Abstract
Purpose
To evaluate changes in macular thickness, ganglion cell layer/inner plexiform layer (GCL/IPL) thickness, and retinal nerve fiber layer (RNFL) thickness in normal eyes and glaucomatous eyes using spectral domain optical coherence tomography (SD-OCT).
Methods
We enrolled 89 eyes (all left eyes), including 45 (of 45 patients) eyes with glaucoma and 44 (of 44 patients) normal eyes. The data from macular measurements using spectral domain optical coherence tomography were analyzed according to groups divided by age and glaucoma status. The macular thickness analysis, GCL/IPL thickness, and RNFL thickness values determined by SD-OCT scans were compared among the groups.
Results
Mean macular thickness decreased significantly with age or glaucoma. Mean GCL/IPL thickness decreased significantly in glaucomatous eyes in all sectors but did not decrease with age. Mean RNFL thickness, which was divided into four quadrants (superior, nasal, inferior, and temporal), decreased significantly in glaucomatous eyes at all quadrants and decreased in the temporal quadrant with age in non-glaucomatous eyes. No significant differences were detected between eyes with normal tension glaucoma (NTG) and primary open angle glaucoma (POAG) in all sectors of mean GCL/IPL thickness, RNFL thickness, and macular thickness.
Conclusions
No significant difference in mean thickness was detected between eyes with NTG and POAG. Some of the sectors of RNFL thickness decreased with age or glaucoma. GCL/IPL thickness, however, decreased in glaucomatous eyes but not with age. Therefore, GCL/IPL thickness is less influenced by age when monitoring patients with glaucoma or suspect glaucoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glaucoma is characterized by dysfunction and loss of retinal ganglion cells (RGCs), with resulting structural changes to the optic nerve head, retinal nerve fiber layer (RNFL), and ganglion cell and inner plexiform layers as well as loss of the visual field [1]. The goal of glaucoma management is to slow down the rate of progressive neural losses and to preserve visual function. Because the macula has the highest density of RGCs [2, 3], macular imaging could be a valuable method to assess neural damage in patients with glaucoma. Some studies suggest that glaucomatous damage can be detected in the inner retina or ganglion cell complex, i.e., combined thickness of the RNFL, ganglion cell layer (GCL), and inner plexiform layer (IPL), early during the disease process using spectral domain optical coherence tomography (SD-OCT) [4, 5]. It has been suggested that the macular GCL thickness may be the most relevant parameter to measure in patients with glaucoma [6]. Given the resolution of SD-OCT, accurate measurement of the GCL alone is not practical, but it can be used to calculate the combined thickness of the GCL and IPL. Although GCL/IPL thickness measurements may better reflect RGC counts, no study has evaluated the thickness changes with age and between glaucomatous and healthy eyes.
The purpose of this study was to evaluate the changes in mean macular thickness, GCL/IPL thickness, and RNFL thickness with regard to age in normal and glaucomatous eyes using SD-OCT.
Materials and methods
Subjects and protocols
The medical records of patients who underwent SD-OCT examinations at Dankook University Hospital between January 2012 and November 2014 were reviewed. All patients underwent a chart review in addition to SD-OCT examinations. This study was approved by the institutional review board of Dankook University Hospital, Korea and complied with the tenets of the Declaration of Helsinki.
In total, 25 eyes (of 25 patients) with normal tension glaucoma (NTG) and 20 eyes (of 20 patients) with primary open angle glaucoma (POAG) were included, and their data were compared with those of 27 age-matched normal eyes (of 27 patients). The 27 age-matched normal eyes were compared again with those of 17 younger normal eyes (of 17 patients). Only left eyes were selected and analyzed. The eyes were divided into four groups by age and glaucoma status (Group 1: 20–29 years of age, Group 2: 60–69 years of age, Group 3: 60–69 years of age with NTG, Group 4: 60–69 years of age with POAG).
The inclusion criteria for glaucomatous eyes were: best-corrected visual acuity of 20/40 or better with a spherical equivalent within ± 3 diopters; a normal anterior segment on a slit-lamp examination; open angle on gonioscopic examination; age ≥ 60 years; and diagnosis of glaucoma by the principal investigator or co-investigator. The glaucoma diagnosis was based on a characteristic glaucomatous structural change to the optic disc accompanied by glaucomatous visual field defects. The criteria for a glaucomatous visual field defect were: glaucoma hemifield test outside the normal limit, pattern standard deviation with a P value < 5%, or a cluster of < 3 points in the pattern deviation plot in a single hemifield (superior or inferior) with a P value of < 5%, one of which must have a P value of < 1%. Anyone of the preceding criteria, if repeatable, was considered sufficient evidence of a glaucomatous visual field defect.
Exclusion criteria were as follows in addition to those who do not meet the inclusion criteria: a history of ocular inflammation or trauma, and the presence of concurrent retinal disease (i.e., vascular disorder or macular degeneration), optic nerve disease other than glaucoma, or a brain disorder that could influence the visual field results.
The inclusion criteria for age and refractive error matched healthy eyes were a best-corrected visual acuity of 20/40, a normal anterior segment on a slit-lamp examination, no RNFL defects in red-free fundus photographs, no visual field defects, and intraocular pressure ≤ 21 mmHg.
Optical coherence tomography
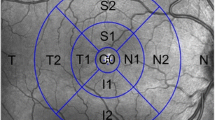
Cirrus HD-OCT software ver. 6.0.0.599 (Carl Zeiss Inc., Zena, Germany) was used to acquire the mean macular thickness, GCL/IPL thickness, and RNFL thickness measurements. This device has been described in detail previously [7]. The protocols used were Macular Cube 512 × 128 for the mean macular thickness measurements (Fig. 1) and GCL/IPL thickness measurements and Optic Disc Cube 200 × 200 for the mean RNFL thickness measurements. The Cirrus HD-OCT images were reviewed and included if signal strength was > 6, if movement artifacts and segmentation errors were absent, and there was good centering on the fovea for the macular cube protocols. The Cirrus HD-OCT ganglion cell analysis (GCA) algorithm was used to process the data and detects and measures the thickness of the macular GCL/IPL within a 14.13-mm2 elliptical annulus area centered on the fovea (Fig. 2). The size and shape of the annulus were chosen because this conforms more closely to actual anatomy, and this annulus corresponds to the area where the RGC layer is thickest in normal eyes [8]. The GCA algorithm processes data from three-dimensional volume scans using the Cirrus macular 512 × 128 × 1024 acquisition protocol. Processing is performed in three dimensions. A region of interest for the algorithm is chosen using the existing retinal pigment epithelium and inner limiting membrane segmentation in Cirrus software to include the retinal tissue and exclude the choroid and vitreous from consideration [9, 10].
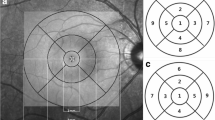
The Optic Disc Cube 200 × 200 consists of 40,000 axial scans (in a 6 × 6 × 2 mm cube) centered on the optic disc. The average RNFL thickness and RNFL thickness in quadrants and clock-hour sectors on a measurement circle 3.46 mm in diameter were calculated, and their deviation from a normative database is provided in a color-coded scheme [11] (Fig. 3). This means 3 o’clock would be temporal retina and 9 o’clock would be nasal.
Example of optic nerve head and retinal nerve fiber layer analysis with optic disc cube 200 × 200 protocol in a normal patient. The average RNFL thickness and RNFL thickness in quadrants and clock-hour sectors on a measurement circle 3.46 mm in diameter are calculated, and their deviation from a normative database is provided in a color-coded scheme. RNFL retinal nerve fiber layer, C/D ratio cup/disc ratio
Statistical analysis
Statistical analyses were performed using PASW Statistics for Windows ver. 18.0 (SPSS Inc., Chicago, IL, USA). The independent sample t-test was used to compare differences in mean age, sex, refractive error, mean deviation (MD), pattern standard deviation (PSD), best-corrected visual acuity (BCVA) between normal and glaucomatous eyes, associations of the mean GCL/IPL thickness, and the mean macular thickness measurements between normal eyes and eyes with glaucoma. A P value < 0.05 was considered significant.
Results
The mean (standard deviation) age was 25.35 ± 0.70 years (range 25–27 years) for Group 1 (normal young age), 64.63 ± 5.43 years (range 60–73 years) for Group 2 (normal old age), 64.08 ± 2.84 years (range 60–70 years) for Group 3 (glaucomatous eyes, NTG), and 63.00 ± 3.16 years (range 60–69 years) for Group 4 (glaucomatous eyes, POAG). The demographic and clinical information for each group is summarized in Table 1. No significant differences were observed in age, sex, refractive error, MD, PSD, or BCVA between Groups 2–4. Refractive error and BCVA were significantly higher, whereas age was significantly lower in Group 1 than those in Group 2.
The mean GCL/IPL thickness and the mean macular thickness measurements in the four groups are shown in Table 2. Groups 3 and 4 had significantly thinner mean GCL/IPL thicknesses than that of Group 2 in all macular sectors. Mean macular thickness was thinner in Groups 3 and 4 than that in Group 2 (P < 0.001). Mean macular thickness (P = 0.002) was thinner in Group 2 than those in Group 1, but none of GCL/IPL thickness was significantly thinner in Group 2 than Group 1.
The mean RNLF thickness measurements in the four groups are shown in Table 3. Groups 3 and 4 had significantly thinner mean RNFL thicknesses than that in Group 2 at almost all clock-hour sectors, except the 8 (nasal-inferior) and 9 (nasal) o’clock sectors in Group 3 and 3 (temporal) and 9 o’clock sectors in Group 4. The 1 (superior-temporal), 3, 4 (temporal-inferior), and 8 o’clock sectors were significantly thinner in Group 2 than those in Group 1. Since the 3 o’clock and 4 o’clock sectors correspond to temporal quadrants, we can see that the quadrant average and the clock-hour sector average are consistent.
No significant differences were observed between Groups 3 and 4 in all sectors for mean GCL/IPL thickness, RNFL thickness, or macular thickness. Representative examples of OCT images are shown in Fig. 4.
Representative examples of OCT images for each group. The sectors of RNFL thickness decreased with age or glaucoma. GCL/IPL thickness, however, decreased in glaucomatous eyes but not with age. Unit: µm. NTG normal tension glaucoma, GCL/IPL ganglion cell-inner plexiform layer, RNFL retinal nerve fiber layer
Discussion
The diagnosis of glaucoma in its early stages is challenging. Misdiagnosis can lead to failure to identify individuals with the condition during the early stages until the significant functional loss has occurred. Thus, detecting glaucoma early allows for early treatment to delay vision loss. Efforts to detect glaucoma early have resulted in new imaging methods. One of these methods is SD-OCT. Use of OCT to detect glaucoma has been investigated previously in several studies, which suggested it as an additional tool for managing the disease [12,13,14,15].
Glaucoma is characterized by loss of RGCs and their respective axons, which make up the RNFL [16]. Considering the importance of evaluating the RNFL in patients with glaucoma, numerous experimental and clinical studies using OCT have documented RNFL loss or thinning in glaucomatous eyes compared to controls and even in those in the early stages of the disease [17,18,19,20,21,22].
Mwanza et al. [23] showed that GCL/IPL thickness is a good indicator of glaucoma and that its glaucoma diagnostic performance is similar to that of RNFL. Subsequent studies by other groups [24,25,26,27] built on this work to confirm these findings and to show the usefulness of GCL/IPL for diagnosing glaucoma.
In this study, we investigated the changes in macular thickness and GCL/IPL and RNFL parameters with regard to age in normal eyes and glaucomatous eyes using SD-OCT.
We found that GCL/IPL and macular thicknesses were thinner in eyes with glaucoma than in normal eyes, which was expected and was similar to results reported by Mwanza et al. [23].
Another finding was that 1, 3, 4, and 8 sectors of RNFL thickness decreased, but none of the sectors of GCL/IPL thickness decreased with age. Other studies have shown that decreased RNFL thickness with age mainly appears in the superior and inferior quadrants. Alasil et al. [28] and Feuer et al. [29] reported that the superior quadrant of RNFL thickness decreases the most. However, the subjects in these studies were mostly Caucasian or Hispanic and varied in age from 10 to 90 years. We used all Asians (Koreans) and only two age range groups of 20 and 60 s.
In conclusion, our results suggest that GCL/IPL thickness is less affected by age than RNFL thickness when monitoring patients with glaucoma and suspected glaucoma. And also because GCL/IPL thickness was decreased only in glaucomatous eyes and not with age, it correlates with one of the definitions of glaucoma, which is selective damage of ganglion cell layer. Further studies will be needed with the large scale of subjects to support this result.
References
Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma: a review. JAMA 311:1901–1911
Knighton RW, Gregori G (2012) The shape of the ganglion cell plus inner plexiform layers of the normal human macula. Invest Ophthalmol Vis Sci 53:7412–7420
Hood DC, Raza AS, de Moraes CG, Odel JG, Greenstein VC, Liebmann JM et al (2011) Initial arcuate defects within the central 10 degrees in glaucoma. Invest Ophthalmol Vis Sci 52:940–946
Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G et al (2009) Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology 116(12):2305–2314
Kotera Y, Hangai M, Hirose F, Mori S, Yoshimura N (2011) Three dimensional imaging of macular inner structures in glaucoma by using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 52(3):1412–1421
Nakano N, Hangai M, Nakanishi H, Mori S, Nukada M, Kotera Y et al (2011) Macular ganglion cell layer imaging in preperimetric glaucoma with speckle noise-reduced spectral domain optical coherence tomography. Ophthalmology 118(12):2414–2426
Medeiros FA, Lisboa R, Weinreb RN, Liebmann JM, Girkin C, Zangwill LM (2013) Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology 120:736–744
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300:5–25
Geitzenauer W, Kiss CG, Durbin MK, Abunto MT, Callan TM, Stetson PF et al (2010) Comparing retinal thickness measurements from Cirrus spectral domain- and Stratus time domain-optical coherence tomography. Retina 30:596–606
Legarreta JE, Gregori G, Punjabi OS, Knighton RW, Lalwani GA, Puliafito CA (2008) Macular thickness measurements in normal eyes using spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging 39(Suppl):S43–S49
Kouros NM, Sara N, Nariman N, Cirineo N, Knipping S, Giaconi J et al (2013) Macular ganglion cell/Inner plexiform layer measurements by spectral domain optical coherence tomography for detection of early glaucoma and comparison to retinal nerve fiber layer measurements. Am J Ophthalmol 156(6):1297–1307
Lin SC, Singh K, Jampel HD, Hodapp EA, Smith SD, Francis BA et al (2007) Optic nerve head and retinal nerve fiber layer analysis: a report by the American Academy of Ophthalmology. Ophthalmology 114:1937–1949
Chen TC, Zeng A, Sun W, Mujat M, deBoer JF (2008) Spectral domain optical coherence tomography and glaucoma. Int Ophthalmol Clin 48:29–45
Lu AT, Wang M, Varma R, Schuman JS, Greenfield DS, Smith SD et al (2008) Combining nerve fiber layer parameters to optimize glaucoma diagnosis with optical coherence tomography. Ophthalmology 115:1352–1357
Leite MT, Rao HL, Zangwill LM, Weinreb RN, Medeiros FA (2011) Comparison of the diagnostic accuracies of the spectralis, cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology 118(7):1334–1339
Quigley HA, Dunkelberger GR, Green WR (1989) Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 107:453–464
Kanamori A, Kakamura M, Escano MF, Seya R, Maeda H, Negi A (2003) Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol 135:513–522
Quigley HA, Pease ME (1996) Change in the optic disc and nerve fiber layer estimated with the glaucoma-scope in monkey eyes. J Glaucoma 5:106–116
Mistlberger A, Liebmann JM, Greenfield DS, Pons ME, Hoh ST, Ishikawa H et al (1999) Heidelberg retina tomography and optical coherence tomography in normal, ocularhypertensive, and glaucomatous eyes. Ophthalmology 106:2027–2032
Carpineto P, Ciancaglini M, Zuppardi E, Falconio G, Doronzo E, Mastropasqua L (2003) Reliability of nerve fiber layer thickness measurements using optical coherence tomography in normal and glaucomatous eyes. Ophthalmology 110:190–195
Schuman JS, Hee MR, Puliafito CA, Wong C, Pedut-Kloizman T, Lin CP et al (1995) Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol 113:586–596
Guedes V, Schuman JS, Hertzmark E, Wollstein G, Correnti A, Mancini R et al (2003) Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology 110:177–189
Mwanza JC, Durbin MK, Budenz DL, Sayyad FE, Chang RT, Neelakantan A et al (2012) Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology 119:1151–1158
Kotowski J, Folio LS, Wollstein G, Ishikawa H, Ling Y, Bilonick RA et al (2012) Glaucoma discrimination of segmented Cirrus spectral domain optical coherence tomography (SD-OCT) macular scans. Br J Ophthalmol 96:1420–1425
Takayama K, Hangai M, Durbin M, Nakano N, Morooka S, Akagi T et al (2012) A novel method to detect local ganglion cell loss in early glaucoma using spectral domain optical coherence tomography. Invest Ophthalmol VisSci 53:6904–6913
Akashi A, Kanamori A, Nakamura M, Fujihara M, Yamada Y, Negi A (2013) Comparative assessment for the ability of Cirrus, RTVue and 3D-OCT to diagnose glaucoma. Invest Ophthalmol Vis Sci 54:4478–4484
Jeoung JW, Choi YJ, Park KH, Kim DM (2013) Macular ganglion cell imaging study: glaucoma diagnostic accuracy of spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 54:4422–4429
Alasil T, Wang K, Keane PA, Lee H, Baniasadi N, de Boer JF et al (2013) Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma 22(7):532–541
Feuer WJ, Budenz DL, Anderson DR, Cantor L, Greenfield DS, Savell J et al (2011) Topographic differences in the age-related changes in the retinal nerve fiber layer of normal eyes measured by Stratus optical coherence tomography. J Glaucoma 20:133–138
Acknowledgements
This study was approved by the institutional review board of Dankook University Hospital, Korea and complied with the tenets of the Declaration of Helsinki.
Author’s contribution
JWJ, MWL collected the data, made the analysis and composed the manuscript. KJC designed the study, reviewed and approved the manuscript. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI15C1524).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Rights and permissions
About this article
Cite this article
Jang, J.W., Lee, M.W. & Cho, K.J. Comparative analysis of mean retinal thickness measured using SD-OCT in normal young or old age and glaucomatous eyes. Int Ophthalmol 38, 2417–2426 (2018). https://doi.org/10.1007/s10792-017-0744-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-017-0744-7