Abstract
Purpose
The purpose of this prospective, randomized, double-masked, study was to compare the visual performance of patients after bilateral implantation of six different IOLs.
Methods
The following IOLs were used in the study: SV25T0 (n = 19), ATLISA 809M (n = 18), ATLISA Tri 839MP (n = 19), ZKB00 (n = 20), ZLB00 (n = 20) and Symfony ZXR00 (n = 20). Visual performance was assessed by the monocular distance-corrected visual acuity at 4 m (CDVA), distance-corrected intermediate visual acuity (DCIVA) at 60 cm and distance-corrected near visual acuity (DCNVA) at 40 cm. Binocular defocus curves were obtained for a range of defocus from + 1.50 D to − 4.50 D, in 0.50 D steps. Quality of life was assessed with the VF-14 questionnaire.
Results
CDVA was better with the Symfony ZXR00 than with the SV25T0 (p = 0.032), ATLISA Tri 839MP (p = 0.032) and ATLISA 809M (p = 0.018). The Symfony ZXR00 offered the best DCIVA, followed by the ZKB00. The best and worst DCNVA results corresponded to the ZLB00, and the SV25T0 and Symfony ZXR00, respectively. Defocus curves at distance were good in all groups, although the Symfony had a wider range of clear vision (− 1.50 D to + 0.50 D), with no decay. For intermediate vision, only the Symfony obtained sharp visual acuity. The ATLISA 809M, ATLISA Tri 839MP and ZLB00 were superior at near distance.
Conclusions
The extended depth of focus of the Symfony ZXR00 offers a superior range of clear vision at far and intermediate distances than other multifocal designs, with worse results at near distance. Visual outcomes reflect the particular optical, geometrical and power distribution characteristics of each IOL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a large number of presbyopia correcting intraocular lens designs introduced over the last decade. To satisfy the demands for clear intermediate vision, multifocal intraocular lens (MIOL) manufacturers have adopted different strategies. Thus, on the one hand, there has been a corresponding modification of the near add powers of diffractive MIOL models, with a trend towards lower additions in new lens designs, which has also benefited patients in terms of photic phenomena such as halos and glare [1]. On the other hand, trifocal designs have been introduced, with various combinations of intermediate and near add powers to accommodate all visual requirements. Finally, the first extended depth of focus (EDOF) intraocular lens (IOL) received the approval of the U.S. Food and Drug Administration in 2016 (Symfony, Abbott Medical Optics, Inc., Abbott Park, IL, now Johnson and Johnson Surgical Vision, Santa Ana, CA), with many manufacturers currently offering their own EDOF IOL design. In effect, the increasing interest in EDOF designs prompted the American Academy of Ophthalmology to issue a special report in 2017, providing a consensus on the criteria for the definition and performance of EDOF IOLs, as well as for the adequate conditions to determine diverse visual function parameters, mainly defocus curves, contrast sensitivity and intermediate visual acuity [2].
The diversity in surface profiles (aspheric, spherical), focality (bifocal, trifocal, EDOF), physical principle (refractive, diffractive, hybrid, apodized) and additions are attempts to fulfil all the visual performance needs of patients at varying working distances and levels of illumination. Comparative assessments of these varying designs are fraught with difficulties. For instance, to allow for meaningful comparison of near vision performance, near visual acuity charts should be placed at the near focus of each IOL, and the corresponding angular of resolution needs to be adjusted [1]. Similar adjustments must be conducted when assessing near contrast sensitivity and stereoscopic acuity. Defocus curves are considered superior to traditional visual acuity measurements to assess and fully characterize visual function in patients with presbyopia IOLs. Defocus curves determine the visual acuity of patients through different levels of defocus induced with trial lenses (between + 1.50 D and − 2.50 D), defining the depth of focus as the range of lens powers through which a visual acuity of 0.2 logMAR or better is maintained [2,3,4].
The aim of the present study is to evaluate the comparative visual performance of six different presbyopia lenses in a cataract population in a prospective, randomized, double-masked study.
Materials and methods
Study sample
All patients included in the study attended the Ophthalmology Department of Santa Creu and Sant Pau Hospital, Barcelona, Spain, for cataract intervention. Inclusion criteria were defined as age over 60 years, bilateral cataract, good motivation for spectacle independence, potential visual acuity of 20/25 or better and preoperative corneal astigmatism less or equal to 1.25 D. Preoperative exclusion criteria were ocular fundus abnormalities, such as, retinal detachment, history of glaucoma, dry eye requiring ophthalmologic treatment and corneal pathologies. Patients with a history of intraocular or corneal surgery, irresponsive or abnormal pupils, or diabetic retinopathy were also excluded. Intra-operative exclusion criteria were any surgical complication, including pupillary trauma and loss of vitreous. Postoperatively, patients were excluded if the lens could not be placed in the capsular bag. Patients were also excluded if they reported critical visual demands, such as night drivers, and if they had difficulty attending the required follow-up visits.
Participants provided written informed consent after the details of the study were described to them. The study was conducted in accordance with the Declaration of Helsinki tenets of 1975 (as revised in Tokyo in 2004). The study protocol was granted the approval of the Santa Creu and Sant Pau Hospital review board.
Intraocular lenses
Six IOL designs were assessed, including an EDOF lens (Table 1). IOL implantation followed a 1:1:1:1:1:1 block randomization design determined with the IBM SPSS software 25.0 (IBM Corp., Armonk, NY) for Windows.
Surgical intervention
All surgeries were conducted by the same experienced ophthalmic surgeon (M.A.G). For corneal astigmatism over 1.00 D, a clear main corneal incision of 2.75 mm was performed in the steepest corneal meridian, and a secondary paired incision at 180°. Otherwise, incisions were performed to avoid inducing astigmatism. Following phacoemulsification, all IOLs were placed in the capsular bag with the corresponding injectors. Interventions aimed at complete correction of refractive error. Follow-up visits were programmed accordingly. No instances of posterior capsular opacification at 6 months were observed.
Visual acuity evaluation
The Early Treatment Diabetic Retinopathy Study chart (ETDRS) (Optec 6500, Stereo Optical Inc., Chicago, IL) was used to measure monocular corrected distance visual acuity (CDVA) at 4 m. In turn, the tumbling E chart of the Department of Employment and Industry of the Catalan Government was employed to determine distance-corrected intermediate (DCIVA) and near (DCNVA) visual acuities at 60 and 40 cm, respectively. As appropriate, results were converted to logMAR notation.
Defocus curves
Binocular defocus curves were obtained by adding trial lenses from + 1.50 D to − 4.50 D (in 0.50 D steps) over the best-corrected distance refraction. The range of acceptable vision was defined as the range of lens powers over which mean visual acuity (VA) was 20/32 (0.2 logMAR) or better [2], with results over 0.05 logMAR considered clear vision. The same ETDRS chart used for CDVA measurements, placed at 4 m, was employed to evaluate defocus curves. Letters were randomly presented to avoid memorization and patients were asked: “Are you able to read any more letters from the line below?” Given the non-negligible impact of lens magnification, particularly for shorter distances, a correction accounting for a back vertex distance of 12 mm was implemented, as previously described [5].
All examinations for this study were conducted by the same experienced optometrist (C.V.) 6 months after surgery. The optometrist was masked to lens type. Visual acuity and defocus measurements were performed under photopic conditions (85 cd/m2).
In addition, photopic and mesopic pupil diameters were determined with the infrared Colvard pupillometer (Oasis Medical Inc., San Dimas, CA) while patients fixated a distant target, and axial length was measured with infrared interferometry (IOLMaster 500, Carl Zeiss Meditec Iberia S.A.U., Tres Cantos, Madrid, Spain).
Finally, patient satisfaction and quality of life were assessed with the Visual Function 14 (VF-14) questionnaire, which provides an index of functional impairment in patients with cataract [6].
Data analysis
The IBM SPSS software 25.0 was used for statistical analysis. Only one eye per patient, randomly selected, was considered for statistical analysis, except for the evaluation of defocus curves. Data were fist examined for normality using the Kolmogorov–Smirnov test. After ascertaining a Gaussian distribution, descriptive statistics were reported as mean ± standard deviation (SD). The ANOVA test was used to compare IOL groups and, when statistical significance was found, the post hoc Bonferroni test was employed for pair-wise analysis and to account for the possibility of committing a type-I error. Cut-off for statistical significance was a p value < 0.05.
Results
Sample demographics
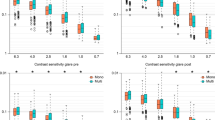
One hundred and sixteen patients met the eligibility criteria and enrolled into the study. There were 33 males and 83 females, with ages ranging from 60 to 86 years. Patients were randomly selected for bilateral implantation with one of the following IOLs: AcrySof ReSTOR SV25T0 (n = 19), ATLISA 809M (n = 18), ATLISA Tri 839MP (n = 19), Tecnis ZKB00 (n = 20), Tecnis ZLB00 (n = 20) and Tecnis Symfony ZXR00 (n = 20). Table 2 shows a summary of demographic data, including age, gender distribution, IOL power and photopic pupil diameter for each lens group. No statistically significant differences were found in any of these variables amongst groups.
Visual acuity evaluation
Monocular visual acuity results at far (4 m), intermediate (60 cm) and near (40 cm) distances for each IOL group are summarized in Table 3 and Fig. 1. Statistically significant differences were encountered in visual acuity amongst the IOL groups at all distances under evaluation.
Overall, patients with the Symfony ZXR00 obtained superior monocular CDVA than patients with any of the other IOLs. Significant differences were found between the Symfony ZXR00 and the SV25T0 (p = 0.032), the ATLISA 809M (p = 0.018) and the ATLISA Tri 839MP (p = 0.032). Differences between these pairs of lenses reached clinical significance (a difference of 0.05 logMAR or more) in all instances. The other IOLs performed similarly at distance.
Regarding monocular DCIVA at 60 cm, the best outcome was also obtained with the Symfony ZXR00, with statistically (and clinical) significant differences with the SV25T0 (p < 0.001), the ZLB00 (p = 0.001), the ATLISA 809M (p < 0.001) and the ATLISA Tri 839MP (p = 0.002). The ZKB00 provided the second best performance at intermediate distance, with statistically (and clinical) significant differences with the SV25T0 (p = 0.033) and the ATLISA 809M (p = 0.043).
Finally, the best outcome for DCNVA at 40 cm corresponded to the ZLB00 group, although differences were only statistically significant between this IOL and the Symfony ZXR00 (p < 0.001). On the contrary, the SV25T0 offered the poorest performance for near distance, with statistically significant differences between this lens and all the other lens groups (all p < 0.05), except the Symfony ZXR00. In effect, the Symfony ZXR00 also obtained poor results in terms of DCNVA, with statistically significant differences with all the other IOLs (all p < 0.05) except with the SV25T0. All statistically significant differences also reached clinical significance. The ZKB00, ATLISA 809M and ATLISA Tri 839MP had similar results at near distance.
Defocus curves evaluation
Binocular visual acuity values for each defocus step (as provided with trial lenses) are summarized in Table 4 and shown in Fig. 2. Figure 2 also displays, for reference, the regions corresponding to simulated distance (0 D, infinite), intermediate (− 1.5 D, 66 cm) and near vision (− 3 D, 33 cm). Overall, no particular IOL group was superior at all distances, although the Symfony ZXR00 offered the largest range of lens powers (from − 1.50 D to + 0.50 D) over which VA was better than 0.05 logMAR, without any significant decay of the curve. In contrast, the range of clear vision for the other lens groups extended from − 0.50 D to + 0.50 D, being slightly larger in patients with the SV25T0. Accordingly, whereas the shape of the defocus curve of the Symfony ZXR00 corresponds to a single focus extending from intermediate to far distances, all the other lens groups present a biphasic curve profile (a clearly defined focus for distance vision and a second focus located approximately at the area of near or intermediate vision, depending on each lens add power).
All IOLs provided good VA (< 0.050 logMAR) at distance, with the Symfony ZXR00 offering statistically significant better VA than the ZKB00 (p = 0.005), the ZLB00 (p = 0.012) and the ATLISA 809M (p = 0.007). At the intermediate region, only the Symfony ZXR00 group offered good VA (< 0.050 logMAR), with statistically significant differences with all the other lens groups (all p < 0.05). At the other range of the spectrum, the ATLISA 809M provided the worst performance at 66 cm, with statistically significant differences with all the other lens groups (all p < 0.05), except for the SV25T0. It is worth noting that, whereas the ZKB00 did not provide good results at 66 cm, it was the best IOL at the distance of 50 cm (− 2.00 D). Finally, at the near vision region of 33 cm (− 3.00 D), none the IOLs under study offered clear vision (< 0.050 logMAR), although VA was acceptable (< 0.200 logMAR) with the ATLISA 809M, followed by the ATLISA Tri 839MP and the ZLB00.
Interestingly, of the IOLs characterized by biphasic curves, only the ATLISA 809M had the near focus at 33 cm, with a range of acceptable vision between 28.5 cm and 40 cm (− 3.50 D to − 2.50 D). Similarly, the near vision focus of the ATLISA Tri 839MP and the ZLB00 corresponded to 40 cm (− 2.50 D), with a range of acceptable vision from − 3.00 D to − 2.00 D. Finally, the ZKB00 and the SV25T0 had their near focus at 50 cm (− 2.00 D), with statistically significant differences in VA between the ZKB00 and both the SV25T0 (p = 0.044) and the ATLISA 809M (p < 0.001). The ATLISA 809M had the worst outcome at this distance.
Finally, statistically significant differences were found amongst the different IOLs in terms of quality of life, as measured with the VF-14 questionnaire (p < 0.001). Pair-wise, statistically significant differences were encountered between the Symfony (median score of 99, range from 94 to 100) and the SV25T0 (median score of 95, range from 72 to 100) (p = 0.003), the ATLISA Tri 839MP (median score of 97, range from 84 to 100) (p = 0.039) and the ATLISA 809M (median score of 95, range from 78 to 100) (p = 0.015).
Discussion
Monocular visual acuity evaluation
Visual acuity at distance and with the best correction was similarly good for all IOL designs, although the EDOF lens Symfony ZXR00 outperformed some of the diffractive MIOLs with a bifocal (SV25T0 and ATLISA 809M) and trifocal (ATLISA Tri 839MP) designs. These findings are partly in agreement with previous research reporting comparable performance in terms of VA at distance with bifocal, trifocal and EDOF designs [7,8,9].
Regarding intermediate distance, the best results were obtained with the EDOF design, with an estimated addition of + 1.73 D (58 cm). These findings are in agreement with previous reports documenting best intermediate visual acuity with different EDOF designs [8,9,10]. After the Symfony ZXR00, the second best outcome at this distance was obtained with the diffractive and aspheric ZKB00, with a low addition of + 2.75 D, in agreement with previous researchers describing better performance at intermediate distances for low add power MIOL designs [1, 8, 11]. Many patients over 65 years of age report the need for good intermediate visual acuity to operate electronic devices such as desktop computers, which are commonly viewed at 60 cm. Therefore, this was the selected distance to evaluate intermediate vision, rather than the 70 cm favoured in previous studies or the 65 cm defined as intermediate as per presbyopia spectacle conventions.
Finally, DCNVA was assessed at 40 cm, which may be considered a comfortable reading distance. The add powers of the different IOLs under evaluation provided near foci at distances ranging from 26.7 cm (ATLISA 809M) to 40 cm (SV25T0). Interestingly, however, the worst outcome at 40 cm was obtained with the diffractive-refractive SV25T0, with add power of + 2.50 D, and, less unexpectedly, with the Symfony ZXR00, which has an add power of approximately + 1.73 D and does not show a biphasic defocus profile. Better and similar results were obtained with IOLs of high add power, with the ZLB00 (add power of + 3.25 D) displaying the best outcome. These findings are in agreement with those reported by Kretz and co-workers, with slightly better DCNVA values with the ZLB00 in the present study (0.052 logMAR, versus 0.100 logMAR) [12].
It must be noted that for intermediate, and particularly for near visual acuity evaluation, the selection of the appropriate distance is critical, as the outcome of each lens design at near is governed, amongst other factors, by add power. Therefore, comparison between studies assessing different distances and add powers may be a challenging task. On this context, for instance, published literature has explored near vision in a range of distances from 33 to 40 cm [13,14,15].
Defocus curves evaluation
As previously described, the bifocal and trifocal lens designs under evaluation displayed a biphasic defocus curve profile, with a clearly defined distant focus and a second focus located adjacent to the near or intermediate regions, depending on the specific add power of each lens [1, 8, 16]. As may be observed in Fig. 2, only the EDOF IOL showed a profile with a single extended focus, without decay and loss of VA in the range from intermediate to distance vision [8]. Indeed, the Symfony ZXR00, with a clear vision range from − 1.75 D to + 0.50 D, would be an ideal choice for those patients with high visual demands at far and intermediate distances. In comparison, all the other IOLs presented a decay in the defocus curve between the near and distant foci, which was associated with a moderate visual acuity loss. However, with the exception of the ATLISA 809M, VA in the area between foci remained over the acceptable threshold of 0.2 logMAR. Interestingly, in all biphasic curves the near focus was less pronounced than the distant focus, implying that visual acuity at this focus was poorer. This is particularly evident for the lens SV25T0, which presents an almost non-identifiable near peak at approximately − 2.00 D (50 cm). As for the rest of the IOLs, the slope of the decay of the curves for defocus steps beyond their respective near foci is very different, with the ATLISA Tri 839MP displaying the steepest decay immediately beyond − 3.00 D and the ATLISA 809M showing a less pronounced decay, thus allowing acceptable vision up to − 3.50 D of induced defocus.
In turn, whereas patients with the trifocal ATLISA Tri 839MP achieved lower VA values at the − 1.50 D defocus step than those with the ZKB00, and similar than those with the ZLB00, the ATLISA Tri 839MP outperformed both ZKB00 and ZLB00 at the − 1.00 D defocus step. In contrast, published literature describes trifocal designs as the lens of choice for far and intermediate distances, similar in performance to EDOF designs at distances from 50 cm to 67 cm [13,14,15] or even superior at 67 cm [17]. Overall, in view of the results of the present study, the best option for intermediate distance would be the Symfony ZXR00.
None of the IOLs under study allowed clear vision (defined as < 0.05 logMAR) at near distance, although the best outcomes were obtained with the ATLISA 809M, the ATLISA Tri 839MP and the ZLB00. Specifically, the ATLISA 809M presented a near VA peak at 33 cm, with a range of acceptable vision between 28.5 cm and 40 cm (− 3.50 D to − 2.50 D). In comparison, both the ATLISA Tri 839MP and the ZLB00 displayed a peak at 40 cm, with a range of acceptable vision between 33 cm and 50 cm (− 3.00 D to − 2.00 D). However, it is relevant to mention, particularly when considering patients requiring good vision at intermediate distances, that the ATLISA 809M showed a very marked biphasic profile, with a sharp decay in the defocus curve in the region between both foci, corresponding to intermediate vision.
It must be noted that the evaluation of defocus curves is not devoid of limitations. For instance, VA values may not reflect real-life conditions. Indeed, although accommodation after cataract surgery is not possible, convergence and pupil miosis take place, resulting in an overestimation of the depth of focus, mainly at short distances [3, 18, 19]. In addition, although the recommendations of the American Academy of Ophthalmology concerning defocus curve testing require monocular measurements [2], the present study describes binocular measurements, as these may be expected to better simulate daily tasks conducted by patients following cataract intervention [20]. Similarly, it may be argued that defocus curves describe visual performance of patients after binocular summation and do not provide a direct measurement of each specific lens performance. However, as the purpose of this study was to explore the outcome of patients with different lens designs, we believe the results of the defocus analysis to be relevant and easily interpretable.
In conclusion, the joint assessment of distance, intermediate and near visual acuity, and of defocus curves has proved particularly relevant when comparing different IOLs designs, including bifocal, trifocal and EDOF lenses. These findings have revealed optimal combinations of patient requirements and lens designs, which may be useful to the ophthalmologist when selecting the best IOL option for each patient.
References
Alfonso JF, Fernandez-Vega L, Puchades C, Montés-Micó R (2010) Intermediate visual function with different multifocal intraocular lens models. J Cataract Refract Surg 36:733–739. https://doi.org/10.1016/j.jcrs.2009.11.018
MacRae S, Holladay JT, Glasser A, Calogero D, Hilmantel G, Masket S et al (2017) Special report: American academy of ophthalmology task force consensus statement for extended depth of focus intraocular lenses. Ophthalmology 124:139–141. https://doi.org/10.1016/j.ophtha.2016.09.039
Pieh S, Kellner C, Hanselmayer G, Lackner B, Schmidinger G, Walkow T et al (2002) Comparison of visual acuities at different distances and defocus curves. J Cataract Refract Surg 28:1964–1967. https://doi.org/10.1016/S0886-3350(02)01317-2
Akella SS, Juthani VV (2018) Extended depth of focus intraocular lenses for presbyopia. Curr Opin Ophthalmol 29:318–322. https://doi.org/10.1097/ICU.0000000000000490
Gupta N, Wolffsohn JSW, Naroo S (2008) Optimizing measurement of subjective amplitude of accommodation with defocus curves. J Cataract Refract Surg 34:1329–1338. https://doi.org/10.1016/j.jcrs.2008.04.031
Steinberg EP, Tielsch JM, Schein OD, VF-14 (1994) An index of functional impairment in patients with cataract. Arch Ophthalmol 112:630–638. https://doi.org/10.1001/archopht.1994.01090170074026
Vilar C, Hida WT, de Medeiros AL, Magalhaes KRP, de Moraes Tzelikis PF, Chaves MAPD et al (2017) Comparison between bilateral implantation of a trifocal intraocular lens and blended implantation of two bifocal intraocular lenses. Clin Ophthalmol 11:1393–1397. https://doi.org/10.2147/OPTH.S139909
Pedrotti E, Carones F, Aiello F, Mastropasqua R, Bruni E, Bonacci E et al (2018) Comparative analysis of visual outcomes with 4 intraocular lenses: monofocal, multifocal, and extended range of vision. J Cataract Refract Surg 44(2):156–167. https://doi.org/10.1016/j.jcrs.2017.11.011
Savini G, Schiano-Lomoriello D, Balducci N, Barboni P (2018) Visual performance of a new extended depth-of-focus intraocular lens compared to a distance-dominant diffractive multifocal intraocular lens. J Refract Surg 34(4):228–235. https://doi.org/10.3928/1081597X-20180125-01
Hogarty DT, Russell DJ, Ward BM, Dewhurst N, Burt P (2018) Comparing visual acuity, range of vision and spectacle independence in the extended range of vision and monofocal intraocular lens. Clin Exp Ophthalmol 46:854–860. https://doi.org/10.1111/ceo.13310
Yoo YS, Whang WJ, Byun YS, Piao JJ, Kim DY, Joo CK et al (2018) Through-focus optical bench performance of extended depth-of-focus and bifocal intraocular lenses compared to a monofocal lens. J Refract Surg 34(4):236–243. https://doi.org/10.3928/1081597X-20180206-04
Kretz FT, Koss MJ, Auffarth GU, ZLB00 Study Group (2015) Intermediate and near visual acuity of an aspheric, bifocal, diffractive multifocal intraocular lens with +3.25 D near addition. J Refract Surg 31(5):295–299. https://doi.org/10.3928/1081597X-20150423-02
Escandón-García S, Ribeiro FJ, McAlinden C, Queirós A, González-Méijome JM (2018) Through-focus vision performance and light disturbances of 3 new intraocular lenses for presbyopia correction. J Ophthalmol 2018:6165493. https://doi.org/10.1155/2018/6165493
Ferreira-Ríos I, Zuñiga-Posselt K, Serna-Ojeda JC, Chávez-Mondragón E (2018) Objective and subjective results following implantation of the FineVision trifocal intraocular lens in Mexican patients. Int Ophthalmol 38:2617–2622. https://doi.org/10.1007/s10792-017-0725-x
Mojzis P, Kukuckova L, Majerova K, Ziak P, Piñero P (2017) Postoperative visual performance with a bifocal and trifocal diffractive intraocular lens during a 1-year follow-up. Int J Ophthalmol 10(10):1528–1533. https://doi.org/10.18240/ijo.2017.10.08
García-Bella J, Ventura-Abreu N, Morales-Fernández L, Talavero-González P, Carball-Álvarez J, Sanz-Fernández JC et al (2018) Visual outcomes after progressive apodized diffractive intraocular lens implantation. Eur J Ophthalmol 28(3):282–286. https://doi.org/10.5301/ejo.5001030
Monaco G, Gari M, Di Censo F, Poscia A, Ruggi G, Scialdone A (2017) Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg 43(6):737–747. https://doi.org/10.1016/j.jcrs.2017.03.037
Breyer DRH, Kaymak H, Ax T, Kretz FTA, Auffarth GU, Hagen PR (2017) Multifocal intraocular lenses and extended depth of focus intraocular lenses. Asia Pac J Ophthalmol 6(4):339–349. https://doi.org/10.22608/APO.2017186
Charman WN, Whitefoot H (1977) Pupil diameter and the depth-of-field of the human eye as measured by laser speckle. Opt Acta 24:1211–1216. https://doi.org/10.1080/713819479
Wolffsohn JS, Jinabhai AN, Kingsnorth A, Sheppard AL, Naroo SA, Shah S et al (2013) Exploring the optimum step size for defocus curves. J Cataract Refract Surg 39(6):873–880. https://doi.org/10.1016/j.jcrs.2013.01.031
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gil, M.A., Varón, C., Cardona, G. et al. Visual acuity and defocus curves with six multifocal intraocular lenses. Int Ophthalmol 40, 393–401 (2020). https://doi.org/10.1007/s10792-019-01196-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01196-4