Abstract
Purpose
This study searched for early predictive vascular biomarkers for visual outcomes in eyes with macular edema caused by branch retinal vein occlusion (BRVOME).
Methods
Twenty-four eyes of 24 subjects with BRVOME were treated with the intravitreal injection of ranibizumab (IVR) for at least 6 months. We measured mean blur rate (MBR) in the optic nerve head (ONH) and vessel density (VD) in the macula with laser speckle flowgraphy and optical coherence tomography angiography, respectively.
Results
Six-month post-IVR best-corrected visual acuity (BCVA) was correlated positively with age, pre-IVR BCVA, 1-month post-IVR BCVA, 3-month post-IVR BCVA and pre-IVR systolic blood pressure (P < 0.001, P < 0.001, P < 0.001, P < 0.001 and P = 0.02, respectively) and negatively with pre-IVR overall MBR, 1-month post-IVR overall MBR, 6-month post-IVR overall MBR, 3-month post-IVR deep retinal capillary plexus (DCP) VD and 6-month post-IVR DCP VD (P = 0.03, P = 0.03, P = 0.02, P = 0.01 and P = 0.005, respectively). Furthermore, a multiple regression analysis showed that pre-IVR overall MBR (β = − 0.67, P = 0.009) was among independent prognostic factors predicting 6-month post-IVR BCVA. Six-month post-IVR DCP VD was also correlated with overall MBR at all time points.
Conclusion
ONH blood flow may be a pre-IVR biomarker of both visual outcomes and post-IVR deep macular microcirculation in eyes with BRVOME.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Branch retinal vein occlusion (BRVO) and subsequent macular edema (BRVOME) are common retinal vascular conditions that cause severe visual disturbance. Previously, many kinds of approaches have been introduced to treat BRVOME, such as the intravitreal injection of anti-vascular endothelial growth factor (VEGF) antibodies [1,2,3], intravitreal or sub-Tenon’s injection of triamcinolone acetonide (TA) [4], laser photocoagulation [5, 6] and pars plana vitrectomy [7, 8]. Currently, anti-VEGF therapy is the strongest evidence-based treatment, reaching evidence level I [9]. Anti-VEGF therapy can improve vision by temporarily blocking VEGF action and subsequently reducing macular edema. There are several types of anti-VEGF therapy in use clinically, notably ranibizumab and aflibercept [10, 11]. Ranibizumab is quickly cleared from the blood and does not accumulate after multiple injections, and thus has no significant effect on either serum or plasma VEGF levels [10]. The intravitreal injection of ranibizumab (IVR) has become a common treatment for retinal vein occlusion (RVO) [2, 3]. However, it is still difficult to predict the effect of IVR on visual outcomes in eyes with BRVOME. There has been speculation that macular vascular parameters might be predictive of visual prognosis, but before treatment, it is difficult to accurately evaluate these parameters because of the presence of macular edema and intraretinal hemorrhage in BRVO.
Recently, two non-invasive tools have been introduced that can measure ocular microcirculation: optical coherence tomography angiography (OCTA) and laser speckle flowgraphy (LSFG). OCTA can produce high-quality images of the retinal vasculature [12,13,14,15] and has proven particularly useful in studies of retinal diseases [16,17,18,19,20]. LSFG uses the laser speckle phenomenon to enable the in vivo quantification of circulation in the optic nerve head (ONH), choroid and retinal vessels [21, 22]. LSFG has contributed to recent research on glaucoma and other retinal diseases by clarifying the impact of changes in ocular circulation [23, 24]. A few preliminary reports have suggested that IVR treatment can lower intraocular circulation [25, 26], but none have provided precise data on changes in ocular blood flow, measured with both OCTA and LSFG, in eyes with BRVOME after treatment with IVR.
Many parameters, including ocular vascularity, should be evaluated to predict visual prognosis in BRVOME [27, 28]. In this study, we retrospectively determined whether the outcomes of IVR treatment for BRVOME could be predicted by ocular vascular parameters, measured with OCTA and LSFG.
Materials and methods
Patients
This was a retrospective, cross-sectional, single-center study, and the procedures used were approved by the Ethics Committee of Tohoku University Hospital. The procedures conformed to the tenets of the Declaration of Helsinki, and all patients provided written informed consent. Twenty-four eyes of 24 patients with treatment-naive BRVOME were followed between February 2017 and January 2018. All eyes received one initial 0.5 mg/0.05 mL IVR treatment, followed by additional pro re nata (PRN) IVR treatments. These additional treatments were administered if central macular thickness (CMT) exceeded 300 µm, cystoid spaces formed, or there was an increase in serous retinal detachment. No eyes received any other form of treatment, including scatter or grid laser photocoagulation, steroid therapy, surgical intervention or intravitreal injections of anti-VEGF agents other than ranibizumab. We excluded patients with cataract (i.e., a lens nucleus with Emery–Little grade 2 or higher), diabetic retinopathy or hypertensive retinopathy before IVR. Only patients followed for a minimum of 6 months were included in this study.
Ophthalmic examinations
The patients underwent comprehensive ophthalmologic examinations including measurements of best-corrected visual acuity (BCVA), intraocular pressure (IOP) and axial length (IOL Master, Carl Zeiss Meditec, Oberkochen, Germany), as well as slit-lamp biomicroscopy, color fundus photography, indirect ophthalmoscopy, optical coherence tomography (Cirrus SD-OCT, Carl Zeiss Meditec, Inc., Dublin, CA, USA, or Triton SS-OCT, Topcon Co., Tokyo, Japan), OCTA (RS-3000 Advance, Nidek, Gamagori, Japan) and LSFG (LSFG-NAVI, Softcare Co., Ltd., Fukuoka, Japan), before IVR and 1, 3, 6 months after IVR. BRVO was classified as ischemic or non-ischemic based on fluorescein angiography findings of a non-perfusion area greater than or less than 5 disc diameters, respectively.
Retinal microvasculature imaging with OCTA
All subjects underwent OCTA scans of the macula. In eyes with persistent macular edema, OCTA was performed within 2 months of the last anti-VEGF treatment before successful suppression of macular edema. The RS-3000 software (NAVIS-EX 1.7.0) automatically segments the vascular area into four layers: the superficial retinal capillary plexus (SCP), deep retinal capillary plexus (DCP), outer retina and choroid. Default auto-segmentation of the SCP images includes the vasculature, measured from the internal limiting membrane to 8 µm below the boundary between the inner plexiform layer (IPL) and inner nuclear layer (INL). DCP images include the vasculature from 13 µm below the boundary between the IPL and INL to 84 µm below. We defined microvascular changes in the SCP, DCP and foveal avascular zone (FAZ) area according to previously reported methods based on the RS3000 software [29]. First, for each patient, we obtained a 3 × 3-mm scan centered on the fovea, showing visible perfusion. Then, we converted this to a percentage value to obtain vessel density (VD). We used FAZ area in the SCP, but not in the DCP, because of its possible measurement bias [30]. The pre-IVR OCTA values were obtained from 18 of 24 eyes, after six eyes were excluded because the images had poor quality (due to artifacts, segmentation errors or a signal strength index < 6) and the VD measurements consequently had low reliability. These parameters were corrected for axial length using the built-in software.
ONH microvasculature imaging with LSFG
LSFG-NAVI was used to evaluate ONH microcirculation. The principles of LSFG have previously been described in detail [31,32,33]. We obtained color maps indicating mean blur rate (MBR), a relative measure of blood flow velocity, which were automatically divided with the accompanying LSFG software (LSFG analyzer, version 3.1.59.0) into the large vessel and tissue (i.e., capillary) areas of the ONH. Therefore, LSFG can assess vessel MBR and tissue MBR separately, in addition to overall MBR, in the ONH. LSFG measurements were performed in triplicate at each time point in all eyes. The average of these measurements was then calculated for MBR.
Statistical analyses
The data are presented as mean ± standard deviation. The main outcome measures were 6-month post-IVR BCVA and retinal microcirculation, as measured with LSFG and OCTA. The Tukey–Kramer test was used to evaluate differences in systolic blood pressure (SBP), diastolic blood pressure (DBP), BCVA, CMT, overall MBR, vessel MBR, tissue MBR, SCP VD, DCP VD and FAZ area between each time point. Spearman’s rank correlation test was used to estimate the relationships between 6-month post-IVR BCVA and age, symptom duration, SBP, DBP, BCVA, CMT, overall MBR, vessel MBR, tissue MBR, SCP VD, DCP VD and FAZ area at each time point. Spearman’s rank correlation test was also used to estimate the relationship between 6-month post-IVR OCTA-derived parameters and overall MBR at each time point. Separate multiple linear regression analyses were performed to analyze each independent variable: pre-IVR overall MBR, pre-IVR SBP and 1-month post-IVR DCP VD, and determine their potential effect on 6-month post-IVR BCVA. A receiver operating characteristic (ROC) curve analysis was performed to assess the ability of age, pre-IVR BCVA, pre-IVR overall MBR, pre-IVR SBP and 1-month post-IVR DCP VD to predict whether BCVA reached ≤ 0 logarithmic minimum angle of resolution (logMAR) at 6 months after IVR. Post-IVR time points were defined as the time after initial IVR. All statistical analyses were performed with JMP software (Pro version 10.0.2, SAS Institute Japan Inc., Tokyo, Japan). Differences were considered significant at P < 0.05.
Results
The clinical characteristics of the patients are shown in Table 1. This study included 24 BRVOME eyes of 24 subjects (8 males/16 females; mean age: 67.1 ± 11.9 YO). There were 17 eyes with major BRVO and 7 eyes with macular BRVO. There were 16 eyes with ischemic BRVO and 8 eyes with non-ischemic BRVO. The mean number of IVR treatments was 2.8 ± 1.3 during the first 6 months. During the study, 15 of the 24 BRVOME eyes developed recurrent macular edema. A comparison of clinical parameters before and 1, 3 and 6 months after IVR showed that SBP, DBP and CMT decreased, while BCVA improved; these changes were all statistically significant (P < 0.01, P < 0.001, P < 0.001 and P < 0.01, respectively, Table 1). However, these parameters did not significantly change during the post-IVR period (i.e., 1, 3 and 6 months post-IVR). LSFG and OCTA parameters did not significantly change during any time period.
Six-month post-IVR BCVA was correlated positively with age, pre-IVR SBP, pre-IVR BCVA, 1-month post-IVR BCVA and 3-month post-IVR BCVA (P < 0.001, P = 0.02, P < 0.001, P < 0.001 and P < 0.001, respectively, Table 2) and negatively with pre-IVR overall MBR, 1-month post-IVR overall MBR, 6-month post-IVR overall MBR, pre-IVR vessel MBR, 1-month post-IVR vessel MBR, 6-month post-IVR vessel MBR, 3-month post-IVR DCP VD and 6-month post-IVR DCP VD (P = 0.03, P = 0.03, P = 0.02, P = 0.04, P = 0.02, P < 0.001, P = 0.01 and P = 0.005, respectively, Table 2). Furthermore, there was a close association between pre-IVR overall MBR and 6-month post-IVR BCVA in the non-recurrent group (9 eyes) (r = − 0.71, P = 0.03), but not in the recurrent group (15 eyes) (r = − 0.20, P = 0.49).
Multiple regression analysis confirmed that pre-IVR overall MBR and pre-IVR SBP were independent factors associated with 6-month post-IVR BCVA (β = − 0.67, P = 0.009 and β = 0.73, P = 0.004, respectively, Table 3).
Six-month post-IVR SCP VD was correlated positively with 1-month post-IVR overall MBR (P = 0.02, Table 4), but was not correlated with pre-IVR overall MBR, 3-month post-IVR overall MBR or 6-month post-IVR overall MBR. Six-month post-IVR DCP VD was correlated positively with pre-IVR overall MBR, 1-month post-IVR overall MBR, 3-month post-IVR overall MBR and 6-month post-IVR overall MBR (P = 0.008, P = 0.004, P = 0.02 and P = 0.002, respectively, Table 4). Six-month post-IVR FAZ was not correlated with overall MBR at any time point (Table 4).
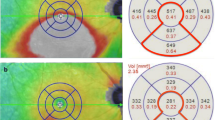
The ROC curves for age, pre-IVR BCVA, pre-IVR overall MBR, pre-IVR SBP and 1-month post-IVR DCP VD are shown in Fig. 1, representing the ability of these parameters to predict a good BCVA outcome (logMAR ≤ 0). The predictive ability of age, pre-IVR BCVA, pre-IVR overall MBR, pre-IVR SBP and 1-month post-IVR DCP VD was 0.92 (95% confidence interval [CI] 0.81–1.03), 0.91 (95% CI 0.78–1.03), 0.75 (95% CI 0.53–0.96), 0.68 (95% CI 0.45–0.92) and 0.64 (95% CI 0.39–0.89), respectively. The cut-off values for age, pre-IVR BCVA, pre-IVR overall MBR, pre-IVR SBP and 1-month post-IVR DCP VD were 70.0 YO, 0.40 logMAR, 22.0 arbitrary units (AU), 150 mmHg and 43.5%, respectively.
ROC curves showing the AUCs for age, pre-IVR BCVA, pre-IVR overall MBR, pre-IVR SBP and 1-month post-IVR DCP VD to predict good 6-month post-IVR BCVA (logMAR ≤ 0). The predictive abilities of age, pre-IVR BCVA, pre-IVR overall MBR, pre-IVR SBP and 1-month post-IVR DCP VD for a good BCVA outcome were 0.92 (95% CI 0.81–1.03), 0.91 (95% CI 0.78–1.03), 0.75 (95% CI 0.53–0.96), 0.68 (95% CI 0.45–0.92) and 0.64 (95% CI 0.39–0.89), respectively. The cut-off values for age, pre-IVR BCVA, pre-IVR overall MBR, pre-IVR SBP and 1-month post-IVR DCP VD were 70.0 YO, 0.40 logMAR, 22.0 AU, 150 mmHg and 43.5%, respectively
Two representative cases are shown in Figs. 2 and 3. These BRVOME cases had similar ages and BCVA and different BRVO types. They differed in pre-IVR overall MBR and subsequently had different 6-month post-IVR BCVA, showing the contrast in outcomes.
A representative case in which 6-month post-IVR BCVA showed poor recovery. This case was a 77-year-old female with macular BRVOME. Pre-IVR decimal BCVA was 0.4. BCVA did not improve; 6-month post-IVR decimal BCVA remained at 0.2. a Fundus photography before IVR. b Pre-IVR overall MBR was 16.6 AU. c Pre-IVR CMT was 290 µm. There were disturbances in the interdigitation zone, ellipsoid zone and ELM line. d Fundus photography 6 months after IVR. e Six-month post-IVR MBR was 18.5 AU. f Six-month post-IVR CMT was 205 µm. Macular structure recovered, but disturbances remained in the interdigitation zone, ellipsoid zone and ELM line in the fovea. g Six-month post-IVR FAZ area in the SCP was 0.61 mm2. h Six-month post-IVR DCP VD was 29.7%
A representative case in which 6-month post-IVR BCVA showed good recovery. This case was a 76-year-old male with major BRVOME. Pre-IVR decimal BCVA was 0.5, and 6-month post-IVR decimal BCVA recovered to 1.2. a Fundus photography before IVR. b Pre-IVR overall MBR was 29.6 AU. c Pre-IVR CMT was 289 µm. There were only slight disturbances in the interdigitation zone, ellipsoid zone and ELM line. d Fundus photography 6 months after IVR. e Six-month post-IVR MBR was 29.2 AU. f Six-month post-IVR CMT was 226 µm. Macular structure recovered and there were no remaining disturbances in the interdigitation zone, ellipsoid zone or ELM line in the fovea. g Six-month post-IVR FAZ area in the SCP was 0.69 mm2. h Six-month post-IVR DCP VD was 49.2%
Discussion
In this study, we used LSFG and OCTA to measure ocular microcirculation in patients with BRVOME before and after they underwent treatment with IVR, and searched for pre-IVR predictors of visual outcomes. We found that BCVA and CMT improved after IVR and that MBR in the ONH was stable throughout the period of the study. Furthermore, 6-month post-IVR BCVA was positively correlated with age, pre-IVR SBP, pre-IVR BCVA, 1-month post-IVR BCVA and 3-month post-IVR BCVA, and negatively correlated with pre-IVR overall MBR, 1-month post-IVR overall MBR, 6-month post-IVR overall MBR, pre-IVR vessel MBR, 1-month post-IVR vessel MBR, 6-month post-IVR vessel MBR, 3-month post-IVR DCP VD and 6-month post-IVR DCP VD. Moreover, a multiple regression analysis showed that pre-IVR overall MBR and pre-IVR SBP were independent prognostic factors predictive of 6-month post-IVR BCVA. Finally, 6-month post-IVR DCP VD was also correlated with overall MBR at all time points.
Does IVR change ocular blood flow in the macula or ONH?
The effect of IVR on ocular blood flow in the macula and ONH remains unclear, although our current results suggest that IVR did not change ONH blood flow in either the vessel or tissue areas. This reinforces a previous study of intravitreal bevacizumab (IVB), a well known anti-VEGF treatment, which found that MBR in the retinal artery, retinal vein or ONH did not change significantly 1 week or 1 month after IVB in eyes with BRVOME [34]. However, that study found that by contrast, MBR in the retinal artery, retinal vein and ONH decreased significantly in eyes with diabetic macular edema (DME) after IVB. This may have been because retinal autoregulation is more impaired in DME than in BRVOME. Many studies have reported that inducible nitric oxide synthase (iNOS) is upregulated in diabetes [35, 36], with subsequent capillary degeneration, pericyte loss and permeability [37, 38]. Vascular resistance increases and the velocity of blood flow in the ophthalmic artery decreases in diabetes [39]. Thus, a further decrease in retinal circulation caused by anti-VEGF therapy might lead to macular ischemia in patients with diabetes [40, 41].
It is relatively difficult to precisely measure SCP and DCP VD before IVR in BRVOME patients, because macular edema and intraretinal hemorrhage obscure images of the retina, reducing their reliability. In the current study, the images from six of 24 eyes had poor quality and could not reliably be used for pre-IVR VD measurements. Thus, we confined the analysis to the remaining 18 eyes. A previous analysis of 30 eyes with RVO showed that SCP VD was not reduced, while DCP VD was reduced, at baseline and did not change 1 month after IVR, though seven out of these 30 eyes were, like in our study, excluded from the final OCTA analysis because of poor image quality [42]. The current study also confirmed another analysis (that included 48 eyes with RVO treated with ranibizumab or aflibercept under a PRN protocol) that showed that macular VD in neither the SCP nor DCP changed significantly for 12 months [43]. Thus, we believe that IVR should allow the improvement in BCVA and CMT in eyes with BRVOME, without changing blood flow in the macula or ONH.
Predicting 6-month post-IVR BCVA
Predicting visual outcomes based on pre-IVR ocular vascular parameters remains difficult in BRVOME patients. In the current study, MBR in the ONH was stable throughout the follow-up period. Univariate and multivariate analyses showed that pre-IVR overall MBR was closely associated with 6-month post-IVR BCVA, which was enhanced in the non-recurrent group. Though it is difficult to explain this finding at this stage of our research, we speculate that when ocular blood flow is preserved in eyes with BRVOME, it improves their capacity for neurological recovery after the resolution of macular edema with IVR. Interestingly, 6-month post-IVR DCP VD was correlated with overall MBR at all time points. Importantly, the closest association was between 6-month post-IVR DCP VD and 6-month post-IVR overall MBR. This suggests that ONH blood flow reflects the macular condition of the DCP. We believe these findings are reasonable because preservation of the deep macular vasculature has recently been suggested to be critical for ensuring good visual function in BRVOME patients. A previous study reported that good BCVA at 12 months was significantly associated with good VD in the SCP and DCP, both at baseline and at 12 months, in an analysis of 48 eyes with RVO treated with ranibizumab or aflibercept under a PRN protocol [43]. Another study used a stepwise multiple regression analysis and found that the vascular perfusion area in the DCP was the most closely correlated parameter with BCVA in eyes with resolved BRVOME [44]. Finally, a study used a multivariate analysis to show that deep parafoveal VD was associated with BCVA in eyes with BRVO or central RVO (CRVO) [45]. Thus, pre-IVR measurement of overall MBR might be a complementary tool to predict the post-IVR visual prognosis in patients with BRVOME. We concentrated on overall MBR in this study, because overall MBR is easier to measure and showed close associations with DCP VD in our data.
Parameters other than ocular vascular parameters have previously been investigated in efforts to predict visual outcomes. Pre-treatment visual function and patient age have been reported to be closely associated with visual outcomes, and BRVOME eyes with better pre-IVR BCVA or younger age appeared to have better post-IVR BCVA [27, 28, 46]. These findings were confirmed in the current study. Previous studies also reported that a longer duration of symptoms was a negative predictive factor for visual outcomes after IVR [47], but this was not confirmed in our study. The integrity of the external limiting membrane (ELM) has also been shown to be an independent factor predictive of post-IVR BCVA [46]. One of our representative cases (Case 1), which showed no improvement in post-IVR BCVA, also had a disrupted ellipsoid zone and ELM line before IVR. This suggests that analysis of the outer retinal layers might also have the potential to predict post-IVR visual function. Furthermore, foveal subretinal hemorrhage, an uncommon feature of BRVOME, may cause subsequent damage to the foveal photoreceptor layer, resulting in poor visual function [48]. In the current study, pre-IVR SBP was closely associated with 6-month post-IVR BCVA, i.e., higher SBP leads to worse BCVA after IVR. This finding is understandable because hypertension is known as a risk factor for the 10-year incidence of RVO [49], and in patients with CRVO, better resolution of macular edema after IVB is seen in individuals with SBP less than 120 mmHg [50].
Limitations and advantages
Our study was limited by a small sample size, short follow-up period and the use of pre-IVR OCTA data from 18 of 24 eyes after six eyes were excluded due to poor pre-IVR image quality. Furthermore, though we aimed to predict visual outcomes based on ocular vascular parameters, rather than non-vascular parameters such as age or pre-IVR BCVA, we were not able to find more than a moderate relationship between pre-IVR overall MBR and 6-month post-IVR BCVA. AUCs greater than 0.75 were obtained only for age, pre-IVR BCVA and pre-IVR overall MBR, but the AUCs for age and pre-IVR BCVA were higher than for pre-IVR overall MBR. Nevertheless, these results are the first to show, using both OCTA and LSFG, that blood flow in the ONH is stable during IVR therapy and is associated with post-IVR BCVA as well as post-IVR DCP VD. Furthermore, reliable pre-IVR OCTA data were harder to obtain than reliable pre-IVR LSFG data; 25% (6/24) of the pre-IVR OCTA data were unusable, versus 0% for pre-IVR LSFG data. Though some investigators have reported that short-term post-IVR parameters, such as measurements on the first post-treatment day or the time to initial resolution of the macular edema, may predict the long-term effects of IVR in BRVOME [51, 52], we believe that pre-IVR prediction would be the ideal method for patients and ophthalmologists and that measuring ONH blood flow with LSFG still has promise as a simple-to-use tool for such prediction. Furthermore, we calculated the statistical power of our multiple regression analysis with software (Free Statistics Calculators v. 4.0; https://www.danielsoper.com/statcalc/default.aspx), and the observed statistical power of our analysis was 0.94, confirming that our main results were reliable.
We observed that SBP and DBP did not increase, but rather decreased after the initial IVR treatment. Though previous studies have reported that IVR has no impact on blood pressure [53, 54], since more than 50% of RVO patients have hypertension [55], it is important not to increase blood pressure during therapy for BRVOME. We believe that IVR might be a safe approach that does not cause severe systemic complications in eyes with BRVOME.
Conclusion
We found that post-IVR BCVA was associated with age, pre-IVR SBP and pre-IVR BCVA, meaning that younger age, lower blood pressure or better visual acuity led to better visual outcomes after IVR treatment for BRVOME. Importantly, we also found that overall MBR did not change after IVR treatment, but that it may be a predictor of late post-IVR BCVA in BRVOME, as well as of late post-IVR DCP VD. Furthermore, we observed a close association between late post-IVR BCVA and late post-IVR DCP VD. Thus, there was a mutual relationship between visual function, deep macular microcirculation and ONH blood flow. LSFG measurements of ONH microcirculation may be valuable to predict visual outcomes in BRVOME patients undergoing IVR treatment. We believe that the current results could shed light on the improvement in prediction of visual outcomes after IVR treatment in BRVOME patients.
Conflict of interest
The authors declare that they have no conflict of interest.
References
Campochiaro PA, Clark WL, Boyer DS, Heier JS, Brown DM, Vitti R, Kazmi H, Berliner AJ, Erickson K, Chu KW, Soo Y, Cheng Y, Haller JA (2015) Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology 122(3):538–544
Campochiaro PA, Hafiz G, Channa R, Shah SM, Nguyen QD, Ying H, Do DV, Zimmer-Galler I, Solomon SD, Sung JU, Syed B (2010) Antagonism of vascular endothelial growth factor for macular edema caused by retinal vein occlusions: two-year outcomes. Ophthalmology 117(12):2387–2394 e2381-2385
Pece A, Isola V, Piermarocchi S, Calori G (2011) Efficacy and safety of anti-vascular endothelial growth factor (VEGF) therapy with intravitreal ranibizumab (Lucentis) for naive retinal vein occlusion: 1-year follow-up. Br J Ophthalmol 95(1):56–68
Hayashi K, Hayashi H (2005) Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol 139(6):972–982
Finkelstein D (1986) Argon laser photocoagulation for macular edema in branch vein occlusion. Ophthalmology 93(7):975–977. https://doi.org/10.1016/S0161-6420(86)33651-0
Parodi MB, Spasse S, Iacono P, Di Stefano G, Canziani T, Ravalico G (2006) Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology 113(12):2237–2242
Kumagai K, Furukawa M, Ogino N, Uemura A, Larson E (2007) Long-term outcomes of vitrectomy with or without arteriovenous sheathotomy in branch retinal vein occlusion. Retina 27(1):49–54
Tachi N, Hashimoto Y, Ogino N (1999) Vitrectomy for macular edema combined with retinal vein occlusion. Doc Ophthalmol 97(3–4):465–469
Ehlers JP, Kim SJ, Yeh S, Thorne JE, Mruthyunjaya P, Schoenberger SD, Bakri SJ (2017) Therapies for macular edema associated with branch retinal vein occlusion: a report by the American Academy of Ophthalmology. Ophthalmology 124(9):1412–1423
Wang X, Sawada T, Sawada O, Saishin Y, Liu P, Ohji M (2014) Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol 158(4):738–744 e731
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Le K, Maia M, Visich JE (2014) Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 98(12):1636–1641
Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, Lombardi LH, Gattey DM, Armour RL, Edmunds B, Kraus MF, Fujimoto JG, Huang D (2014) Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 121(7):1322–1332
Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, Potsaid B, Liu JJ, Lu CD, Kraus MF, Fujimoto JG, Huang D (2014) Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 121(7):1435–1444
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D (2012) Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 20(4):4710–4725
Chung CY, Li KKW (2018) Optical coherence tomography angiography wide-field montage in branch retinal vein occlusion before and after anti-vascular endothelial-derived growth factor injection. Int Ophthalmol 38(3):1305–1307
Scarinci F, Nesper PL, Fawzi AA (2016) Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol 168:129–138
Roisman L, Rosenfeld PJ (2016) Optical coherence tomography angiography of macular telangiectasia type 2. Dev Ophthalmol 56:146–158
Matet A, Daruich A, Dirani A, Ambresin A, Behar-Cohen F (2016) Macular telangiectasia type 1: capillary density and microvascular abnormalities assessed by optical coherence tomography angiography. Am J Ophthalmol 167:18–30
Lin TC, Chung YC, Lin CY, Lee FL, Chen SJ (2016) Focal nonperfusion of deep retinal capillary plexus in eyes with epiretinal membranes revealed by optical coherence tomography angiography. Ophthalmic Surg Lasers Imag Retina 47(5):404–409
Zhang Q, Wang RK, Chen CL, Legarreta AD, Durbin MK, An L, Sharma U, Stetson PF, Legarreta JE, Roisman L, Gregori G, Rosenfeld PJ (2015) Swept source optical coherence tomography angiography of neovascular macular telangiectasia type 2. Retina 35(11):2285–2299
Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S (2010) Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol 88(7):723–729
Nagahara M, Tamaki Y, Tomidokoro A, Araie M (2011) In vivo measurement of blood velocity in human major retinal vessels using the laser speckle method. Invest Ophthalmol Vis Sci 52(1):87–92
Kunikata H, Nakazawa T (2016) Recent clinical applications of laser speckle flowgraphy in eyes with retinal disease. Asia Pac J Ophthalmol (Phila) 5(2):151–158
Nakazawa T (2016) Ocular blood flow and influencing factors for glaucoma. Asia Pac J Ophthalmol (Phila) 5(1):38–44
Micieli JA, Tsui E, Lam WC, Brent MH, Devenyi RG, Hudson C (2012) Retinal blood flow in response to an intravitreal injection of ranibizumab for neovascular age-related macular degeneration. Acta Ophthalmol 90(1):e13–20
Fukami M, Iwase T, Yamamoto K, Kaneko H, Yasuda S, Terasaki H (2017) Changes in retinal microcirculation after intravitreal ranibizumab injection in eyes with macular edema secondary to branch retinal vein occlusion. Invest Ophthalmol Vis Sci 58(2):1246–1255
Jaissle GB, Szurman P, Feltgen N, Spitzer B, Pielen A, Rehak M, Spital G, Heimann H, Meyer CH (2011) Predictive factors for functional improvement after intravitreal bevacizumab therapy for macular edema due to branch retinal vein occlusion. Graefe Arch Clin Exp Ophthalmol 249(2):183–192
Winegarner A, Wakabayashi T, Fukushima Y, Sato T, Hara-Ueno C, Busch C, Nishiyama I, Shiraki N, Sayanagi K, Nishida K, Sakaguchi H, Nishida K (2018) Changes in retinal microvasculature and visual acuity after antivascular endothelial growth factor therapy in retinal vein occlusion. Invest Ophthalmol Vis Sci 59(7):2708–2716
Al-Sheikh M, Falavarjani KG, Tepelus TC, Sadda SR (2017) Quantitative comparison of swept-source and spectral-domain OCT angiography in healthy eyes. Ophthalmic Surg Lasers Imag Retina 48(5):385–391
Spaide RF, Curcio CA (2017) Evaluation of segmentation of the superficial and deep vascular layers of the retina by optical coherence tomography angiography instruments in normal eyes. JAMA Ophthalmol 135(3):259–262
Fujii H (1994) Visualisation of retinal blood flow by laser speckle flow-graphy. Med Biol Eng Compu 32(3):302–304
Sugiyama T, Utsumi T, Azuma I, Fujii H (1996) Measurement of optic nerve head circulation: comparison of laser speckle and hydrogen clearance methods. Jpn J Ophthalmol 40(3):339–343
Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H (1995) Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp Eye Res 60(4):373–383
Nitta F, Kunikata H, Aizawa N, Omodaka K, Shiga Y, Yasuda M, Nakazawa T (2014) The effect of intravitreal bevacizumab on ocular blood flow in diabetic retinopathy and branch retinal vein occlusion as measured by laser speckle flowgraphy. Clin Ophthalmol 8:1119–1127
Carmo A, Cunha-Vaz JG, Carvalho AP, Lopes MC (2000) Nitric oxide synthase activity in retinas from non-insulin-dependent diabetic Goto-Kakizaki rats: correlation with blood-retinal barrier permeability. Nitric Oxide 4(6):590–596
Abu El-Asrar AM, Desmet S, Meersschaert A, Dralands L, Missotten L, Geboes K (2001) Expression of the inducible isoform of nitric oxide synthase in the retinas of human subjects with diabetes mellitus. Am J Ophthalmol 132(4):551–556
Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Ball S, Berkowitz BA, Kern TS (2007) Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 50(9):1987–1996
Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrosio AF, Forrester JV (2007) Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci 48(11):5257–5265
Gracner T (2004) Ocular blood flow velocity determined by color Doppler imaging in diabetic retinopathy. Ophthalmologica 218(4):237–242
Shimura M, Yasuda K (2010) Macular ischaemia after intravitreal bevacizumab injection in patients with central retinal vein occlusion and a history of diabetes and vascular disease. Br J Ophthalmol 94(3):381–383
Huang ZL, Lin KH, Lee YC, Sheu MM, Tsai RK (2010) Acute vision loss after intravitreal injection of bevacizumab (avastin) associated with ocular ischemic syndrome. Ophthalmologica 224(2):86–89
Costanzo E, Parravano M, Gilardi M, Cavalleri M, Sacconi R, Aragona E, Varano M, Bandello F, Querques G (2019) Microvascular retinal and choroidal changes in retinal vein occlusion analyzed by two different optical coherence tomography angiography devices. Ophthalmologica 242(1):8–15
Winegarner A, Wakabayashi T, Fukushima Y, Sato T, Hara-Ueno C, Busch C, Nishiyama I, Shiraki N, Sayanagi K, Nishida K, Sakaguchi H (2018) Changes in retinal microvasculature and visual acuity after antivascular endothelial growth factor therapy in retinal vein occlusion. Invest Ophthalmol Vis Sci 59(7):2708–2716
Wakabayashi T, Sato T, Hara-Ueno C, Fukushima Y, Sayanagi K, Shiraki N, Sawa M, Ikuno Y, Sakaguchi H, Nishida K (2017) Retinal microvasculature and visual acuity in eyes with branch retinal vein occlusion: imaging analysis by optical coherence tomography angiography. Invest Ophthalmol Vis Sci 58(4):2087–2094
Kang JW, Yoo R, Jo YH, Kim HC (2017) Correlation of microvascular structures on optical coherence tomography angiography with visual acuity in retinal vein occlusion. Retina 37(9):1700–1709
Liu H, Li S, Zhang Z, Shen J (2018) Predicting the visual acuity for retinal vein occlusion after ranibizumab therapy with an original ranking for macular microstructure. Exp Ther Med 15(1):890–896
Chatziralli I, Theodossiadis G, Chatzirallis A, Parikakis E, Mitropoulos P, Theodossiadis P (2018) Ranibizumab for retinal vein occlusion: predictive factors and long-term outcomes in real-life data. Retina 38(3):559–568
Muraoka Y, Tsujikawa A, Murakami T, Ogino K, Miyamoto K, Yoshimura N (2013) Branch retinal vein occlusion-associated subretinal hemorrhage. Jpn J Ophthalmol 57(3):275–282
Zhou JQ, Xu L, Wang S, Wang YX, You QS, Tu Y, Yang H, Jonas JB (2013) The 10-year incidence and risk factors of retinal vein occlusion: the Beijing eye study. Ophthalmology 120(4):803–808
Madanagopalan VG, Kumari B (2018) Predictive value of baseline biochemical parameters for clinical response of macular edema to bevacizumab in eyes with central retinal vein occlusion: a retrospective analysis. Asia Pac J Ophthalmol (Phila) 7(5):321–330
Minami Y, Nagaoka T, Ishibazawa A, Yoshida A (2017) Correlation between short- and long-term effects of intravitreal ranibizumab therapy on macular edema after branch retinal vein occlusion: a prospective observational study. BMC Ophthalmol 17(1):90
Shiono A, Kogo J, Sasaki H, Yomoda R, Jujo T, Tokuda N, Kitaoka Y, Takagi H (2018) Optical coherence tomography findings as a predictor of clinical course in patients with branch retinal vein occlusion treated with ranibizumab. PLoS ONE 13(6):e0199552
Sengul A, Rasier R, Ciftci C, Artunay O, Kockar A, Bahcecioglu H, Yuzbasioglu E (2017) Short-term effects of intravitreal ranibizumab and bevacizumab administration on 24-h ambulatory blood pressure monitoring recordings in normotensive patients with age-related macular degeneration. Eye (Lond) 31(5):677–683
Singh RP, Habbu K, Ehlers JP, Lansang MC, Hill L, Stoilov I (2016) The impact of systemic factors on clinical response to ranibizumab for diabetic macular edema. Ophthalmology 123(7):1581–1587
Arakawa S, Yasuda M, Nagata M, Ninomiya T, Hirakawa Y, Doi Y, Kiyohara Y, Ishibashi T (2011) Nine-year incidence and risk factors for retinal vein occlusion in a general Japanese population: the Hisayama study. Investig Ophthalmol Vis Sci 52(8):5905–5909
Acknowledgements
The principal investigator, Dr. Toshifumi Asano, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. No authors have any potential conflicts of interest to disclose. This paper was supported by JST grants from JSPS KAKENHI Grants-in-Aid for Scientific Research (C) (H.K.40361092). The funders had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
TA, HK and TN were involved in design and conduct of the study. TA, HK, MY, KN, TA and TN were involved in preparation, collection, management, analysis and interpretation of the data. TN approved the manuscript. HK wrote the main manuscript text. TA and HK prepared all tables and all figures, and all authors reviewed the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asano, T., Kunikata, H., Yasuda, M. et al. Ocular microcirculation changes, measured with laser speckle flowgraphy and optical coherence tomography angiography, in branch retinal vein occlusion with macular edema treated by ranibizumab. Int Ophthalmol 41, 151–162 (2021). https://doi.org/10.1007/s10792-020-01562-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01562-7