Abstract
Purpose
To investigate refractive and visual outcomes as well as rotational stability following implantation of Eyecryl phakic toric intraocular lens (pIOL) for moderate-to-high myopic astigmatism.
Methods
The efficacy, safety, predictability, stability, and adverse events of Eyecryl toric pIOL were evaluated in patients with spherical refraction from − 4.50 to − 18.50 diopters (D) and cylindrical refraction from − 0.50 to − 5.50 D.
Results
This study included 60 eyes of 31 patients. The mean manifest refraction spherical equivalent (MRSE) dropped from − 10.45 ± 2.74 D preoperatively to − 0.34 ± 0.51 D and − 0.40 ± 0.56 D at 6 and 12 months postoperatively, respectively. There was an 81% decrease in astigmatism after surgery. The safety and efficacy of indices were 1.36 ± 0.43 and 1.20 ± 0 .32. At the final follow-up, the rate of eyes within ± 1.00 D and ± 0.50 D of the desired MRSE were 85% and 68.33%, respectively. Vision-threatening complications were not observed during the follow-up.
Conclusions
The implantation of pIOL was effective, safe, and predictable in patients with moderate-to-high myopic astigmatism during 1-year follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical correction of moderate-to-high myopia and astigmatism includes laser refractive surgery and toric phakic intraocular lens (pIOL) implantation. Toric pIOLs are superior to laser refractive surgery for patients with high myopia and astigmatism as they can correct more cylindrical values and induce less corneal aberrations [1,2,3]. Toric pIOLs have anterior (AC) and posterior chamber (PC) models. Due to concerns regarding progressive endothelial loss with AC models, PC pIOLs have gained in popularity [4].
Clinical outcomes of a PC toric pIOL model of intraocular collamer lens (ICL) (Staar Surgical Co.) showed its safety, efficacy, and predictability for the correction of myopic astigmatism [5,6,7,8]. In addition to ICL, there is another PC toric pIOL, Eyecryl phakic toric IOL (Biotech Vision Care, Ahmedabad, India), which is also a foldable and hydrophilic PC pIOL, and is implanted in the ciliary sulcus similar with ICL.
Early refractive results of Eyecryl toric pIOL presented similar efficacy and predictability compared to other toric ICL models [9, 10]. However, there is no study investigating its refractive results and rational stability after 1-year follow-up.
The aim of this study was to evaluate the safety, efficacy, predictability, and rational stability of Eyecryl phakic toric IOL 1 year after the implantation.
Methods
This study adhered to the tenets of the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Taksim Training and Research Hospital. The medical record of patients who had implantation of Eyecryl Phakic Toric IOL for the correction of moderate-to-high myopic astigmatism at Beyoglu Eye Training and Research Hospital was reviewed retrospectively. Patients who had at least 1 year of follow-up were included in the study.
All participants underwent a thorough ophthalmologic examination that included uncorrected (UDVA) and best corrected visual acuity (BCVA) measurement, cycloplegic refraction, slit lamp evaluation, intraocular pressure (IOP) measurement using a Goldmann applanation tonometer, posterior segment assessment through a dilated fundus, Sirius (Costruzione Strumenti Oftalmici, Italy) corneal topography, endothelial cell density (ECD) (CEM 530; Nidek Co. Ltd., Aichi, Japan), and postoperative pIOL vault was measured with a Visante OCT (Carl Zeiss Meditec AG, Jena, Germany). A dilated anterior segment photography was performed postoperatively at each follow-up. The photography was then transferred to the Goniotrans, a free standalone application (available at https://www.goniotrans.com/), to determine the rotational stability of the lens (Fig. 1). A free application named (AstigMATIC) was used for vector analysis [11]. Patients were seen postoperatively at 1 week, and at 1, 3, 6, and 12 months.
Inclusion criteria were: stable refraction for a minimum of 1 year before the pIOL implantation, minimum age of 21 years old, an ECD > 2000 cells/mm2, and an anterior chamber depth (ACD) ≥ 3 mm measured from the corneal endothelium to the anterior surface of the lens. Patients who had an ocular pathology, such as cataract, glaucoma, previous ocular surgery, or keratoconus, were excluded from the study.
Eyecryl phakic toric IOL (Biotech Vision Care, Ahmedabad, India) is a foldable, hydrophilic acrylic, plate-haptic posterior chamber pIOL.
IOL power calculation was performed in software provided by the manufacturer based on manifest refraction and keratometry. The size of toric pIOL was determined by horizontal white-to-white (WTW) distance and ACD measured with Sirius and Visante OCT, respectively.
Surgical procedure
All cases were performed by the same surgeon (AA) using sub-tenon anesthesia. Before the operation, the 0- and 180-horizontal axis was marked manually at the slit lamp to prevent the effect of potential cyclotorsion. At the beginning of the operation, a Mendez ring was placed on the eye to mark the desired implantation axis. Mydriasis was achieved with preoperative topical cyclopentolate and phenylephrine drops.
After creating paracentesis, sodium hyaluronate 1% (Provisc; Alcon Inc., Ft. Worth, TX, USA) was injected in the anterior chamber. The toric pIOL was inserted in the anterior chamber using the injector system through a temporal corneal incision of 2.75 mm. The haptics of pIOL was cautiously tucked under the iris, and the pIOL was rotated until the markers of pIOL were matched with the markers of the implantation axis. Viscoelastic material was removed by washing out with a buffered salt solution, and corneal incisions were hydrated.
Statistical analysis
Statistical analysis was performed using SPSS for Windows (version 21.0, IBM Corp.). The estimation of sample size was based on the number of eyes required to a mean decrease of 4.3% ± 11.5% after 12 months in ECD to achieve 80% power with a 5% level of significance. Accordingly, a total of 57 eyes was required to conclude the study. The Shapiro–Wilk test was used to evaluate the distribution of parameters. The results were described as mean ± standard deviation.
Results
Sixty eyes of 31 patients (11 males [35.4%], 20 females [64.5%]) were included in the study. Preoperative data of patients are provided in Table 1.
Refractive outcomes
The improvement in the manifest spherical refraction equivalent (MRSE) and its stability are shown in Table 2. MRSEs between each pair of follow-ups were compared, and no statistically significant differences were found except for MRSE between 3 and 12 months (Wilcoxon test; P = 0.04). At 12 months postoperatively, all eyes except one eye were within ± 2.00 D of the desired MRSE. The number of eyes within ± 1.00 D and ± 0.50 D were 51 (85%) and 41 (68.33%), respectively.
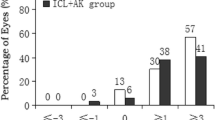
The change in manifest cylindrical refraction over time is shown in Table 2. When the mean cylindrical refraction at the last visit was compared to the preoperative mean, we detected an 81% decrease in astigmatism. The distribution of refractive astigmatism preoperatively and at 12 months postoperatively is illustrated in Fig. 2. All except two eyes had ≤ 1.00 D of astigmatism, and 73.3% of eyes had ≤ 0.50 D of astigmatism at the last visit.
Vector analysis was performed using the Alpins method. Figure 3 shows a vector analysis of refractive astigmatism of the patients at the last visit. The vector mean of target induced astigmatism (TIA) and surgically induced astigmatism (SIA) were 0.99 D at 0° and 0.73 D at 178°, respectively. The difference vector (DV), which is the difference between TIA and SIA, was 0.26 D at 4°. The correction index (CI), which is calculated by dividing SIA by TIA, was 0.89 (CI < 1.0 means under-correction, CI > 1.0 means overcorrection).
Vector analysis of refractive astigmatism of patients at 12 months after surgery. The analysis was performed by the Alpins method and comprised different vectors, including target induced astigmatism (a), surgically induced astigmatism (b), difference vector (c) (which is the difference between target induced astigmatism and surgically induced astigmatism), and correction index (d) (which is calculated by dividing surgically induced astigmatism by target induced astigmatism)
Visual acuity
The logMAR UDVA was improved from 1.94 ± 0.18 preoperatively to 0.18 ± 0.14, 0.19 ± 0.13, 0.17 ± 0.13, and 0.18 ± 0.13 at 1, 3, 6, and 12 months postoperatively, respectively. ANOVA analysis for postoperative values found that P = 0.27.
The number of eyes with a UDVA of ≤ 0.0 logMAR (≥ 20/20 Snellen) at 12 months (nine eyes; 15.0%) was equal to the number of eyes with a preoperative BCVA of ≤ 0.0 logMAR (≥ 20/20 Snellen) (nine eyes; 15.0%). The number of eyes with a UDVA of ≤ 0.3 logMAR (≥ 20/40 Snellen) at 12 months (51 eyes; 85.0%) was equal to the number of eyes with a preoperative BCVA of ≤ 0.3 logMAR (≥ 20/40 Snellen) (51 eyes; 85.0%). The efficacy index (postoperative UDVA/preoperative BCVA) was 1.20 ± 0.40, 1.16 ± 0.31, 1.22 ± 0.35, and 1.20 ± 0.32, at 1, 3, 6, and 12 months postoperatively, respectively.
The logMAR BCVA was improved from 0.25 ± 0.21 preoperatively to 0.13 ± 0.20, 0.14 ± 0.21, 0.13 ± 0.20, and 0.14 ± 0.20 at 1, 3, 6, and 12 months postoperatively, respectively. At the end of the study, all eyes except two eyes had a BCVA of ≤ 0.5 logMAR (≥ 20/63 Snellen), 88.3% of eyes had a BCVA of ≤ 0.3 logMAR (≥ 20/40 Snellen). Additionally, the proportion of eyes with a BCVA of ≤ 0.0 logMAR (≥ 20/20 Snellen) improved from 15% before surgery to 43.3% at 12 months. The safety index (postoperative BCVA/preoperative BCVA) was 1.39 ± 0.41, 1.33 ± 0.36, 1.37 ± 0.32, and 1.36 ± 0.43 at 1, 3, 6, and 12 months postoperatively, respectively.
Figure 4 shows the gain and loss lines of BCVA at 12 months after surgery. While only one (1.8%) eye had lost one line, loss of two or more lines was not observed during the follow-ups.
Rotational stability
The mean absolute rotation of lenses was 2.56° ± 2.25°, 3.61° ± 3.54°, 3.55° ± 3.64°, 3.25° ± 3.64°, and 3.51° ± 3.30° at 1 day and 1, 3, 6, and 12 months postoperatively, respectively. The absolute rotation in axis orientation between follow-ups is given in Table 3. Accordingly, ≤ 90% of lenses had a misalignment of ≤ 5° between all follow-ups, and the mean value of the absolute axis orientation error was < 3° for all visit intervals.
Endothelial cell density
Table 2 shows the change in the mean ECD over time. The repeated measures ANOVA for ECD returned a P-value of 0.223, confirming that there was no statistically significant difference from the preoperative baseline to the last visit.
Vault
At the 1-, 3-, 6-, and 12-month follow-ups, the mean vault of the lens was 561 ± 190 μm (range 205–940 μm), 578 ± 176 μm (range 180–920 μm), 564 ± 167 μm (range 220–960 μm), and 539 ± 169 μm (range 220–940 μm), respectively. The change in the vault was not statistically significant (P = 0.07, the repeated measures ANOVA).
Intraocular pressure
Table 2 shows the change in the mean IOP over time. There were no differences in IOP from the preoperative visit to the last visit (P = 0.24).
Adverse events
The pupillary block was seen in one eye (1.6%) and was treated successfully with laser iridectomy 1 day after surgery. Two eyes (3.3%) of two patients developed asymptomatic anterior subcapsular lens opacities. Since these patients showed no change in BCVA during the follow-up, pIOLs were not removed. Glaucoma was seen in one patient at 1 month after the surgery. The patient was diagnosed with steroid-induced glaucoma, and the IOP was controlled with antiglaucoma eye drops. No vision-threatening complications (i.e., retinal detachment) were observed over the course of the 1-year follow-up.
Discussion
In the current study, the visual and refractive outcomes and rotational stability of a new toric pIOL were analyzed in patients with moderate-to-high myopic astigmatism. We aimed to determine the predictability, stability, and safety of the Eyecryl phakic toric IOL during 1 year of follow-up.
In our study, the mean MRSE improved from − 10.55 ± 2.97 D preoperatively to − 0.25 ± 0.69 D, − 0.31 ± 0.49 D, − 0.38 ± 0.77 D, and − 0.43 ± 0.59 D at 1, 3, 6, and 12 months postoperatively, respectively. The correlation between the attempted and achieved MRSE was high (r2 = 0.93). At the last visit, all eyes except one eye were within ± 2.00 D of the desired MRSE, and the number of eyes within ± 1.00 D and ± 0.50 D were 51 (85%) and 41 (68.33%), respectively. The FDA toric ICL clinical study reported an improvement in the MRSE from − 9.36 ± 2.66 D before surgery to 0.05 ± 0.46 D at 1 year after surgery, with 76.9% of eyes within ± 0.50 D and 97.3% within ± 1.00 D [5]. Similar to the FDA toric ICL study, Alfonso et al. also found an improvement in MRSE; from − 6.16 ± 2.99 D before surgery to − 0.04 ± 0.24 D at 1 year after surgery, with 94.5% of eyes within ± 0.50 D and 100% within ± 1.00 D [6]. Both mentioned studies reported slightly better outcomes of postoperative MRSE than those found in our study.
The manifest cylindrical refraction decreased from − 1.95 ± 0.96 D preoperatively to − 0.23 ± 0.55 D, − 0.25 ± 0.45 D, − 0.32 ± 0.4 5 D, and − 0.37 ± 0.51 D 1, 3, 6, and 12 months postoperatively, respectively. An 81% decrease in the manifest astigmatism was achieved after 1 year. Similarly, the FDA toric ICL clinical study found a 73.6% decrease in manifest astigmatism and residual manifest astigmatism of − 0.53 ± 0.48 D after 1 year [5]. Schallhorn et al. [12] also stated − 0.58 ± 0.31 D of manifest astigmatism at 1 year after toric ICL implantation.
To determine more accurately, the impact of the surgery on the refractive astigmatism of patients, vector analysis using the Alpins method was performed. A CI of 0.89 revealed a slight under-correction of refractive astigmatism. Lee et al. [13] also reported a similar CI (0.91) for V4c toric ICL in their study. Similar results for V4c and V4 toric ICLs were found by Hyun et al. [14].
In our study, the visual outcomes were satisfactory in terms of the efficacy index and safety index. The efficacy index was 1.20 ± 0.32; the safety index was 1.36 ± 0.43 at 12 months postoperatively. By the last follow-up, while only one eye (1.6%) had lost one line of BCVA, 46.5% of eyes had gained at least one line of BCVA. The proportion of eyes with a UDVA of ≤ 0.0 logMAR (≥ 20/20 Snellen) and ≤ 0.3 logMAR (≥ 20/40 Snellen) were 15.0% and 85.0% after 12 months. The FDA toric ICL study reported that 7.5% of eyes had lost at least one line, and 76.4% of eyes had gained at least one line after 12 months. They also reported the proportion of eyes with a UDVA of ≤ 0.0 logMAR and ≤ 0.3 logMAR were found to be 83% and 96%, respectively. In comparison with patients’ visual acuity and MRSE at baseline in the FDA toric study and the study by Alfonso et al., our patients had higher MRSE and lowered visual acuity values [5, 6]. One of the inclusion criteria for the FDA toric study was the requirement of a BCVA of ≤ 0.3 logMAR; however, there were nine eyes (15.0%) with a BCVA of > 0.3 logMAR in our study [5]. Moreover, our study included 30 eyes (50.0%) with preoperative MRSE of > − 10.00 D, whereas the rate of patients with preoperative MRSE of > − 10.00 D was 36.7% in the FDA toric study.
Similar to our study, Alfonso et al. [6] reported that one eye had lost two lines, and more than 50% of eyes gained at least one line of BCVA with an efficacy index of 1.08 and the safety index of 1.18. Unlike our results, these authors found that 60.0% of eyes had a UDVA of ≤ 0.0 logMAR, and 94.5% of eyes had a UDVA of ≤ 0.3 logMAR after 12 months. In our study, patients had a lower preoperative UDVA and BCVA compared to those in the study by Alfonso et al. (0.01 ± 0.01, 0.61 ± 0.19 vs. 0.11 ± 0.11, 0.74 ± 0.18, respectively) [6].
To achieve satisfactory refractive and visual outcomes after a toric IOL, analysis of the rotational stability of a toric IOL is crucial. Hence, the alignment axis of the Eyecryl toric pIOL was measured at each follow-up, and the absolute rotation was calculated. We found that the mean absolute rotation was 3.25° ± 3.64° and 3.51° ± 3.30° at 6 and 12 months after surgery. More than 90% of lenses rotated ≤ 5° between all follow-ups. Rotational stability of the Eyecryl toric pIOL showed comparable results to those found in other studies [13,14,15].
The most concern regarding PC pIOL implantation is anterior subcapsular cataract [16, 17]. In our study, although two eyes (3.6%) developed anterior subcapsular opacities, the patients were asymptomatic. In the FDA toric ICL study, anterior subcapsular opacities were seen in six eyes (2.9%). Of these patients, five cases were asymptomatic, and only one case underwent ICL removal due to symptomatic anterior subcapsular cataract [5]. Alfonso et al. [6] reported no eyes with anterior subcapsular opacities 1 year after ICL implantation.
In our study, pupillary block was seen in one eye 1 day after surgery. The patient had presented with pain. On examination, the painful eye had a shallow anterior chamber and increased IOP. Following laser iridectomy, the pain was relieved, and IOP had returned to normal values. Although pupillary block is observed as the complication of PC pIOLs without a central port, it has also been reported after PC pIOLs with a central port [18, 19].
Limitations of the current study included a relatively low patient population and retrospective design. Also, a 1 year of follow-up might not be sufficient to evaluate the long-term complications of a pIOL. Nevertheless, to our knowledge, the current study was the first to evaluate refractive and visual outcomes of the Eyecryl phakic toric IOL as well as its rotational stability for 1 year after implantation.
In conclusion, the Eyecryl toric pIOL demonstrated relatively good visual acuity and refractive results regarding the efficacy index and the safety index. Its rotational stability is similar to other PC pIOLs. However, prospective controlled studies with a longer follow-up and larger population are now needed to thoroughly investigate and compare the results of the Eyecryl toric pIOL.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Dick HB, Alio J, Bianchetti M, Budo C, Christiaans BJ, El-Danasoury MA, Guell JL, Krumeich J, Landesz M, Loureiro F, Luyten GPM, Marinho A, Rahhal MS, Schwenn O, Spirig R, Thomann U, Venter J (2003) Toric phakic intraocular lens European multicenter study. Ophthalmology 110:150–162
Pesudovs K (2005) Wavefront aberration outcomes of LASIK for high myopia and high hyperopia. J Refract Surg 21:S508–S512
Keir NJ, Simpson T, Jones LW, Fonn D (2009) Wavefront-guided LASIK for myopia: effect on visual acuity, contrast sensitivity, and higher order aberrations. J Refract Surg 25:524–533
Saxena R, Boekhoorn SS, Mulder PGH, Noordzij B, van Rij G, Luyten GPM (2008) Long-term follow-up of endothelial cell change after Artisan phakic intraocular lens implantation. Ophthalmology 115:608–613
Sanders DR, Schneider D, Martin R, Brown D, Dulaney D, Vukich J, Slade S, Schallhorn S (2007) Toric implantable collamer lens for moderate to high myopic astigmatism. Ophthalmology 114:54–61
Alfonso JF, Fernandez-Vega L, Fernandes P, Gonzalez Meijome JM, Montes-Mico R (2010) Collagen copolymer toric posterior chamber phakic intraocular lens for myopic astigmatism: one year follow-up. J Cataract Refract Surg 36:568–576
Kamiya K, Shimizu K, Kobashi H, Igarashi A, Komatsu M (2013) Three-year follow-up of posterior chamber toric phakic intraocular lens implantation for moderate to high myopic astigmatism. PLoS One 8(2):e56453
Mertens E (2011) Toric phakic implantable collamer lens for correction of astigmatism: 1-year outcomes. Clin Ophthalmol 5:369–375
Yaşa D, Köse B, Ağca A (2020) Rotational stability of a new posterior chamber toric phakic intraocular lens. J Ophthalmol 2020:7
Urdem U, Agca A (2019) Refractive results and endothelial cell density after Eyecryl phakic intraocular lens implantation. Beyoglu Eye J 4(1):17–22
Gauvin M, Wallerstein A (2018) AstigMATIC: an automatic tool for standard astigmatism vector analysis. BMC Ophthalmol 18(1):255
Schallhorn S, Tanzer D, Sanders DR, Sanders ML (2007) Randomized prospective comparison of visian toric implantable collamer lens and conventional photorefractive keratectomy for moderate to high myopic astigmatism. J Refract Surg 23:853–867
Lee H, Kang D, Choi J, Ha B, Kim E, Seo K, Kim T (2018) Rotational stability and visual outcomes of V4c toric phakic intraocular lenses. J Refract Surg 34:489–496. https://doi.org/10.3928/1081597X-20180521-01
Hyun J, Lim DH, Eo DR, Hwang S, Chung ES, Chung TY (2017) A comparison of visual outcome and rotational stability of two types of toric implantable collamer lenses (TICL): V4 versus V4c. PLoS One 12:e0183335
Hashemian SJ, Bigzadeh F, Foroutan A, Tajoddini S, Ghaempanah MJ, Jafari ME (2013) Outcomes and complications of implantable collamer lens and toric implantable collamer lens for the correction of high myopia with and without astigmatism (one year prospective study). Iranian J Ophthalmol 25(1):8–18
Jime´nez-Alfaro I, del Castillo JMB, Garcı´a-Feijoo´ J, de Bernabe´ JGG, de la Iglesia JMS (2001) Safety of posterior chamber phakic intraocular lenses for the correction of high myopia: anterior segment changes after posterior chamber phakic intraocular lens implantation. Ophthalmology. 108:90–99
Lackner B, Pieh S, Schmidinger G, Simader C, Franz C, Dejaco- Ruhswurm I, Skorpik C (2004) Long-term results of implantation of phakic posterior chamber intraocular lenses. J Cataract Refract Surg 30:2269–2276
Smallman DS, Probst L, Rafuse PE (2004) Pupillary block glaucoma secondary to posterior chamber phakic intraocular lens implantation for high myopia. J Cataract Refract Surg 30(4):905–907
Gonzalez-Lopez F, Bilbao-Calabuig R, Alen R, Mompean B (2017) Pupillary block glaucoma secondary to central port occlusion following insertion of a phakic implantable copolymer lens. J Cataract Refract Surg 43(11):1468–1470
Funding
No financial support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by BT and AA. The first draft of the manuscript was written by MES, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethical approval was received from the University of Health Sciences Turkey, Istanbul Training and Research Hospital Ethics Committee.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sucu, M.E., Agca, A. & Tulu, B. One-year follow-up of a new posterior chamber toric phakic intraocular lens implantation for moderate-to-high myopic astigmatism. Int Ophthalmol 41, 2941–2949 (2021). https://doi.org/10.1007/s10792-021-01853-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01853-7