Abstract
Purpose
Retinal detachment (RD) is a vision-threatening complication of open globe injuries (OGI). This study sought to assess clinical, radiographic, and intraoperative risk factors for RD after OGI. A secondary goal was to test the retinal detachment after open globe injury (RD-OGI) score.
Methods
Records of patients undergoing OGI repair at a single trauma center over 3 years were reviewed using a retrospective case series design. Eyes that were enucleated or lost to follow up within 30 days of OGI without evidence of RD were excluded. Potential risk factors for RD development were assessed by logistic regression or chi-square tests were appropriate and were entered into a multivariate logistic regression if significant on univariate analysis. Risk of RD for each eye was categorized by its RD-OGI score.
Results
Seventy-three eyes (72 patients) were included. In univariate analysis, afferent pupillary defect, worse visual acuity, posterior injury, vitreous hemorrhage, and posterior segment volume loss (PSVL) on CT were strong predictors of RD. In multivariate analysis, only PSVL on CT (adjusted OR 10.8, P = 0.025) maintained a statistically significant association with RD risk. At 1 year, 5% of low-risk eyes, 20% of moderate-risk eyes, and 67% of high-risk eyes developed RD. These rates were not significantly different from the RD-OGI derivation or validation cohorts (P = 0.90 and P = 0.67, respectively).
Conclusion
PSVL on CT increases the risk of RD after OGI. The RD-OGI Score was a good prognostic tool for assessing RD risk after OGI in this population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinal detachment (RD) is a potentially blinding complication of open globe injury (OGI). With more than 200,000 OGIs occurring worldwide each year, reliable data on the risk of RD following OGI are helpful for prognostic purposes [1]. Despite the seriousness of retinal detachment, surprisingly little work has been done to identify specific risk factors for RD after OGI. Standardized classification of OGI was first established in 1997 by the Ocular Trauma Classification Group [2]. In 2002, the Ocular Trauma Score (OTS) was established, providing a predictive scoring system for visual prognosis after OGI. The clinical factors upon which the score is based include initial visual acuity (VA) and the presence or absence of globe rupture, endophthalmitis, perforating injury, afferent pupillary defect (APD), and, notably, retinal detachment. Worse visual acuity and the presence of any of the aforementioned features result in poorer 6-month visual prognosis [3]. One study of 52 eyes that underwent OGI repair identified prior cataract removal, VA less than hand motions, and the presence of an intraocular foreign body as risk factors associated with significantly higher rates of RD after OGI [4]. Another similar study of 55 eyes identified posterior injury, APD, vitreous hemorrhage (VH), and hyphema as risk factors specifically for RD after OGI [5]. Recently, the retinal detachment after open globe injury (RD-OGI) score was established, providing a predictive tool for identifying eyes at risk of RD up to 1 year after presentation of OGI [6, 7]. Part of its appeal lies in the simplicity of the variables involved: presenting VA, zone of injury, and the presence of VH. Worse visual acuity, higher zone of injury, and the presence of VH at the time of presentation after OGI were all associated with significantly higher rates of RD on multivariate analysis. RD-OGI Scoring is described in Table 1, and eyes that have experienced OGI are defined as low (0–2), moderate (2.5–4.5), or high-risk (5–7.5) for RD. This score was initially established with a large cohort (893 eyes) from a single tertiary care center and was later validated with an additional 66 eyes from the same institution between 2012 and 2014 [6, 7].
The primary goal of this study was to evaluate a range of risk factors for future RD at presentation of OGI, including radiographic as well as clinical variables. A second goal was to test the predictive value of the RD-OGI score in an external population and compare the results to the original derivation and validation cohorts.
Methods
This was a retrospective case series conducted at a single academic, level-one trauma center. The study was conducted in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA) and was approved by the institutional review board (IRB) of the University of Louisville. An informed consent waiver was approved by the IRB in accordance with United States Code of Federal Regulations 45 CFR 46.116 (d).
Data collection
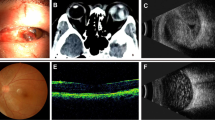
Electronic medical records of patients presenting with OGI between January 1, 2016, and January 1, 2019, were reviewed. Patients were followed from date of injury until the date of most recent follow-up. Inclusion criteria included clinical diagnosis of a ruptured globe with a diagnosis of RD at any time following OGI or with a minimum follow-up of at least 30 days after OGI if no RD was present. Eyes that underwent enucleation within 30 days or lacked follow-up of at least 30 days were excluded if they were not already diagnosed with retinal detachment after OGI. Clinical, demographic, and radiographic data were collected, including age, sex, date of injury, affected eye, presenting VA, zone of injury, presence of VH, afferent pupillary defect status, presence of uveal prolapse, presence of lens trauma, orbit computed tomography (CT) findings, presence of intraocular foreign body, date of RD, anterior vitrectomy at the time of OGI, ocular surgeries post OGI (including pars plana vitrectomy (PPV), and date of last follow-up. Vitreous loss was not routinely reported, and thus, that variable was not collected. This was also the case with mechanisms of injury (i.e., globe laceration versus blunt rupture). In cases of uveal prolapse, intraoperative management, namely repositing of tissue versus excision with or without repositing, was also collected. CT findings were grouped into intact globe, shallow anterior chamber (AC), posterior segment volume loss (PSVL), and intraocular foreign body. PSVL was defined as a distortion of the normal circular shape of the sclera on CT or decrease in the expected size of the vitreous cavity compared to the fellow eye (Figs. 1 and 2). Zones of injury were consistent with the RD-OGI score derivation cohort’s standards: Zone I injury is isolated to the cornea (including the limbus), zone II injury involves the sclera no more than 5 mm posterior to the limbus, and zone III injury involves the sclera more than 5 mm posterior to the limbus.
Statistical analysis
Data analysis was completed using the statistical software, R (The R Foundation, Vienna, Austria). Chi-squared or Fischer’s exact test were used to evaluate the correlation between categorical variables and RD, and univariate logistic regression for continuous variables. Variables that were correlated with RD (with statistical significance at the P = 0.05 level) were entered into a multivariable logistic regression model. Visual acuities were converted to logMAR values [8].
An RD-OGI score was computed for each eye. Eyes were assigned to low-risk (0–2), moderate-risk (2.5–4.5), or high-risk (5–7.5) categories. Rates of RD within each risk category were compared between this cohort and the RD-OGI score derivation and validation cohorts using Chi-square analysis.
Results
Eighty-two patients (83 eyes) treated for OGI within the established time frame were initially identified. Five patients were excluded due to enucleation within 30 days of injury, and five were excluded with less than 30 days of follow-up time after injury. There were 50 males and 23 females with a median age of 37 years (range 2–88 years; interquartile range [IQR] 20–51 years). Twenty-six eyes (36%) developed RD (Table 2).
In univariate analysis, afferent pupillary defect (unadjusted odds ratio [OR] 9.25; 95% confidence interval [95%CI] 2.68–33.32; P < 0.0001), worse visual acuity (P = 0.003), higher zone of injury (Zone II vs. Zone I: OR 15.6; 95%CI 1.34–763.6; P = 0.0047) (Zone III vs. Zone I: OR 38.4; 95%CI 4.77–1640.1; P < 0.0001), presence of vitreous hemorrhage (OR 12.0; 95%CI 2.41–113.6; P = 0.00031), and PSVL versus intact globe on CT radiographs (OR 8.18; 95%CI 2.28–31.1; P < 0.0001) were associated with increased RD risk (Table 3). Intraocular foreign body, PPV following OGI, and anterior vitrectomy during OGI were not associated with increased (or decreased) risk of RD. Indications for PPV following OGI included VH, IOFB, and scleral fixation of intraocular lens (IOL). Excision of uveal tissue after uveal prolapse was associated with higher risk of RD compared to cases without uveal prolapse (OR 7.65; 95%CI 1.32–47.9; P = 0.012) or cases in which the uvea was reposited (OR 3.75; 95%CI 1.4–17.2; P = 0.039). Indications for uveal excision versus reposition were not routinely reported, but included suspected contamination or epithelialization of tissue and technical difficulty of repositing tissue intraoperatively. There was no significant difference in RD risk between cases in which the uvea was reposited and cases which had no uvea prolapse. On multivariate analysis, only PSVL on CT scan remained statistically significant (adjusted OR 10.8, P = 0.025).
Of the 73 eyes with OGI that were included, 69 had clinical data to support RD-OGI score calculation and risk category assignment. Scoring identified 19 low-risk eyes, 20 moderate-risk eyes, and 30 high-risk eyes. Within 1 year, 5% (1/19) of low-risk eyes, 20% (4/20) of moderate-risk eyes, and 67% (20/30) of high-risk eyes had developed RD, versus 3%, 29%, and 73% in the RD-OGI derivation cohort, respectively (Table 4) [3]. The validation cohort reported RD rates of 0% for low-risk, 35% for moderate-risk, and 86% for high-risk eyes [4]. Chi-squared analysis of observed RD rates in this cohort compared to expected RD rates (as predicted by the RD-OGI score derivation cohort) showed no significant difference (P = 0.90). The same analysis of observed RD rates in this cohort compared to those of the RD-OGI score validation cohort showed no significant difference between the moderate and high-risk categories (P = 0.67). Low-risk categories could not be compared between this cohort and the RD-OGI score validation cohort using Chi-square analysis, as the reported rate of RD in the low-risk category was 0% in the validation cohort. Survival curves for each risk category are displayed in Fig. 3.
Discussion
This retrospective study of risk of retinal detachment after open globe injury found PSVL on CT scan to be an independent risk factor for retinal detachment. Afferent pupillary defect (APD), vitreous hemorrhage (VH), worse visual acuity (VA), excision of prolapsed uveal tissue, and higher zone of injury were predictive on univariate analysis but did not remain significant on multivariate regression. The RD-OGI score derivation cohort, which included 893 eyes, determined that worse VA, presence of VH, and higher zone of injury were associated with higher rates of RD after multivariate analysis [6]. It is most likely that our study was insufficiently powered to conclude that these presenting clinical factors are significant predictors of RD after OGI, especially considering the fact that the RD-OGI score (which is determined by presenting VA, presence of VH, and zone of injury) was predictive of RD in this study’s cohort as well as the derivation and validation cohorts without significant difference between the three. Previous clinical studies have noted strong associations between VH and RD risk after OGI, and animal models have also clearly established a causative role in the pathogenesis of retinal detachment after penetrating globe injuries [9,10,11,12,13]. Thus, the lack of association seen here appears spurious and perhaps stems from the limits of sample size or documentation in cases where VH may have been present but not appropriately noted at presentation or immediately post-operatively. Worse visual acuity on presentation is a well-established risk factor for RD after OGI and requires no further discussion [4, 6,7,8, 14, 15]. The presence of APD has been previously identified as a risk factor for RD after OGI [2]. Uveal excision may also prove to be predictive of RD after OGI on future analysis, though confounding variables may be a factor, such as early epithelialization due to duration of injury. One study reported the presence of an intraocular foreign body (IOFB) as having a significantly increased risk of RD after OGI [4], but the RD-OGI score derivation study [6] and this cohort found no significant difference in RD rates between eyes with and without IOFB.
The finding of an increased risk of RD with PSVL on CT scan is intriguing. Although there have been several descriptions of orbital CT findings for open globe detection and their impact on visual outcomes, orbital CT findings have not previously been associated with increased RD risk after OGI. Orbital CT is the imaging modality of choice upon initial evaluation of suspected ruptured globe due to its wide availability, high resolution, rapid acquisition, and excellent safety in the setting of possible ferromagnetic foreign bodies [16]. A notable change in globe contour on CT (which we have defined as evidence of PSVL) has been reported as predictive of OGI (specifically globe rupture) prior to surgical exploration, though not specifically for RD [17, 18]. The language used to describe the change in contour of the globe is not always consistent between radiologists, and it is sometimes described as scleral deformity or as a fractional decrease in globe volume [19, 20]. PSVL on CT implies a significant or even total loss of pressure in the eye. Since the vitreous cavity represents most of the volume of the eye, it is not surprising that a majority of globes with PSVL on CT are zone III injuries (Table 5), which create a direct communication between the vitreous cavity and the outside world and allow for significant vitreous loss. Interestingly, on multivariate analysis, zone III site of injury was not a statistically significant risk factor for RD, while PSVL was. One can infer that the latter finding conveys more information about the status of the eye after trauma than the former. Major volume loss likely indicates not only a posterior site of injury, and thus direct violation of the retina from trauma, but also more forceful trauma, greater vitreous loss, and thus greater vitreoretinal traction, which probably carry a greater risk of retinal tears or dialyses. To the best of our knowledge, this is a novel finding. While vitreous loss itself is likely the underlying pathophysiological mechanism for development of RD after OGI, PSVL noted on orbital CT may serve as a reasonable surrogate finding in cases where vitreous loss is not readily apparent during repair or is not reliably documented. More consistent language use among radiologists to describe this finding may be useful, but the observation of a change in the contour of the globe should be easily identified on review of the imaging by ophthalmologists.
Finally, our data support the RD-OGI score as a valid prediction model for RD after OGI. Comparison of RD rates between low, moderate, and high-risk categories between our cohort and the RD-OGI derivation and validation cohorts was not significantly different. The elegant simplicity and apparent validity of the RD-OGI score are notable strengths that make it a useful tool for clinicians.
Study limitations
This study’s limitations largely arise from the inherent bias of retrospective studies. While most of these clinical findings are standardized and largely objective, they are subject to some interrater variability. This caveat also applies to radiographic reports, as some radiologists may note PSVL where others may not. In general, this is a highly unsubtle finding, but some variability may exist in documentation. PSVL was not quantified, and the degree of vitreous loss could play a role in risk of RD development. Ultimately, vitreous loss is likely the true underlying pathophysiological mechanism for RD after OGI rather than the change in the contour of the globe noted on CT scan, and the presence or absence of vitreous loss was not consistently documented among patients within this cohort.
Potential confounding variables for which this study did not account include mechanism of OGI (blunt vs laceration vs. penetrating), indication for excising versus repositing uveal tissue, surgical techniques, time from injury or presentation to intervention, and underlying ocular disease/history (i.e., previous history of RD). Additionally, the study’s power is limited by a sample size of 73 eyes. This study did not account for time to presentation after injury which could influence RD risk, although previous work has not found an increased RD risk when time from presentation (not time from injury to presentation) was compared to time of repair as starting points. Since most OGIs present within hours of injury, cases of delayed repair are uncommon, but this is an area of future consideration [5].
Data availability
De-identified patient data were contained in a single spreadsheet, which is available from any author upon reasonable request.
References
Négrel A, Thylefors B (1998) The global impact of eye injuries. Ophthalmic Epidemiol 5:143–169
Pieramici DJ, Sternberg P Jr et al (1997) A system for classifying mechanical injuries of the eye (globe). Am J Ophthalmol 123(6):820–831
Kuhn F, Maisiak R, Mann L, Mester V, Morris R, Witherspoon CD (2002) The Ocular Trauma Score (OTS). Ophthalmol Clin North Am 15(2):163–vi. https://doi.org/10.1016/s0896-1549(02)00007-x
Kono Kono JO, Maier M, Schmidt T (2001) Clinical predictors of retinal detachment after open globe injury. Klin Monbl Augenheilkd 218:553–556. https://doi.org/10.1055/s-2001-17137
Lin H, Lema GM, Yoganathan P (2016) Prognostic indicators of visual acuity after open globe injury and retinal detachment repair. Retina 36(4):750–757. https://doi.org/10.1097/IAE.0000000000000798
Stryjewski TP, Andreoli CM, Eliott D (2014) Retinal detachment after open globe injury. Ophthalmol 121(1):327–333. https://doi.org/10.1016/j.ophtha.2013.06.045
Brodowska K, Stryjewski TP, Papavasileiou E et al (2017) Validation of the retinal detachment after open globe injury (RD-OGI) score as an effective tool for predicting retinal detachment. Ophthalmol 124(5):674–678. https://doi.org/10.1016/j.ophtha.2004.05.010
Schulze-Bonsel K, Feltgen N, Burau H et al (2006) Visual acuities “hand motion” and “count fingers” can be quantified with the Freiburg visual acuity test. Invest Vis Sci Ophthalmol 47:1236–1240. https://doi.org/10.1167/iovs.05-0981
Cardillo JA, Stout JT, LaBree L et al (1997) Post-traumatic proliferative vitreoretinopathy: the epidemiologic profile, onset, risk factors, and visual outcome. Ophthalmol 104:1166–1173. https://doi.org/10.1016/S0161-6420(97)30167-5
Feng K, Hu Y, Wang C et al (2013) Risk factors, anatomical, and visual outcomes of injured eyes with proliferative vitreoretinopathy: eye injury vitrectomy study. Retina 33(8):1512–1518. https://doi.org/10.1097/IAE.0b013e3182852469
Cleary PE, McInckler DS, Ryan SJ (1980) Ultrastructure of traction retinal detachment in rhesus monkey eyes after a posterior penetrating injury. Am J Ophthalmol 90(6):829–845. https://doi.org/10.1016/S0002-9394(14)75198-0
Cleary PE, Ryan SJ (1979) Histology of wound, vitreous, and retina in experimental posterior penetrating eye injury in the rhesus monkey. Am J Ophthalmol 88(2):221–231. https://doi.org/10.1016/0002-9394(79)90469-0
Hui YN, Goodnight R, Sorgente N et al (1989) Fibrovascular proliferation and retinal detachment after intravitreal injection of activated macrophages in the rabbit eye. Am J Ophthalmol 108(2):176–184. https://doi.org/10.1016/0002-9394(89)90014-7
Entezari M, Rabei HM, Badalabadi MM et al (2006) Visual outcome and ocular survival in open-globe injuries. Injury 37:633–637. https://doi.org/10.1016/j.injury.2006.02.043
Lesniak SP, Bauza A, Son JH et al (2011) Twelve-year review of pediatric traumatic open globe injuries in an urban U.S. population. J Pediatr Ophthalmol Strabismus 49:73–79. https://doi.org/10.3928/01913913-20110712-02
Dunkin JM, Crum AV, Swanger RS, Bokhari SA (2011) Globe trauma. Semin Ultrasound CT MR 32:51–56. https://doi.org/10.1053/j.sult.2010.09.003
Arey ML, Mootha VV, Whittemore AR et al (2007) Computed tomography in the diagnosis of occult open-globe injuries. Ophthalmol 114(8):1448–1452. https://doi.org/10.1016/j.ophtha.2006.10.051
Yuan WH, Hsu HC, Cheng HC et al (2014) CT of globe rupture: analysis and frequency of findings. AJR Am J Roentgenol 202(5):1100–1107. https://doi.org/10.2214/AJR.13.11010
Joseph DP, Pieramici DJ, Beauchamp NJ (2000) Computed tomography in the diagnosis and prognosis of open-globe injuries. Ophthalmol 107(10):1899–1906. https://doi.org/10.1016/S0161-6420(00)00335-3
Bodanapally UK, Addis H, Dreizin D et al (2017) Prognostic predictors of visual outcome in open globe injury: Emphasis on facial CT findings. AJNR Am J Neuroradiol 38(5):1013–1018. https://doi.org/10.3174/ajnr.A5107
Funding
The authors declare no outside sources of funding for this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and initial data collection were performed by Travis Bales. Analysis was performed by Harpal S. Sandhu, MD. The first draft of the manuscript was written by Travis Bales with substantial contribution and revision by Harpal S. Sandhu, MD. Additional data collection and subsequent manuscript revision was performed by Tyler Ogden. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Research ethics and patient consent
In this retrospective study, care was taken to protect the identities of human subjects. Patient names were substituted with a numerical code. De-identified patient data were contained in a single spreadsheet. The study was approved by the University of Louisville Institutional Review Board (Study ID# 19.0068) and conducted in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). An informed consent waiver was approved by the IRB in accordance with United States Code of Federal Regulations [45 CFR 46.116 (d)].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bales, T., Ogden, T. & Sandhu, H.S. Clinical, radiographic, and intraoperative risk factors for retinal detachment after open globe injury. Int Ophthalmol 41, 815–823 (2021). https://doi.org/10.1007/s10792-020-01635-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01635-7