Abstract
Purpose
The aim of the present study was to investigate the changes in intraocular pressures (IOP) in patients who underwent pulsatile and non-pulsatile cardiopulmonary bypass (CPB).
Methods
A total of 42 patients operated for elective coronary bypass surgery (CABG) on CPB were randomly allocated to pulsatile (Group P) and non-pulsatile (Group N) groups. Pulsatile flow was applied to Group P patients during crops-clamp period. The IOP measurements were made before and after the induction of anesthesia, before the onset of CPB, on the 5th, 15th, 30th, 45th, and 60th min of CPB, after CPB and at the end of the operation. The results of repetitive measurements were analyzed at different intervals and in two groups.
Results
The second IOP measurements of right and left eyes displayed statistically significant decreases from the baseline level [11.9 ± 2.9 (p = 0.0001) and 12.5 ± 3.2 (p = 0.0001), respectively]. The significant decrease in the IOP values persisted in the repeated measurements except for the 5th min of CPB values [17.0 ± 3.5 (p = 0.346) and 16.7 ± 3.6 (p = 0.399)]. Comparison of two groups demonstrated significant differences at pre-CPB (right 12.8 ± 2.3 vs. 10.8 ± 2.4; p = 0.013 and left 13.3 ± 2.4 vs. 11.5 ± 2.5; p = 0.023), and 5th min of CPB measurements (right 18.5 ± 3.1 vs. 15.9 ± 3.4; p = 0.015; left 18.2 ± 3.0 vs. 15.7 ± 3.6; p = 0.019).
Conclusion
We noted a steady decrease in repeated IOP measurements except for the transient increase in CPB values on 5th min. The IOP values were higher in pulsatile CPB group in pre-CPB and 5th min of CPB measurements; however, the difference was not significant in the repeated measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cardiopulmonary bypass (CPB) procedure provides extracorporeal circulation and can avoid the challenge of operating on the beating heart. Remarkable hemodynamic changes can occur in end organs during CPB. Postoperative ocular complications occur rarely after cardiac surgery on CPB [1]. Their pathophysiology is poorly understood and hazardous outcomes such as permanent loss of vision may be seen [2]. The incidence in literature ranges from 0.06 to 0.33 % in recently reported series [2–4].
Multiple physiologic alterations such as arterial embolism, hypotension, and hypothermia encountered during CPB may be responsible for the development of these complications. Most frequent type of injury in visual pathway in patients undergoing cardiac surgery with CPB is anterior ischemic optic neuropathy [2, 5].
One of the important factors associated with ocular complications during cardiac surgery is the relationship between CPB and intraocular pressures (IOP) [2, 6]. Induction of CPB may influence dynamics of aqueous humor fluid, choroidal blood volume, and muscular tone of extraocular muscles [6]. Increased intraocular pressure is considered to be an important risk factor attributed to the diminished perfusion pressures of the optic nerve which may eventually lead to ischemic optic neuropathy [2]. In relevant publications, increased or unchanged IOPs were reported during CPB [7–9]. However, vast majority of these publications have been made several decades ago and owing to the current developments in anesthetic and surgical techniques, impact of CPB on IOP may have changed.
The aim of the current study was to investigate changes in IOP during pulsatile and non-pulsatile CPB in patients undergoing elective coronary bypass surgery (CABG).
Patients and methods
Study design
This prospective, observational, cohort study included 42 patients scheduled for elective CABG with CPB. The approval of the local institutional review board and written informed consents of all patients have been obtained prior to the study. Patients with glaucoma, traumatic eye, and keratopathy were excluded from the study.
Patients were randomized to pulsatile (Group P) and non-pulsatile (Group N) CPB groups by closed envelope method. Pulsatile flow was applied to Group P patients during crops-clamp period. Concomitant hemodynamic and arterial blood gas measurements were recorded.
Measurement of intraocular pressure
The IOP measurements were made by the same ophthalmologist with a Tonopen® XL hand held tonometer (Medtronic SOLAN, Jacksonville, FL, USA). The calibration of the tonometer was made before each operation in the operating room before the operation was begun. The IOP measurements were made at the following times: (T1) before anesthesia induction (baseline), (T2) after anesthesia induction, (T3) before onset of CPB, at the (T4) 5th, (T5) 15th, (T6) 30th, (T7) 45th, and (T8) 60th min of CPB, (T9) after CPB and (T10) at the end of the operation.
Preoperative demographic features and operative details were gathered in a prospectively recorded database. The placements of all patient lines (arterial and venous) were made in the operating room. All patients were operated under general anesthesia and conventional cardiac surgery was performed via median sternotomy. For induction and maintenance of anesthesia, intravenous midazolam, fentanyl, and vecuronium were used. When necessary, supplemental sevoflurane (0.5–1.0 %) was used to maintain mean arterial pressures and heart rate within ±25 % of pre-induction values. Remifentanil infusion (1–1.5 µg/kg/min) was begun during CPB as rewarming was started. Cardiopulmonary bypass was established with non-pulsatile (Group N) or pulsatile (Group P) fashion at 2.0–2.4 L/min/m2 flow rates. Moderate hypothermia (26–27 °C) was used in all cases. Mean arterial pressure was maintained around 50–70 mmHg during CPB and α-stat blood gas management was used. Myocardial protection was maintained with antegrade and retrograde isothermic blood cardioplegia. As a standard of our practice, we preferred antegrade induction with 1000 mL at the time of the aortic cross-clamping and afterwards either continuous retrograde or intermittent antegrade (500 mL/20 min) infusion was used until aortic cross-clamp removal. Antifibrinolytic therapy was administered with aminocaproic acid as institutional routine.
Statistical analysis
Continuous variables were described as means (±standard deviation) or medians (interquartile range) as appropriate; categorical variables were described as a percentage. The repeating measurements were compared with ANOVA test for repeating measurements and groups were compared with Mann–Whitney U test and Χ 2 analyses. The data were managed and analyzed using Statistical Package for Social Sciences version 11.0 (SPSS Inc., Chicago, IL, USA). A p value of 0.05 was considered statistically significant.
Results
The average age of our series was 57.1 ± 9.3 (range 41–78). Thirty-one of them (73.8 %) were male and 11 (26.2 %) were female. Seventeen patients (40.5 %) were assigned to Group P and 25 (59.5 %) were assigned to Group N. The preoperative characteristics and comparison between the groups are shown in Table 1.
As presented in Table 2, total intraoperative fluid volume was significantly higher (p = 0.022) and positive fluid balance during surgery was more likely (p = 0.049) in Group P. Two groups displayed similar features in terms of durations of CPB and ACC as well as number of distal vessels undergoing distal anastomoses (Table 2).
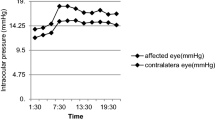
The initial mean right and left IOP values were 18.8 ± 3.9 and 18.3 ± 3.6 mmHg, respectively, and the second measurements statistically, significantly decrease from the baseline level [11.9 ± 2.9 (p = 0.0001) and 12.5 ± 3.2 (p = 0.0001), respectively]. The significant decrease in the IOP values persisted in the repeated measurements except for the 5th min of CPB values, which did not differ significantly from the baseline levels [17.0 ± 3.5 (p = 0.346) and 16.7 ± 3.6 (p = 0.399)]. Comparison of two groups demonstrated that there were significant differences at pre-CPB (right 12.8 ± 2.3 vs. 10.8 ± 2.4; p = 0.013 and left 13.3 ± 2.4 vs. 11.5 ± 2.5; p = 0.023), 5th min of CPB measurements (right 18.5 ± 3.1 vs. 15.9 ± 3.4; p = 0.015; left 18.2 ± 3.0 vs. 15.7 ± 3.6; p = 0.019) (Table 3).
Discussion
The objective of the present study was to evaluate the changes in IOP in patients that underwent pulsatile and non-pulsatile CPB. Results of the present study indicated a persistent diminution in repeated IOP measurements except for the transient increase in CPB values on 5th min. The IOP values were found to be higher in pulsatile CPB group in pre-CPB period on measurements performed on the 5th min of CPB. However, the difference between two groups was insignificant for IOP measurements performed at other intervals.
Owing to the redistribution of peripheral and splanchnic circulation, remarkable changes can be seen in blood circulation in CABG with CPB. Hypoperfusion of the peripheral tissues and alteration of metabolic activity attributed to vasoconstriction, hypothermia, administration of heparin, decrease in hemoglobin concentration due to haemodilution and centralization of circulation may occur and eye, as an end organ, is prone to be influenced by the final ischemia and hypoperfusion. Cardiopulmonary bypass may lead to deprivation of peripheral tissue energy metabolism, but these changes did not have any impact on postoperative clinical outcome [10].
Pekel et al. stated that sub-foveal choroidal thickness and ocular pulse amplitude are unchanged, while intraocular pressure decreased one week and one month after CPB [1]. The postoperative decrease in IOP may be linked with the use of diuretic and antihypertensive drugs after CPB or ischemia of ciliary bodies. Elevated IOP levels during CPB were reported to sustain for several days postoperatively [11]. In contrast, Hayashi et al. proposed that IOP values were decreased remarkably during CPB and return to baseline levels occurred at the end of the operation [2].
Increased duration of CPB was linked with a greater systemic inflammatory response, increased vascular resistance and increased risk of ocular complications. The duration of CPB is reported to be longer in patients diagnosed with ocular problems including anterior ischemic optic neuropathy [1]. The blood flow in the optic nerve head was found to be reduced during CPB and there was a negative association between blood flow and extracorporeal circulation time [1].
Main strength of the present study was assessment of intraocular pressure after pulsatile and non-pulsatile subtypes of CPB. Our results indicated that there was a transient increase in IOPs on 5th min after CPB in Group P. Clinical relevance of raised IOP and course of IOP during CPB remain to be investigated in further trials. We noted that pulsatile CPB group had significantly higher IOP levels on 5th min after the onset of procedure. In parallel to findings of Hayashi et al., there was a decrease in bilateral IOP values during CPB [2].
Regulation of IOP during CPB is supposed to be multifactorial. Hypothermia, hyperosmolarity of the infused fluid and reduced arterial pressure can diminish the amount of aqueous produced [6]. Reduced protein content of blood plasma, decreased colloidal osmotic pressure and haemodilution by the pump priming fluid may increase aqueous production. These factors and hormonal changes during CPB may contribute to the increase on 5th min after CPB [6]. An experimental study has shown that IOP and intracranial pressure were increased during haemodilution [12]. However, mechanical and non-mechanical factors associated with the permanent decrease in IOP throughout CPB needs to be elucidated in further trials.
In the relevant literature, controversial results have been reported on alteration of IOP during CPB [7–9]. Levy et al. proposed that IOP was unchanged during CPB and a decrease in IOP was observed in conjunction with systemic hypotension. In contrast, Larkin and Stellpflug reported that IOP tended to increase during CPB procedure [6, 8]. Lillaesen demonstrated that IOP increased only at the onset of CPB and did not change significantly in the other periods [9].
The steady decrease in IOP in our series may be linked with anesthetic techniques, CPB settings such as temperature, oxygenator and determinants of hemodynamic status. In addition, drainage of aqueous humor may be increased in conjunction with a decrease in central venous pressure.
The morbidities such as anterior ischemic optic neuropathy and permanent loss of vision may complicate the cardiopulmonary bypass. Increased awareness on the possibility of these complications is important for establishing early diagnosis, starting appropriate treatment without delay and developing safer surgical techniques.
There were remarkable changes in intraocular pressure due to blood pH, PCO2, and PO2 alterations induced by hyperventilation and hypercapnea under general anesthesia [13]. Hypercapnea is supposed to cause elevation of intraocular pressure, while hyperventilation may decrease it [14].
The main strength of our study is evaluation of impacts of pulsatile and non-pulsatile CPB on IOPs separately. Weaknesses of the present study include small sample size, lack of long-term follow-up and inability to test our findings with Doppler ultrasonography and measurement of ocular perfusion pressure. The main limitations of the Tonopen® XL tonometry are high cost as well as inter-session and inter-observer variabilities. Moreover, time interval between surgery and ocular examinations may have affected the results.
Conclusion
To conclude, repetitive IOP measurements showed a steady decrease in both groups except for the transient increase in 5th min CPB values. The IOP values were higher in pulsatile CPB group in pre-CPB and 5th min of CPB measurements but the difference was not significant in the repeating measurements. Further trials are warranted to clarify the physiological mechanisms underlying IOP changes during CPB and their relationship with ophthalmological complications.
References
Pekel G, Alur I, Alihanoglu YI, Yagci R, Emrecan B (2014) Choroidal changes after cardiopulmonary bypass. Perfusion 29:560–566
Hayashi H, Kawaguchi M, Hasuwa K, Inoue S, Okamoto M, Matsuura T et al (2010) Changes in intraocular pressure during cardiac surgery with and without cardiopulmonary bypass. J Anesth 24:663–668
Nuttall GA, Garrity JA, Dearani JA, Abel MD, Schroeder DR, Mullany CJ (2001) Risk factors for ischemic optic neuropathy following cardiopulmonary bypass: a matched case/control study. Anesth Analg 93:1410–1416
Sweeney PJ, Breuer AC, Selhorst JB, Waybright EA, Furlan AJ, Lederman RJ et al (1982) Ischemic optic neuropathy: a complication of cardiopulmonary bypass surgery. Neurology 32:560–562
Holy SE, Tsai JH, McAllister RK, Smith KH (2009) Perioperative ischemic optic neuropathy: a case control analysis of 126,666 surgical procedures at a single institution. Anesthesiology 110:246–253
Larkin DF, Connolly P, Magner JB, Wood AE, Eustace P (1987) Intraocular pressure during cardiopulmonary bypass. Br J Ophthalmol 71:177–180
Levy NS, Rawitscher R (1977) The effect of systemic hypotension during cardiopulmonary bypass on intraocular pressure and visual function in humans. Ann Ophthalmol 9:1547–1552
Stellpflug H, Busse H, Niedermeier M, Dittrich H (1980) Intraocular pressure during operations using the heart–lung machine (author’s transl). Klin Monatsbl Augenheilkd 176:402–408
Lilleaasen P, Hørven I (1982) Intra-ocular pressure levels during extracorporeal circulation in man. Scand J Thorac Cardiovasc Surg 16:51–53
Mandak J, Pojar M, Cibicek N, Lonsky V, Palicka V, Kakrdova D et al (2008) Impact of cardiopulmonary bypass on peripheral tissue metabolism and microvascular blood flow. Perfusion 23:339–346
Deutch D, Lewis RA (1989) Intraocular pressure after cardiopulmonary bypass surgery. Am J Ophthalmol 107:18–22
Hørven I, Lundar T, Lilleaasen P, Hysing E, Stokke O (1981) The effect of haemodilution on arterial blood pressure, central venous pressure, intracranial and intra-ocular pressures in pigs. Scand J Thorac Cardiovasc Surg 15:273–277
Abbott MA, McLaren AD, Algie T (1994) Intra-ocular pressure during cardiopulmonary bypass—a comparison of crystalloid and colloid priming solutions. Anaesthesia 49:343–346
Petounis AD, Chondreli S, Vadaluka-Sekioti A (1980) Effect of hypercapnea and hyperventilation on human intraocular pressure general anaesthesia following acetazolamide administration. Br J Ophthalmol 64:422–425
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Nuhoglu, F., Gumus, F., Sinikoglu, S.N. et al. Changes in intraocular pressure during cardiopulmonary bypass. Int Ophthalmol 37, 1155–1160 (2017). https://doi.org/10.1007/s10792-016-0376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-016-0376-3