switching glaucoma medication to a preservative-free version [19, 20]. These medications have specific disadvantages such as high cost and difficult use, and unfortunately, there are no commercially available preservative-free topical glaucoma medications in some countries. In these regions, surgery and laser procedures, which also bring additional adverse effects to the ocular surface, or switching medication to containing less toxic preservatives were suggested to alleviate iatrogenic DED [3, 21]. However, there are still insufficient data showing the long-term results of alternative preservatives proving less toxicity than BAK.
For the reasons mentioned above, in the current study, we aimed to compare the effects of two prostaglandin analogs containing different preservatives on the IOP and ocular surface parameters in patients with newly diagnosed primary open-angle glaucoma (POAG) and ocular hypertension (OHT).
Materials and methods
Subjects
Patients who were newly diagnosed as having POAG and OHT in the ophthalmology clinic of Ordu State Hospital between April 2014 and June 2016 were included in this prospective study. Informed consent was obtained from all suitable patients who agreed to participate. This study was approved by the ethics board of Firat University, and it adhered to the tenets of the Declaration of Helsinki.
Subjects with a history of ocular surface disease, severe DED such as Sjogren or Graft Versus Host disease, ocular surgery, and contact lens wear were not included in the study. Also, during the study period, patients who changed their anti-glaucoma treatment, who underwent any ocular surgery (including laser treatment), who had an ocular surface disease except for DED, or used additional topical eye drops were excluded from the study.
Patients were randomly divided into two groups and treated with 0.02% BAK-preserved latanoprost 0.005% (Xalatan®, Pfizer Ophthalmics, New York, USA) or 0.001% PQ-preserved travoprost 0.004% (Travatan®, Alcon, Geneva, Switzerland) eye drops once per a day.
Each subject underwent a comprehensive ophthalmologic examination including visual acuity testing, refraction, slit-lamp biomicroscopy, dilated fundus examination, IOP measurement, anterior angle evaluation with gonioscopy, central corneal thickness, retinal nerve fiber layer (RNFL), and reliable visual field testing. Examinations and measurements of patients at follow-up visits were performed by a single ophthalmologist who was blinded in terms of the type of medication. IOP measurement was done with a calibrated Goldmann applanation tonometer at 9.00 and 16.00 o’clock in each visit. The mean IOP value was used in statistical analysis by averaging of the diurnal values. Optic cup evaluation of patients was performed using classic dilated fundus examinations on slit-lamp biomicroscopy with a 90 D lens. The mean cup-disc ratio value was calculated by averaging of the vertical and horizontal cup-disc ratio. Central corneal thickness (CCT) was measured with anterior segment imaging of optical coherence tomography (OCT) (Optovue RTVue Optical Coherence Tomography, Optovue Inc., Fremont, CA). RNFL was obtained with spectral-domain OCT (Optovue RTVue Optical Coherence Tomography, Optovue Inc., Fremont, CA). Visual field defects were detected on standard automated perimetry tested with the 24-2 Swedish Interactive Threshold Algorithm (SITA) (Humphrey Visual Field Analyzer; Carl Zeiss Meditec, Dublin, CA). The diagnosis of POAG relied upon the detection of signs of glaucomatous optic neuropathy and characteristic glaucomatous visual field defects.
Ocular surface alterations of patients were assessed through the severity of symptoms and the evaluation of tear film stability, tear volume, and ocular surface damage. The severity of symptoms was assessed with ocular surface disease index (OSDI, Allergan Inc.) questionnaire which includes 12 questions related to visual disturbance and visual function [22]. Each question was graded on a scale of 0 to 4: 0 = the presence of symptoms none of the time; 1 = some of the time; 2 = half of the time; 3 = most of the time; 4 = all the time. The formula (sum of scores for all questions answered) × 25/ (total number of questions answered) was used to obtain the total OSDI score. Standard tear film breakup time (TBUT) using sodium fluorescein-impregnated strips were used for the evaluation of tear film stability. After application of dyed strip to the lower conjunctival sac, patients were instructed to blink naturally three times and then cease blinking. The value of TBUT was the interval time in terms of seconds between the last blink and the appearance of the first break in the tear film. Ocular surface damage was assessed by staining of the cornea and conjunctiva using fluorescein and lissamine green dye, respectively. After application of the impregnated strips to the lower conjunctival sac, the staining severity of the ocular surface was graded and recorded using the Ocular staining score [23]. The scoring system evaluates the ocular surface regarding a scale of 0–3 in each following three zones, cornea, nasal and temporal conjunctiva. In corneal staining, 0 means 0 dots; 1 = 1–5 dots; 2 = 6–30 dots; 3 = > 30 dots. In conjunctival staining, 0 means 0–9 dots; 1 = 10–32 dots; 2 = 33–100 dots; 3 = > 100 dots. In the presence of patches of confluent staining, staining in the pupillary area, or one or more filaments, one point for each is added to the sum of scores of three zones, and the total score is calculated over a maximum of 12 points. A Schirmer test without anesthesia was used at the evaluation of tear volume. Sterilized Schirmer paper strips were folded at the notch and, while the folded end was placed to the inferior fornix, the notch was placed to the temporal one-third of the lower lid margin. After 5 min, the Schirmer test score was measured with the length of wetting distance from the notch. These tests were performed before treatment and following the 1st, 3rd, 6th, and 12th months of treatment. Tests were performed in the same environment and at 10-min intervals so as not to affect the results of each other.
The test scores of one eye per patient were used in data analysis. The data of the right eye were used in patients with bilateral POAG, and the data of the affected eye were used if the disease was unilateral.
Statistical analysis
An outlier check was performed before starting the analyses. The normal distribution of quantitative variables was assessed using histograms, P–P plots, and the Shapiro–Wilk test. Student's t test was used for quantitative variables with normal distribution, whereas the Mann–Whitney U test was performed for quantitative variables with non-normal distribution. Pearson’s Chi-square test was used for qualitative variables. The main effects (time and drug) and time-drug interaction were analyzed using two-way repeated measures analysis of variance (ANOVA). Multiple comparisons were performed using Bonferroni post hoc test. For quantitative variables, descriptive statistics are expressed as mean and standard deviation for normally distributed variables, and as median and interquartile range for non-normally distributed variables. For qualitative variables, descriptive statistics are given as counts and percentages. The significance level was determined as 0.05 in all statistical analyses. All statistical analyses were performed using TURCOSA (Turcosa Analytics Ltd Co, Turkey, www.turcosa.com.tr) and R v-3.3.1 statistical software.
Results
During the study period, 102 patients were diagnosed and followed-up for POAG and OHT. Fifty-eight patients were excluded from the study according to the exclusion criteria. The results of the remaining 44 eyes of 44 patients (27 female and 17 male) were used for statistical analyses. Twenty-two subjects were in the PQ-preserved travoprost group, and 22 subjects were in the BAK-preserved latanoprost group. The demographic data of patients including age, sex ratio, and the glaucoma screening parameters at baseline examination including best corrected visual acuity (BCVA) levels in logMAR, cup/disc ratio, central corneal thickness values, and IOP levels were similar between the two groups. The investigation methods of DED before the glaucoma treatment including OSDI, TBUT, and ocular surface staining were similar between the groups (Table 1). At the initial examination, only Schirmer test score was different between groups, as 9.14 in the PQ-preserved travoprost group and 13.41 in the BAK-preserved latanoprost group (p = 0.014).
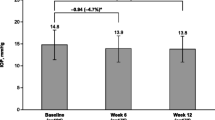
Following the beginning of the glaucoma treatment, the values of all dry eye examination methods performed in the study were worsened in both groups. IOP levels were decreased with the treatment as expected. Impairment in the dry eye parameters and IOP level reduction was observed in the first month of the treatment. In the following visits, including the 3rd month, 6th month, and 1st year of treatment, the values were stable. No significant further change was detected during the study period. The comparisons of investigation parameters including IOP, OSDI score, TBUT, ocular surface staining scores, and Schirmer test scores at baseline and each visit during the follow-up for all subjects are shown in Table 2.
The comparisons of the study parameters of the two groups and alterations with respect to time are given in detail in Table 3 and graphically in Fig. 1. Table 4 shows the comparison of alterations in study parameters between two drugs. The effects of the two drugs with respect to time on IOP, TBUT, ocular surface staining score, and Schirmer scoring were similar.
Discussion
The awareness of iatrogenic DED is not sufficient among both patients and physicians, although the adverse effects of preservatives are well known. In the study by Lemij et al. of 164 patients with glaucoma, although 89% of the patients were satisfied with their glaucoma treatment, 44% had ocular surface disease based on examination findings and 28% had corneal fluorescein staining [24]. In addition to the low awareness of their situation, 38% of patients used artificial eye drops, and 56% of the artificial tears they used contained preservatives. The study did not mention who prescribed the prescription for artificial tears, whether it was a primary care physician or an ophthalmologist, or whether patients took drops without physician advice; awareness of iatrogenic DED needs to be increased. Perhaps both patients and ophthalmologists focus their attention on glaucoma progression in the follow-up of these patients, causing them to overlook iatrogenic DED.
The coexistence of glaucoma and dry eye is important for the course and management of both diseases. Both diseases have a high prevalence in the elderly and make their managements mutually challenging due to their negative effects on each other [5, 6, 25]. Glaucoma therapy modalities such as topical medications, and laser and surgical procedures cause ocular surface damage, causing dry eye or exacerbating already existing DED [26,27,28,29]. Ocular discomfort due to ocular surface damage also causes glaucoma therapy failure by reducing the patients' adherence to glaucoma treatment [30]. Furthermore, changes in the ocular surface both reduce the IOP-lowering effects of anti-glaucomatous drugs and adversely affect the success of surgery [31]. Boso et al. found a 1.59 mm Hg decrease in IOP after treatment of DED in patients with glaucoma under topical treatment [32].
Conjunctival scarring due to long-term use of IOP-lowering medications causes failure in filtration surgery and poor surgical outcomes [33]. Overall, a vicious circle occurs between these two diseases causing treatment failure for both diseases. It is important to treat dry eye by breaking this circle in patients with both glaucoma and DED. With an improvement in ocular surface health, patient comfort and treatment adherence may increase, achieving target IOP will be easier, and the success of possible future surgical procedures may improve.
In the present study, we compared Xalatan® eye drops containing 0.02% BAK, which has well-known adverse effects on the ocular surface, and Travatan® eye drops containing another preservative molecule, 0.001% Polyquad, produced as an alternative to BAK. The effects of the two prostaglandin analogs on IOP were found to be similar. The target IOP at 1 month was reached in both groups. IOP levels remained similarly stable in both groups during the visits in the following year. Both drugs negatively affected the ocular surface in terms of investigation parameters for DED including OSDI score, TBUT, ocular surface staining, and Schirmer scores. These effects were seen in the 1st month following the initiation of the drugs and remained stable for 1 year.
Polyquad (PQ), Purite, and SofZia are alternative preservatives to BAK used in artificial tears and glaucoma medications. The antimicrobial activity of Purite and SofZia is through oxidative stress, and it was suggested that both molecules had fewer ocular adverse effects than BAK [34,35,36,37]. Both molecules have antimicrobial activity in the bottle; however, following application of the drop to the eye, SofZia and Purite turn to inactivated particles on the ocular surface through enzymatic pathways and stabilization by light exposure, respectively [17, 38].
Polyquad® (Polyquanternium, Alcon Inc., Fort Worth, TX) is another quaternary ammonium compound derived from BAK and was first used in contact lens solutions [15]. Nowadays, it has been widely used in eye drops including artificial tears and glaucoma medications. It is a hydrophilic cationic polymer and impairs cell membrane integrity via its detergent-type property [39,40,41]. It separates from BAK by its large size and lacks a hydrophilic domain [42]. The fact that PQ is approximately twenty-seven times larger than BAK limits the entry of PQ into human cells and gains itself less toxicity [42, 43]. In vitro analyses showed that PQ-containing glaucoma medications demonstrated significantly better cell viability, less apoptosis, and less oxidative damage in human conjunctival and corneal epithelial cells than BAK [38, 43, 44]. The evidence of lesser ocular surface toxicity of PQ compared with BAK was also shown in various animal models [16, 45,46,47]. Rossi et al. presented 6-months’ results of 44 patients with POAG or OHT in whom glaucoma medication was switched to PQ-preserved travoprost from BAK-preserved latanoprost. PQ-preserved travoprost was found more tolerable and safer in terms of OSDI, TBUT (8 s to 10 s), and corneal staining (punctate keratitis 70.5% to 29.6%) [48]. Lopes et al. reported that 81.5% of 173 patients who previously used BAK-preserved latanoprost preferred PQ-preserved travoprost after the change of topical glaucoma treatment [49]. Marsovszky et al. compared the results of OSDI scores, lid parallel conjunctival folds, Schirmer test, and TBUT and confocal microscopic findings of healthy subjects using BAK-preserved travoprost and PQ-preserved travoprost [50]. Based on ocular surface parameters and confocal findings, including the counting of wing and basal cells, central and peripheral Langerhans cell, PQ was found superior to BAK. It was considered that PQ caused less disturbance in corneal homeostasis and ocular surface compared with BAK. In both studies, IOP levels remained stable or decreased after switching.
Before analyzing the results, we expected that PQ would cause less deterioration in dry eye parameters, and would cause less dry eye than BAK. However, the analysis showed that the two drugs had similar effects during the study period and after 1 year. Evidence of PQ toxicity to the ocular surface was also reported in the literature. Paimela et al. demonstrated PQ toxicity to human corneal epithelial cell by comparing Travatan, Systane Ultra, and BAK 0.01%. Similar results in terms of cell viability were obtained for three agents [39]. In the same study, increased nuclear factor-kappa B (NF-κB)-dependent inflammation in cultured human corneal epithelial cells by PQ was found. In another in vitro study, it was shown that PQ damaged cell integrity and reduced the metabolic rates of human corneal epithelial cells [51]. Miyashiro et al. found a higher percentage of patients preferred BAK-free travoprost over latanoprost, but on clinical examination, no significant changes had occurred in terms of ocular hyperemia, corneal staining, and OSDI scores. BAK-free travoprost was found superior in only TBUT values from all examination parameters [52]. Goldberg et al. reported that after switching BAK-preserved glaucoma medication to BAK-free glaucoma medication, the need for lubricants decreased and an improvement in questionnaire scores and TBUT was observed. Interestingly, there was also an improvement in all study parameters in the non-switched group [53]. In a comparison study by El Hajj Moussa et al., OSDI scores were better in PQ-preserved travoprost group than with preservative-free tafluprost in 32 patients with newly diagnosed glaucoma 6 months after starting glaucoma treatment [54]. The superiority of PQ has been demonstrated in in vitro and animal experiments. However, when looking at clinical studies, PQ was found to be significantly superior, mostly according to patient preferences and questionnaire scores. In addition, when evaluated in terms of examination findings, its superiority decreases even more than expected.
A finding showing that PQ does as much ocular surface damage as BAK was that the similarity of the results between groups although the gender distribution inequality between the groups. Even it was statistically similar, there was a difference in gender distribution between groups. The female/male ratio was 11/11 in the PQ-preserved travoprost group vs 16/6 in the BAK-preserved latanoprost group (p = 0.12) (Table 1). It is well known that females are more prone to dry eyes [55]. Considering the female dominance in the BAK-preserved latanoprost group in the present study, dry eye parameters, which were already expected to be worse in BAK-preserved latanoprost users, should have been even more impaired.
The following are the limitations of the present study. Relatively few subjects were included in the analysis. The gender distribution inequality has a negative impact on the reliability of the study even statistically insignificant. Similarly, the difference in Schirmer test score at initial examination has a negative effect. If other dry eye diagnostic methods, such as meniscometry, tear osmolarity, tear film interferometry, and impression cytology could be used to evaluate the presence and severity of DED, more accurate results would be obtained. Eye drops belonging to the same group but containing two different prostaglandin analogs were compared in the study because there was no available product with the same active ingredient in our country during the study. If drugs with the same active ingredient but containing different preservatives were compared, and even if a preservative-free anti-glaucoma medication could be added to the comparison, more accurate results could be achieved.
In conclusion, the harmful effects of BAK on the ocular surface are well known and have been demonstrated in various clinical and experimental studies in the literature. However, PQ produced as an alternative molecule to BAK is not completely safe and is as harmful to the ocular surface as BAK.
Data availability
Data are available with the corresponding author on request.
Abbreviations
- IOP:
-
Intraocular pressure
- DED:
-
Dry eye disease
- BAK:
-
Benzalkonium chloride
- PQ:
-
Polyquad
- POAG:
-
Primary open-angle glaucoma
- OHT:
-
Ocular hypertension (OHT)
- RNFL:
-
Retinal nerve fiber layer
- CCT:
-
Central corneal thickness
- OCT:
-
Optical coherence tomography
- OSDI:
-
Ocular surface disease index
- TBUT:
-
Tear film breakup time
- BCVA:
-
Best corrected visual acuity
- NF-κB:
-
Nuclear factor-kappa B
References
Casson RJ, Chidlow G, Wood JP, Crowston JG, Goldberg I (2012) Definition of glaucoma: clinical and experimental concepts. Clin Exp Ophthalmol 40(4):341–349
Toris CB, Zhan G, Fan S, Dickerson JE, Landry TA, Bergamini MV, Camras CB (2007) Effects of travoprost on aqueous humor dynamics in patients with elevated intraocular pressure. J Glaucoma 16(2):189–195
Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, Kim T, Mehta JS, Messmer EM, Pepose JS et al (2017) TFOS DEWS II iatrogenic report. Ocul Surf 15(3):511–538
Orozco Garcia A, Giorgi-Sandoval LA, Paczka JA, Garcia y Otero Sánchez SA, Valencia-Paredes D, Ponce-Horta AM, Rueda D, Vazquez IF (2020) Dry eye disease prevalence exponentially increases with age in patients under topical glaucoma treatment. Invest Ophthalmol Vis Sci 61(7):335–335
Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC (2010) Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea 29(6):618–621
Leung EW, Medeiros FA, Weinreb RN (2008) Prevalence of ocular surface disease in glaucoma patients. J Glaucoma 17(5):350–355
Erb C, Gast U, Schremmer D (2008) German register for glaucoma patients with dry eye: I: basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol 246(11):1593–1601
Camp A, Wellik SR, Tzu JH, Feuer W, Arheart KL, Sastry A, Galor A (2015) Dry eye specific quality of life in veterans using glaucoma drops. Cont Lens Anterior Eye 38(3):220–225
Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J et al (2017) TFOS DEWS II epidemiology report. Ocul Surf 15(3):334–365
Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V et al (2017) TFOS DEWS II pathophysiology report. Ocul Surf 15(3):438–510
Pisella PJ, Pouliquen P, Baudouin C (2002) Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol 86(4):418–423
Kahook MY (2009) Preservatives in topical ophthalmic medications: historical and clinical perspectives AU—Freeman, P David. Exp Rev Ophthalmol 4(1):59–64
Rosin LM, Bell NP (2013) Preservative toxicity in glaucoma medication: clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin Ophthalmol 7:2131–2135
Baudouin C (2008) Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol 86(7):716–726
Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F (2010) Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res 29(4):312–334
Kim JH, Kim EJ, Kim YH, Kim YI, Lee SH, Jung JC, Lee KW, Park YJ (2015) In vivo effects of preservative-free and preserved prostaglandin analogs: mouse ocular surface study. Korean J Ophthalmol 29(4):270–279
Steven DW, Alaghband P, Lim KS (2018) Preservatives in glaucoma medication. Br J Ophthalmol 102(11):1497–1503
Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T (2007) Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol 17(3):341–349
Janulevičienė I, Derkač I, Grybauskiene L, Paulauskaitė R, Gromnickaite R, Kuzmienė L (2012) Effects of preservative-free taflup rost on tear film osmolarity, tolerability, and intraocular pressure in previously treated patients with open-angle glaucoma. Clin Ophthalmol 6:103–109
Uusitalo H, Chen E, Pfeiffer N, Brignole-Baudouin F, Kaarniranta K, Leino M, Puska P, Palmgren E, Hamacher T, Hofmann G et al (2010) Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol 88(3):329–336
Stewart WC, Stewart JA, Nelson LA (2011) Ocular surface disease in patients with ocular hypertension and glaucoma. Curr Eye Res 36(5):391–398
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL (2000) Reliability and validity of the ocular surface disease index. Arch Ophthalmol 118(5):615–621
Whitcher JP, Shiboski CH, Shiboski SC, Heidenreich AM, Kitagawa K, Zhang S, Hamann S, Larkin G, McNamara NA, Greenspan JS et al (2010) A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry. Am J Ophthalmol 149(3):405–415
Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C (2015) Patient satisfaction with glaucoma therapy: Reality or myth? Clin Ophthalmol 9:785–793
Garcia-Feijoo J, Sampaolesi JR (2012) A multicenter evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol 6:441–446
Yee RW (2007) The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: a review. Curr Opin Ophthalmol 18(2):134–139
Baudouin C, Pisella PJ, Fillacier K, Goldschild M, Becquet F, De Saint JM, Béchetoille A (1999) Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology 106(3):556–563
Kobayashi H, Kobayashi K (2011) A correlation between latanoprost-induced conjunctival hyperemia and intraocular pressure-lowering effect. J Glaucoma 20(1):3–6
Skalicky SE, Goldberg I, McCluskey P (2012) Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol 153(1):1-9.e2
Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B (2006) Patient-reported behavior and problems in using glaucoma medications. Ophthalmology 113(3):431–436
Batra R, Tailor R, Mohamed S (2014) Ocular surface disease exacerbated glaucoma: optimizing the ocular surface improves intraocular pressure control. J Glaucoma 23(1):56–60
Mylla Boso AL, Gasperi E, Fernandes L, Costa VP, Alves M (2020) Impact of ocular surface disease treatment in patients with glaucoma. Clin Ophthalmol 14:103–111
Mastropasqua R, Fasanella V, Brescia L, Oddone F, Mariotti C, Di Staso S, Agnifili L (2017) In vivo confocal imaging of the conjunctiva as a predictive tool for the glaucoma filtration surgery outcome. Invest Ophthalmol Vis Sci 58(6):114–120
Aihara M, Otani S, Kozaki J, Unoki K, Takeuchi M, Minami K, Miyata K (2012) Long-term effect of BAK-free travoprost on ocular surface and intraocular pressure in glaucoma patients after transition from latanoprost. J Glaucoma 21(1):60–64
Noecker RJ, Herrygers LA, Anwaruddin R (2004) Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea 23(5):490–496
Horsley MB, Kahook MY (2009) Effects of prostaglandin analog therapy on the ocular surface of glaucoma patients. Clin Ophthalmol 3:291–295
Mundorf T, Wilcox KA, Ousler GW 3rd, Welch D, Abelson MB (2003) Evaluation of the comfort of Alphagan P compared with Alphagan in irritated eyes. Adv Ther 20(6):329–336
Ammar DA, Noecker RJ, Kahook MY (2010) Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther 27(11):837–845
Paimela T, Ryhänen T, Kauppinen A, Marttila L, Salminen A, Kaarniranta K (2012) The preservative polyquaternium-1 increases cytoxicity and NF-kappaB linked inflammation in human corneal epithelial cells. Mol Vis 18:1189–1196
Walsh K, Jones L (2019) The use of preservatives in dry eye drops. Clin Ophthalmol 13:1409–1425
Codling CE, Hann AC, Maillard JY, Russell AD (2005) An investigation into the antimicrobial mechanisms of action of two contact lens biocides using electron microscopy. Cont Lens Anterior Eye 28(4):163–168
Rolando M, Crider JY, Kahook MY (2011) Ophthalmic preservatives: focus on polyquaternium-1. Expert Opin Drug Deliv 8(11):1425–1438
Brignole-Baudouin F, Riancho L, Liang H, Baudouin C (2011) Comparative in vitro toxicology study of travoprost polyquad-preserved, travoprost BAK-preserved, and latanoprost BAK-preserved ophthalmic solutions on human conjunctival epithelial cells. Curr Eye Res 36(11):979–988
Brignole-Baudouin F, Riancho L, Liang H, Nakib Z, Baudouin C (2011) In vitro comparative toxicology of polyquad-preserved and benzalkonium chloride-preserved travoprost/timolol fixed combination and latanoprost/timolol fixed combination. J Ocul Pharmacol Ther 27(3):273–280
Labbé A, Pauly A, Liang H, Brignole-Baudouin F, Martin C, Warnet JM, Baudouin C (2006) Comparison of toxicological profiles of benzalkonium chloride and polyquaternium-1: an experimental study. J Ocul Pharmacol Ther 22(4):267–278
Lee HJ, Jun RM, Cho MS, Choi KR (2015) Comparison of the ocular surface changes following the use of two different prostaglandin F2α analogues containing benzalkonium chloride or polyquad in rabbit eyes. Cutan Ocul Toxicol 34(3):195–202
Liang H, Brignole-Baudouin F, Pauly A, Riancho L, Baudouin C (2011) Polyquad-preserved travoprost/timolol, benzalkonium chloride (BAK)-preserved travoprost/timolol, and latanoprost/timolol in fixed combinations: a rabbit ocular surface study. Adv Ther 28(4):311–325
Rossi GC, Scudeller L, Rolle T, Pasinetti GM, Bianchi PE (2015) From benzalkonium chloride-preserved Latanoprost to Polyquad-preserved Travoprost: a 6-month study on ocular surface safety and tolerability. Expert Opin Drug Saf 14(5):619–623
Lopes JF, Hubatsch DA, Amaris P (2015) Effect of benzalkonium chloride-free travoprost on intraocular pressure and ocular surface symptoms in patients with glaucoma previously on latanoprost: an open-label study. BMC Ophthalmol 15:166
Marsovszky L, Resch MD, Visontai Z, Németh J (2014) Confocal microscopy of epithelial and langerhans cells of the cornea in patients using travoprost drops containing two different preservatives. Pathol Oncol Res 20(3):741–746
Choy CK, Cho P, Boost MV (2012) Cytotoxicity and effects on metabolism of contact lens care solutions on human corneal epithelium cells. Clin Exp Optom 95(2):198–206
Miyashiro MJ, Lo SC, Stewart JA, Stewart WC (2010) Efficacy, safety, and tolerability of travoprost 0.004% BAK-free versus prior treatment with latanoprost 0.005% in Japanese patients. Clin Ophthalmol 4:1355–1359
Goldberg I, Graham SL, Crowston JG, d’Mellow G (2015) Clinical audit examining the impact of benzalkonium chloride-free anti-glaucoma medications on patients with symptoms of ocular surface disease. Clin Exp Ophthalmol 43(3):214–220
El Hajj Moussa WG, Farhat RG, Nehme JC, Sahyoun MA, Schakal AR, Jalkh AE, Abi Karam MP, Azar GG (2018) Comparison of efficacy and ocular surface disease index score between bimatoprost, latanoprost, travoprost, and tafluprost in glaucoma patients. J Ophthalmol 2018:1319628
Sullivan DA, Rocha EM, Aragona P, Clayton JA, Ding J, Golebiowski B, Hampel U, McDermott AM, Schaumberg DA, Srinivasan S et al (2017) TFOS DEWS II sex, gender, and hormones report. Ocul Surf 15(3):284–333
Acknowledgements
All authors attest that they meet the current ICMJE criteria for Authorship.
Funding
No funding or grant support.
Author information
Authors and Affiliations
Contributions
OEM was involved in concept design, data collection, analysis, drafting manuscript, and final approval; KD was involved in analysis and final approval; TP was involved in data collection and final approval; MG was involved in analysis and final approval.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Ethical approval
Approval was obtained from the ethics committee of Firat University of Medical Sciences (Date:09/20/2018 No:15/01). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muz, O.E., Dagdelen, K., Pirdal, T. et al. Comparison of BAK-preserved latanoprost and polyquad-preserved travoprost on ocular surface parameters in patients with glaucoma and ocular hypertension. Int Ophthalmol 41, 3825–3835 (2021). https://doi.org/10.1007/s10792-021-01947-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01947-2