Abstract
Purpose
To evaluate treatment outcomes and complications of intravitreal rituximab (IVR) monotherapy for eyes with vitreoretinal lymphoma (VRL).
Methods
Patients diagnosed with ‘isolated primary VRL’ or ‘VRL with remission of systemic disease’ and treated with IVR (1 mg/0.1 ml) between June 2014 and June 2019 were included in this retrospective, interventional case series. Injections were repeated at monthly intervals until complete resolution. All patients signed a written informed consent form. Institutional review board approval was obtained.
Results
Twelve eyes of 7 patients with VRL were treated with 77 IVR injections at mean 6.42 injections per eye (median = 5; range = 2–13) for complete resolution at mean 8.16 ± 4.62 months (median = 6.97 months; range = 1.97–14.33 months). Mean age at presentation was 53.3 years (median = 54 years; range = 34–74 years). Patients were co-managed with medical oncologist and periodically evaluated. Complications included anterior uveitis (n = 6), raised intraocular pressure (n = 3), posterior synechiae (n = 2), vitreous haemorrhage (n = 1), pre-retinal haemorrhage (n = 1), retinal detachment (n = 1), posterior subcapsular cataract (n = 2) and sectoral iris atrophy (n = 1). Recurrences were seen in 3 eyes (25%), which eventually achieved complete resolution with treatment. None of the patients had systemic involvement or death during follow-up. Mean follow-up was 18.73 ± 8.83 months (median = 21.60 months; range = 7.37–32.67 months).
Conclusion
Intravitreal rituximab monotherapy is effective in management of vitreoretinal lymphoma in patients with isolated ocular disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diagnosing intraocular lymphoma can be challenging because of its rarity and features mimicking intermediate or posterior uveitis, infectious retinitis, and other simulating conditions [1]. A high degree of clinical suspicion and confirmatory tissue diagnosis is essential for an accurate diagnosis [2]. Accurate diagnosis and staging of the disease are essential in choosing the appropriate treatment that may include systemic therapy with inherent adverse effects. The clinician needs to weigh the risk–benefit paradigm with the existing treatment protocols and his own experience with them. Local treatment modalities are gaining importance in treating eyes with the isolated ocular disease or in patients with primary central nervous system lymphoma (PCNSL) in whom CNS lesions have completely resolved since local therapy has a better safety profile, efficient vision preservation and local tumour control [3]. Among local therapy, intravitreal methotrexate has proven to be a good therapeutic agent as reported in many published reports [4,5,6]. However, more frequent injections and adverse effects like corneal epitheliopathy, cataract, hypotony are major drawbacks. Hence, intravitreal rituximab (IVR) has been tried in a few centres to bypass the issues related to methotrexate use.
Rituximab is a chimeric monoclonal antibody (mAb) that binds specifically to CD20, an antigen expressed by most human B lymphocytes. There are very few reports about IVR as primary monotherapy for eyes with VRL [7, 8]. However, most of these are either case reports or small case series, or have used rituximab as secondary/rescue therapy. Herein, we report our study evaluating treatment outcomes and complications of intravitreal rituximab (IVR) as monotherapy for eyes with VRL.
Materials and methods
This was a retrospective, interventional case series of patients diagnosed with VRL and treated with IVR at monthly intervals. Institution review board (IRB) approval was obtained. All patients signed an informed consent form and consented to the treatment after a detailed explanation of the risks and benefits of all the treatment options [intravitreal rituximab or intravitreal methotrexate or external beam radiation therapy (EBRT)] and agreed to the ‘off-label’ nature of the treatment. The study adhered to the tenets of the declaration of Helsinki. Patients with VRL diagnosed and treated with primary IVR between June 2014 and June 2019 were included. Our institutional protocol in dealing with patients suspected of VRL is as follows. A patient suspected of VR lymphoma is systemically investigated for CNS involvement with clinical evaluation, neuroimaging (MRI brain or PET-CT scan), and CSF tap for cytopathology, immunohistochemistry, and MYD88 L265P gene mutation testing. If a diagnosis of PCNSL can be made from the identification of lymphoma cells in CSF, and there is simultaneous ocular involvement, then the patient is spared from the need for invasive ocular tissue biopsy for diagnosis. After a tissue diagnosis and disease staging involving CNS are established, treatment is initiated by a medical oncologist with systemic chemotherapy ± EBRT. In cases of inadequate intraocular treatment response to systemic chemotherapy, intravitreal chemotherapy is considered. If the systemic evaluation is negative, then a tissue diagnosis is established by ocular oncologist with vitreous or retinal tissue biopsy (cytopathology, IHC, MYD88 L265P gene mutation testing). Patients are offered the following treatment options after a thorough explanation of the pros & cons of each option: (i) intravitreal chemotherapy with methotrexate/rituximab or (ii) ocular radiation or (iii) systemic chemotherapy (binocular involvement). The pros and cons of each treatment option are discussed with the patient before arriving at a preferential mode of treatment. A consensual decision involving the medical oncologist who plays an important role in detecting possible CNS involvement at baseline visit and then lifelong surveillance to detect CNS involvement is essential.
MYD88 L265P gene mutation testing was instituted in our centre in 2019 and was done in two cases. Hence, a definitive diagnosis was established in all cases before initiating treatment with at least two of the following three criteria: cytopathological identification of atypical lymphocytes by an experienced ocular pathologist, or immunohistochemistry (CD20 positive cells), or MYD88 L265P gene mutation testing. Treatment protocol required patients to follow-up for IVR injections on a monthly basis. IVR (1 mg/0.1 mL) injection was drawn from a 10-ml vial [Ristova®, Roche Products (India) Pvt. Ltd, Mumbai, India] and administered using a standardized procedure in the operating room under topical anaesthesia (Proparacaine 0.5%) with surgical sterile precautions. The patient was examined the next day for intraocular pressure (IOP) check, slit lamp examination, dilated fundus examination, and clinical findings were noted. Patients were reviewed one month later for a comprehensive eye examination for evaluation of response to treatment. Disease activity was determined by clinical signs and symptoms and augmented with fundus photography (comparison to the previous visit), fundus autofluorescence (FAF showing hyper AF), and OCT (for sub-RPE lesions, subtle macular oedema, intraretinal hyper-reflective foci, disruption of outer retinal layers). IVR injections were repeated if necessary. Complete resolution was defined as “absence of cells in vitreous cavity and resolution of previously documented retinal/subretinal/optic nerve infiltrates with no need for further consecutive injections”. ‘An increase in the vitreous cell count or progressive retinal/subretinal/optic nerve infiltration requiring further consecutive injections’ was defined as progressive disease. ‘Appearance of any new lesion after complete resolution of all lesions noted at presentation’ or ‘the recurrence of vitreous cells after complete resolution’, requiring further consecutive injections were considered as indicators of relapse [9]. The follow-up protocol at our centre is as follows: patients with complete regression are followed-up one monthly for 3 months, 2 monthly for 6 months, 3 monthly for life. The patients are co-managed by an ocular oncologist and medical oncologist. MRI (brain and orbits) is repeated every 3–6 months.
Patients who had primary VRL without systemic involvement, or those patients who received systemic chemotherapy for and were currently in remission systemically, with the ocular disease being the only focus of disease activity were included after evaluation by the medical oncologist. Patients who had active concurrent systemic disease were excluded. Patients with follow-up of fewer than 6 months were excluded. Patient data were reviewed for demographics, history of systemic lymphoma, best-corrected visual acuity (BCVA), clinical features, investigations, treatment details, complications, and outcomes. Treatment outcomes were measured in terms of complete/partial disease resolution, complications and BCVA. During the entire course of management, all patients were periodically reviewed and imaged by a medical oncologist for systemic spread of disease. Statistical analysis: Descriptive statistics like mean, standard deviation, and median were obtained for systemic and ocular factors defined on a continuous scale, while frequencies and percentages were obtained for categorical factors, i.e. gender. All the analyses were performed using SPSS ver 14.0 (IBM Corp., Armonk USA), and the statistical significance was tested at 5%.
Results
Baseline characteristics
Baseline clinical characteristics of patients are listed in Table 1. Ten (83%) eyes had primary VRL; 2 (17%) eyes had primary central nervous system lymphoma (PCSNL) with secondary ocular involvement. Five patients had bilateral involvement. At presentation, all 10 eyes with primary VRL were treatment- naive. One patient with PCNSL (Case # 4) had 6 cycles of systemic chemotherapy, whole brain irradiation, and right occipital lobotomy 5 years back. The mean age at presentation to our clinic was 53.25 ± 13.69 years (median = 53 years; range = 34–74 years). Patients were diagnosed and followed-up from June 2014 to June 2019 with a mean follow-up of 18.73 ± 8.83 months (median = 21.60 months; range = 7.37–32.67 months).
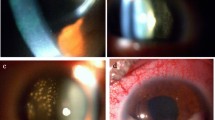
Presenting symptoms included diminution of vision (n = 9 eyes), floaters (n = 3 eyes), metamorphopsia (n = 2), pain (n = 1), redness (n = 1), and photophobia (n = 1), while one patient was asymptomatic. Clinical examination revealed anterior uveitis (n = 2), vitritis (n = 2), multiple, localized subretinal deposits (n = 7; Fig. 1), diffuse subretinal deposits (n = 1), mixed distribution of subretinal deposits (n = 1), ‘pseudo-viral retinitis’ like presentation (n = 1; Fig. 2), large (> 2 quadrant) subretinal deposits (n = 2; Fig. 3), and retinal pigment epithelium (RPE) alterations (n = 2). Optical coherence tomography (OCT) revealed subretinal deposits in patients with clinically subtle sub-RPE features (Fig. 3). All eyes had an established tissue diagnosis before treatment initiation. Vitreous biopsy was done for cytopathological examination in all 10 patients. MYD88 L265P gene mutation testing was done for 2 eyes (as this facility was started in 2019).
At baseline, colour fundus photograph shows vitreous haze with yellowish subretinal mass at macula (black arrow) and placoid lesions (black arrowheads) in the fundus mid-periphery a. Following treatment, lesions resolved completely resulting in subretinal gliosis b. At presentation, B scan ultrasound shows low reflective vitreous echoes with diffuse choroidal mass involving the macula (white arrow) c. Post-treatment OCT shows macular scar with complete regression of lymphoma d. Choroidal biopsy specimen shows large pleomorphic lymphoid cells with scant cytoplasm (asterisk) e (haematoxylin & eosin, 50X)
At presentation, subretinal lymphoma infiltrates (black arrowhead) with atypical ‘pseudo-viral retinitis’ like picture a. Significant regression of lymphoma after intravitreal rituximab injection b. Pre-injection OCT reveals subretinal fluid (black arrow), cystoid retinal thickening (white arrow) and sub-RPE lymphoma aggregates c. Post-injection OCT reveals resolution of subretinal fluid, macular oedema and sub-RPE lymphoma with severe retinal thinning (atrophy) d

source OCT shows typical sub-RPE localization of lymphoma with dome-shaped RPE elevation(white arrow) (c; ‘lumpy-bumpy tumour surface’)
At baseline, colour fundus photograph shows large, peripheral lymphoma (black arrow) with RPE tear (black arrowhead) a. Resolution of the lesions after multiple intravitreal rituximab injections (white arrowhead) b. Swept
IVR Therapy results
Seventy-seven IVR injections were administered with a mean of 6.42 injections per eye (median = 5; range:2–13) at mean 7.13 ± 5.07 months (median: 5.30 months). Although the established treatment protocol required monthly IVR injections, and despite the patients being counselled likewise, the mean inter-injection interval was 41.25 ± 17.99 days (median = 38.5 days; range: 21–124 days). Mean event-free interval, defined as the interval between last injection and last follow-up, was 11.15 ± 8.35 months (median = 9.43 months; range: 2.3–29.67 months). Complete resolution was observed in all 12 eyes. Mean logMAR BCVA at presentation was 0.71 ± 0.89 (range 0–2.30). Mean BCVA at the last visit was 0.81 ± 0.91 (range 0–2.70). Visual acuity improved in 1 eye, deteriorated in 5 eyes, and remained the same in 6 eyes, at the last follow-up. Causes of loss of vision included scarred CNVM (n = 3), extensive subretinal infiltrates involving the macula (n = 2), secondary glaucoma (n = 1), RPE atrophy involving the macula (n = 7), and macular oedema (n = 2). Recurrences were seen in 3 eyes (25%), which eventually achieved complete resolution with treatment. Among patients with recurrence, one had bilateral involvement (Case no.2), whereas the other had unilateral involvement (Case no.6). Case no. 2 required furthermore injections, while Case no. 6 needed more frequent injections at 3-week intervals. Both of them achieved complete resolution at the final follow-up. Ocular complications were seen in 8 of 12 eyes and are summarized in Table 2. Two patients (Case nos. 4 and 6) developed cystoid macular oedema (CME) after 3rd IVR and completion of IVR therapy, respectively. One eye was treated with topical Nepafenac 1% thrice daily (Nevanac®, Alcon Laboratories, Fort Worth, Texas, US) for 4 weeks, and the other received single intravitreal bevacizumab (1.25 mg/ml) with complete resolution of CME. None of the patients had systemic involvement till the last follow-up.
Discussion
In our single-centre study, VRL cases without active CNS/systemic involvement and treated only with IVR were included and followed-up over the last five years. The mean age at diagnosis was 63.5 years, which is similar to the range of 53.5–68 years in studies involving IVR treatment in the past. (7, 8, 10, 11) Female preponderance (71%) in our study also matches that reported by Larkin et al. with 55% female subjects [11]. In two small case series, all cases were females [8, 10].
In previous studies, IVR use showed a good therapeutic response from the first dose itself. A very high response rate is seen in our study, as reported in some other published studies [7, 8, 10]. However, Larkin et al. reported 8% primary non-responders [29]. In our study, complete resolution required a mean of 6.42 injections per eye (median = 5; range:2–13), whereas Larkin et al. and Hashida et al. reported fewer injections with mean 3 (range 1–10) and 4 injections, respectively [10, 11]. While we noticed 25% recurrences in our follow-up, Hashida et al. had a significantly higher recurrence rate of 55% [10]. We feel a longer follow-up of their cohort would have provided potentially different and more insightful results.
Complications in our series are listed in Table 2. Ocular hypertension and anterior uveitis were the predominant complications observed in our study, which is similar to that reported before [10]. In our series, anterior uveitis presented with fine keratic precipitates and sectoral iris atrophy. Posterior subcapsular cataract was seen in 2 eyes as a complication of uveitis. One eye with retinal detachment underwent vitrectomy with gas tamponade. Ocular hypertension was effectively treated by medical management. The survival rate in our study was 100%, which is similar to other studies [7, 8] except Larkin et al.’s study where the survival rate was found to be 76.5%, and this might be attributable to their longer follow-up [11]. Limitations of our study relate to its retrospective nature and small sample size. However, our study had uniform diagnostic criteria and a single treatment protocol.
Intraocular lymphoma is a malignant neoplasm derived from the monoclonal proliferation of B or T lymphocytes. Most intraocular lymphomas are derived from B cell [12]; T Cell origin lymphomas are very rare in the eye [13]. Treatment options include radiation therapy, systemic chemotherapy, intrathecal autologous stem-cell transplant, and ocular chemotherapy (intravitreal injection) [14,15,16]. The International Primary CNS Lymphoma Collaborative Group in 2011 recommended the guidelines for the treatment and follow-up of patients with PVRL with/without PCNSL [17]. In 2015, the European Association of neurooncologists laid the guidelines for the diagnosis and treatment of PCNSL [18]. The International Primary Central Nervous System Lymphoma Collaborative Group retrospectively reviewed 83 VRL patients without CNS involvement and found no difference in CNS relapse or survival between local ocular therapy and extensive systemic therapy and/or whole-brain RT [19]. Similar results were reported by another European multicentre study by Riemens et al. [20]. Studies have shown the efficacy and non-inferiority of local therapy compared with systemic chemotherapy [17] as there is no additional benefit from systemic chemotherapy and ocular recurrences have been reported which subsequently require intraocular chemotherapy. Likewise, in patients with contraindications for systemic chemotherapy or elderly patients with the relapsing intraocular disease, local treatment alone (intravitreal chemotherapy or ocular radiotherapy) is an acceptable and valid approach. Since intrathecal injection and high-dose systemic chemotherapy are only variably effective in curing VRL, intravitreal chemotherapy has a promising therapeutic role in patients with isolated vitreoretinal lymphoma.
Radiation therapy has been used as a treatment option in patients with binocular involvement. Although radiation dose up to 54 Gy has been used, this has been decreased as cases of radiation retinopathy have been reported [21]. Lately, 30–36 Gy have been used, but still, radiation retinopathy can develop at even levels of 20 Gy [22]. Stefanovic et al. reported favourable outcomes in PVRL patients treated with high-dose MTX, whole-brain RT, and ocular RT. However, this study had only six patients [23]. Besides, there are several CNS and intraocular complications related to radiation therapy that makes it a less preferred treatment modality [24]. The complication of whole-brain radiation often induces delayed neurotoxicity with a decrease in cognitive function, ataxia, and sometimes even death, whereas ocular complications include cataract, dry eye, and radiation retinopathy. Ocular recurrences following EBRT for the binocular disease have also been reported. If PVRL patients having concurrent CNS involvement failed systemic chemotherapy and were also unavailable to intraocular chemotherapy, whole brain, and eye radiotherapy may be added. Currently, whether to use intravitreal chemotherapy or ocular radiation as first-line therapy is still controversial. However, we expect this debate to further intensify with the improved life survival of patients with CNS lymphoma. Our study aims to add evidence for IVR as one of the options available in the ocular oncologists’ armamentarium in managing such patients rather than proving its superiority over other treatment options [25,26,27,28,29].
In conclusion, this study adds to the growing evidence for efficacy of intravitreal rituximab in achieving resolution of vitreoretinal lymphoma in cases where the disease is localised to the eye. Intravitreal rituximab is a viable option in the ocular oncologists’ armamentarium for PVRL treatment. However, large prospective, multicentre studies are required to validate these results.
Data availability
Not applicable.
Code availability
Not applicable.
References
Sagoo MS, Mehta H, Swampillai AJ, Cohen VM et al (2014) Primary intraocular lymphoma. Surv Ophthalmol 59(5):503–516
Akpek EK, Ahmed I, Hochberg FH et al (1999) Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmol 106(9):1805–1810
Coupland SE, Damato B (2008) Underst intraocular lymphomas Clin Exp Ophthalmol 36:564–578
Fishburne BC, Wilson DJ, Rosenbaum JT, Neuwelt EA (1997) Intravitreal methotrexate as an adjunctive treatment of intraocular lymphoma. Arch Ophthalmol 115:1152–1156
Smith JR, Rosenbaum JT, Wilson DJ et al (2002) Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmol 109:1709–1716
Frenkel S, Hendler K, Siegal T et al (2008) Intravitreal methotrexate for treating vitreoretinal lymphoma: 10 years of experience. Br J Ophthalmol 92:383–388
Kitzmann AS, Pulido JS, Mohney BG et al (2007) Intraocular use of rituximab. Eye 21(12):1524–1527
Ohguro N, Hashida N, Tano Y (2008) Effect of intravitreal rituximab injections in patients with recurrent ocular lesions associated with central nervous system lymphoma. Arch Ophthalmol 126(7):1002–1003
Abrey LE, Batchelor TT, Ferreri AJ et al (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23:5034–5043
Hashida N, Ohguro N, Nishida K (2012) Efficacy and complications of intravitreal rituximab injection for treating primary vitreoretinal lymphoma. Transl Vis Sci Technol 1(3):1
Larkin KL, Saboo US, Comer GM, Forooghian F, Mackensen F, Merrill P, Sen HN, Singh A, Essex RW, Lake S, Lim LL (2014) Use of intravitreal rituximab for treatment of vitreoretinal lymphoma. Br J Ophthalmol 98(1):99–103
Char DH, Ljung BM, Deschenes J et al (1988) Intraocular lymphoma: immunological and cytological analysis. Br J Ophthalmol 72:905–911
Calfa CJ, Lossos IS, Ruiz P, Davis JL (2007) Ocular involvement as the initial manifestation of T-cell chronic lymphocytic leukemia. Am J Ophthalmol 144:326–329
Vicuna KJ, Frenkel S, Siegal T et al (2008) Maculopathy in patients with primary CNS lymphoma treated with chemotherapy in conjunction with blood-brain barrier disruption. Br J Ophthalmol 92:231–235
Valluri S, Moorthy RS, Khan A, Rao NA (1995) Combination treatment of intraocular lymphoma. Retina 15:125–129
Wang JK, Yang CM, Lin CP et al (2006) An asian patient with intraocular lymphoma treated by intravitreal methotrexate. Jpn J Ophthalmol 50:474–478
Chan CC, Rubenstein JL, Coupland SE et al (2011) Primary vitreoretinal lymphoma: a report from an international primary central nervous system lymphoma collaborative group symposium. Oncol 16(11):1589
Hoang-Xuan K, Bessell E, Bromberg J et al (2015) European association for neuro-oncology Task Force on primary CNS lymphoma Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European association for neuro-oncology. Lancet Oncol 16(7):e322–e332
Grimm SA, Pulido JS, Jahnke K et al (2007) Primary intraocular lymphoma: an international primary central nervous system lymphoma collaborative group report. Ann Oncol 18:1851–1855
Riemens A, Bromberg J, Touitou V et al (2015) Treatment strategies in primary vitreoretinal lymphoma: a 17-center European collaborative study. JAMA Ophthalmol 133:191–197
Berenbom A, Davila RM, Lin HS, Harbour JW (2007) Treatment outcomes for primary intraocular lymphoma: implications for external beam radiotherapy. Eye 21(9):1198–1201
Kaushik M, Pulido JS, Schild SE, Stafford S (2012) Risk of radiation retinopathy in patients with orbital and ocular lymphoma. Int J Radiat Oncol Biol Phys 84:1145–1150
Stefanovic A, Davis J, Murray T et al (2010) Treatment of isolated primary intraocular lymphoma with high-dose methotrexate-based chemotherapy and binocular radiation therapy: a single institution experience. Br J Haematol 151:103–106
Margolis L, Fraser R, Lichter A et al (1980) The role of radiation therapy in the management of ocular reticulum cell sarcoma. Cancer 45:688–692
Pulido JS, Bakri SJ, Valyi-Nagy T, Shukla D (2007) Rituximab penetrates full-thickness retina in contrast to tissue plasminogen activator control. Retina 27:1071–1073
Akiyama H, Takase H, Kubo F et al (2016) High-dose methotrexate following intravitreal methotrexate administration in preventing central nervous system involvement of primary intraocular lymphoma. Cancer Sci 107(10):1458–1464
Kim H, Csaky KG, Chan CC et al (2006) The pharmacokinetics of rituximab following an intravitreal injection. Exp Eye Res 82:760–766
Cartron G, Watier H, Golay J, Solal-Celigny P (2004) From the bench to the bedside: ways to improve rituximab efficacy. Blood 104:2635–2642
Shields CL, Sioufi K, Mashayekhi A, Shields JA (2017) Intravitreal melphalan for treatment of primary vitreoretinal lymphoma. JAMA Ophthalmol 135(7):815
Funding
This study is not financially supported.
Author information
Authors and Affiliations
Contributions
Pukhraj Rishi was involved in conceptualization. Pukhraj Rishi performed methodology. Pukhraj Rishi, Pradeep T Manchegowda and Harshal P Gondhale contributed to formal analysis and investigation. Pradeep T Manchegowda prepared writing—original draft. Pukhraj Rishi, Pradeep T Manchegowda, Ekta Rishi, Kalpita Das, Subramanian Krishnakumar, Thirumalairaj Raja and Jyotirmay Biswas were all involved in writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval
The authors declare that they have no ethical conflicts to disclose.
Consent to participate
Informed consent was obtained from all study participants.
Consent for publication
Consent obtained from all co-authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rishi, P., Manchegowda, P.T., Gondhale, H.P. et al. Intravitreal rituximab monotherapy for management of eyes with vitreoretinal lymphoma: initial experience from India. Int Ophthalmol 41, 2495–2504 (2021). https://doi.org/10.1007/s10792-021-01805-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01805-1