Abstract
The aim of this study was to evaluate plasma adropin levels in patients with pseudoexfoliation (PEX). This retrospective case–control study included 35 patients with PEX and 35 individuals without PEX who served as controls. Plasma adropin levels with triglycerides, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and haemoglobin A1c (HGBA1C) concentrations were measured in both groups. The mean serum adropin levels were 3.24 ± 0.95 ng/mL (range, 1.90–7.88 ng/mL) in patients with PEX syndrome and 5.78 ± 2.85 ng/mL (range, 2.08–5.41 ng/mL) in PEX glaucoma patients. There was no statistically significant difference in mean adropin levels between PEX syndrome and PEX glaucoma patients. However, similar adropin levels were found in the PEX glaucoma patients and the control group (P > 0.05). The mean serum adropin levels were 3.34 ± 0.89 ng/mL (range, 1.90–5.39 ng/mL) in the PEX group and 5.78 ± 2.85 ng/mL (range, 3.08–11.06 ng/mL) in the control group. The mean serum adropin level of the PEX group was significantly lower than that of the control group (P < 0.001). There were no significant differences between the two groups in terms of serum glucose, total cholesterol, LDL, HDL, HGBA1C, triglycerides levels, or body mass index (all P > 0.05). Adropin level is lower in patients with PEX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudoexfoliation (PEX) syndrome is an age-related condition characterised by the accumulation of fibrogranular, extracellular, flake-shaped material that primarily affects the ocular tissues and visceral organs. PEX syndrome is the most common cause of secondary open-angle glaucoma, due to fibrogranular material deposition in the outflow pathway of the trabecular meshwork [1]. Although the aetiology and pathogenesis of PEX syndrome are still not well understood, it is thought to be a systemic disorder [2]. Several investigators have identified PEX syndrome material in extraocular tissues such as the lung, heart, skin, gall bladder, liver, and kidney and around the blood vessels of connective tissue by electron microscopy and immunohistochemistry, suggesting that exfoliation may be the first indicator of a systemic pathology [3, 4].
Cardiovascular disorders such as angina, myocardial infarction, and aortic aneurysm have been found to be linked to PEX syndrome [5, 6]. In addition, some studies have shown a statistically significant association between PEX syndrome and systemic vascular endothelial dysfunction [7].
Adropin is a recently identified protein encoded by the energy homeostasis-associated gene (Enho), which is expressed in the liver and the brain [8]. This new protein also has a protective role in endothelial cells and has been referred to as a novel regulator for these cells [9]. Adropin level has been found to be associated with cardiovascular disease, and it has been shown in previous studies that lower serum adropin levels may be an independent risk factor [10, 11]. Accordingly, the aim of this study was to evaluate serum levels of adropin in patients with PEX in order to search for a possible association.
Materials and methods
This retrospective case–control study included 35 consecutive patients with PEX and 35 individuals without PEX material who served as controls. The research was confirmed by the institutional review board and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent before their participation.
Subjects with previous chronic or recurrent inflammatory eye disease; ocular trauma; ocular infection; retinal disease; corneal abnormality; intraocular surgery within the previous three months; history of smoking; or diabetes mellitus or other systemic disease, such as malignancy, collagen vascular disease, chronic kidney or hepatic failure, pulmonary embolism, or sepsis, were excluded from the study. Pseudoexfoliation syndrome was diagnosed in the presence of evident classical scurf or flaky exfoliation materials on the pupil, lens, or other ocular structures or radial pigment over the lens surface, with or without raised intraocular pressure (IOP). The control group had no history of ocular disease (except for cataract) or elevated IOP (21 mmHg), and no evidence of exfoliation material on the anterior lens capsule or pupillary margin; their optic discs and retinas were normal.
All of the patients’ weights and heights were measured, and body mass index (BMI) was calculated using the formula: weight (kg)/height (m2). Venous blood samples were taken after a minimum 8-h overnight fast and 20 min of supine rest [12]. The samples were drawn into ethylenediaminetetraacetic acid tubes, promptly centrifuged at −4 °C, and serum specimens for adropin were frozen at −80 °C until analysis. Plasma adropin levels were measured using an enzyme-linked immunosorbent assay kit (Phoenix Pharmaceuticals, Belmont, CA) according to the manufacturer’s instructions. The detection range of the kit was 0.01–100 ng/mL; the sensitivity was 0.5 ng/mL; and the intra-assay (within day) and interassay (between days) coefficients of variations were less than 10 % and less than 15 %, respectively. Plasma glucose was measured using an automated glucose oxidase method. Triglycerides, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and haemoglobin A1c (HGBA1C) concentrations were measured by enzymatic methods using an autoanalyzer. Age, sex, and BMI of the participants were recorded.
Anterior and posterior segments of the patients’ eyes were examined by slit-lamp biomicroscope before and after pupillary dilatation. Pseudoexfoliation was diagnosed under biomicroscopic examination. PEX material was visualised on the anterior lens surface, pupillary border, iris, or iridocorneal angle. Diurnal IOPs were measured with Goldmann applanation tonometry at 3-h intervals. The average of two consecutive measurements was recorded as the mean IOP.
At the end of the study, a statistical software program (SPSS for Windows 16.0; SPSS Inc., Chicago, IL) was used for statistical analysis. Continuous variables were presented as mean ± standard deviation and comparisons among group means were performed using Student’s t test. Correlation analyses were performed using Pearson’s or Spearman’s coefficient of correlation. A P value <0.05 was considered statistically significant.
Results
Seventy eyes from 70 patients [35 men (50 %) and 35 women (50 %)] were enrolled in this study. The mean ages of the patients were 65.85 ± 6.02 years in the PEX group (group 1) and 65.11 ± 8.05 years in the control group (group 2). Group 1 consisted of 35 patients (15 women and 20 men) and group 2 consisted of 35 patients (18 women and 17 men). The differences in age and gender between the two groups were not statistically significant (P = 0.7 and 0.4, respectively) (Table 1).
In group 1, 19 patients had PEX glaucoma and were taking at least one anti-glaucomatous agent. Mean IOP levels were 14.1 ± 3.2 mmHg in the patients with PEX syndrome, 15.7 ± 2.8 mmHg in the PEX glaucoma patients, and 13.2 ± 3.7 mmHg in the control group. Mean serum adropin levels were 3.24 ± 0.95 ng/mL (range, 1.90–7.88 ng/mL) in the patients with PEX syndrome and 5.78 ± 2.85 ng/mL (range, 2.08–5.41 ng/mL) in the PEX glaucoma patients. There was no statistically significant difference in mean adropin level between the PEX syndrome and PEX glaucoma patients (Table 2). In addition, similar adropin levels were found in the PEX glaucoma patients and the control group (P > 0.05).
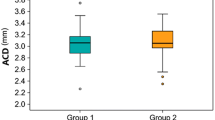
Mean serum adropin levels were 3.34 ± 0.89 ng/mL (range, 1.90–5.39 ng/mL) in the PEX group and 5.78 ± 2.85 ng/mL (range, 3.08–11.06 ng/mL) in the control group. The mean serum adropin level of the PEX group was significantly lower than that of the control group (P < 0.001). The clinical parameters of the groups are given in Table 3. The multivariate analyses showed that age, gender, and BMI did not have an effect on adropin values when used as independent variables (Table 4).
There were no significant differences between groups when serum glucose, total cholesterol, LDL, HDL, HGBA1C triglyceride levels, and BMI were compared. Serum adropin levels had no significant correlations with BMI or biochemical parameters (P = 0.216) (Table 5).
Discussion
PEX syndrome is a condition characterised by the accumulation of fibrogranular material in many other parts of the body (e.g. eye, lung), including blood vessels. It is known to be the most common identifiable cause of secondary open-angle glaucoma, and it is called a syndrome due to exfoliation material deposition in other visceral organs associated with vascular diseases [13, 14].
At first, PEX syndrome was thought to affect only ocular tissue; however, subsequent investigations have demonstrated that pseudoexfoliative material is widely distributed in extraocular tissues, such as the lung, heart, skin, gall bladder, liver, and kidney [15]. Extraocular exfoliative material deposition in the connective tissue of visceral organs has been revealed by electron microscope, and it is consistently associated with elastic collagen, fibres, and fibroblasts [4]. Elastin is part of the extracellular matrix of vessels, and previous studies have shown an association between vascular walls and elastosis with PEX material [16]. Several systemic vascular diseases, including acute myocardial infarction, chronic cerebral ischemia, and aneurysms of the abdominal aorta, have been found to be associated with ocular exfoliation [17, 18].
It is well known nowadays that PEX syndrome is associated with endothelial dysfunction and cardiovascular disease, as described in some previous studies [7, 19, 20]. For example, Atalar et al. evaluated the endothelial function of the brachial artery noninvasively, using ultrasound, in 23 PEX syndrome patients and 20 healthy individuals, and found that systemic endothelial function is impaired in PEX syndrome patients [19]. In another study, Naji et al. found similar result in terms of association between PEX syndrome and systemic vascular endothelial dysfunction [7]. On the other hand, Citirik et al. demonstrated a significantly higher prevalence of PEX in 50 patients with coronary artery disease (CAD) and a higher prevalence of CAD in patients with PEX compared to healthy controls [20]. More recently, French et al. found a statistically significant relationship between PEX syndrome/PEX glaucoma and several cardiovascular diseases such as ischemic heart disease, cardiomyopathy, and aortic aneurysm [21].
Systemic vascular endothelial dysfunction has been demonstrated not only by assessing the endothelial function of the brachial artery, but also with biomarker levels, such as omentin and YKL 40, in PEX patients [22–24]. Bucak et al. showed that lower levels of circulating omentin in patients with PEX syndrome may indicate the inflammatory and systemic nature of the disease [22]. In addition, Türkyılmaz et al. reported elevated serum levels of YKL-40, a new potential biomarker of inflammation and vascular dysfunction, in patients with PEX syndrome [23]. In our study, we evaluated plasma levels of adropin, which has been used as a biomarker of systemic vascular endothelial dysfunction in recent studies [9, 26].
Adropin, a peptide hormone encoded by the Enho gene, was first described in 2008 and is expressed in the liver and the brain [26]. Investigations in mouse models of diet-induced obesity and cell-based systems have strongly demonstrated that adropin is involved in metabolic homeostasis and cardiovascular function, and it has been implicated in the maintenance of insulin resistance and energy homeostasis [9, 26]. The functions of adropin, including regulating angiogenesis and increasing blood flow and capillary density, were demonstrated by Lovren et al., who reported that adropin has a potential role in endothelial protection [9].
Adropin also changes inducible nitric oxide synthase expression and might regulate endothelial cell function by this mechanism [9]. Celik et al. compared consecutive cardiac syndrome X patients with healthy controls and found that plasma adropin and nitrite/nitrate levels were significantly lower in patients with cardiac syndrome X; there was a significant positive correlation between plasma adropin levels and nitrite/nitrate levels [27]. In addition, Zhang showed that lower serum adropin level was significantly associated with CAD, which indicates that adropin might have a role in the prevention of CAD [28]. In another study, Topuz et al. evaluated endothelial function in patients with type 2 diabetes mellitus by measuring plasma adropin levels and compared them with flow-mediated dilatation. They found that lower adropin levels in endothelial dysfunction might be indicative of endothelial dysfunction, such as flow-mediated dilatation [25].
In light of all these findings, we suggest that plasma adropin levels may demonstrate endothelial dysfunction in patients with PEX. To the best of our knowledge, this is the first study of adropin levels in PEX syndrome. We showed that adropin levels were lower in patients with PEX and that it can be used as a marker to identify endothelial dysfunction in patients with PEX. In this study, we also evaluated BMI and serum glucose, total cholesterol, LDL, HDL, HGBA1C, and triglyceride levels, and there were no differences in these parameters between the PEX group and the controls.
Our finding of lower adropin levels in PEX patients supports the notion that PEX syndrome may affect many systems of the body as a result of endothelial dysfunction and that it can damage visceral organs such as ocular tissues. Serum adropin levels, a new potential biomarker of endothelial dysfunction, may indicate the systemic nature of PEX in patients [9, 17]. It can be used as a routine noninvasive method of assessing comorbidities in patients with PEX. We suggest that clinicians could measure adropin levels and identify the possible accompanying systemic disease if PEX material is detected in an ocular examination. It can be an early detection marker for cardiovascular diseases in patients with PEX. Our data could help practitioners stratify ocular PEX patients who have no signs or symptoms associated with any systemic disease.
References
Ritch R, Schlotzer-Schrehardt U (2001) Exfoliation syndrome. Surv Ophthalmol 45:265–315
Hewitt AW, Sharma S, Burdon KP, Wang JJ, Baird PN, Dimasi DP et al (2008) Ancestral LOXL1 variants are associated with pseudoexfoliation in Caucasian Australians but with markedly lower penetrance than in Nordic people. Hum Mol Genet 17:710–716
Amari F, Umihira J, Nohara M et al (1997) Electron microscopic immunohistochemistry of ocular and extraocular pseudoexfoliative material. Exp Eye Res 65:51–56
Schlötzer- Schrehardt UM, Koca MR, Naumann GO, Volkholz H (1992) Pseudoexfoliation syndrome ocular manifestation of a systemic disorder? Arch Ophthalmol 110(12):1752–1756
Mitchell P, Wang JJ, Smith W (1997) Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol 124:685–687
Shrum KR, Hattenhauer MG, Hodge D (2000) Cardiovascular and cerebrovascular mortality associated with ocular pseudoexfoliation. Am J Ophthalmol 129:83–86
Naji M, Naji F, Suran D, Gracner T, Kanic V, Pahor D (2008) Systemic endothelial dysfunction in patients with pseudoexfoliation syndrome. Klin Monbl Augenheilkd 225(11):963–967
Kumar KG, Trevaskis JL, Lam DD et al (2008) Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 8:468–481
Lovren F, Pan Y, Quan A et al (2010) Adropin is a novel regulator of endothelial function. Circulation 122:185–192
Lian W, Gu X, Qin Y, Zheng X (2011) Elevated plasma levels of adropin in heart failure patients. Intern Med 50(15):1523–1527
Celik A, Balin M, Kobat MA et al (2013) Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc Ther 31(3):174–178
Aydin S (2015) A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 72:4–15
Praveen MR, Shah SK, Vasavada AR et al (2011) Pseudoexfoliation as a risk factor for peripheral vascular disease: a case-control study. Eye 25:174–179
Schlötzer-Schrehardt U, Naumann GO (2006) Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol 141(5):921–937
Ovodenko B, Rostagno A, Neubert TA et al (2007) Proteomic analysis of exfoliation deposits. Invest Ophthalmol Vis Sci 48:1447–1457
Streenten BW, Li ZY, Wallace RN, Eagle RC Jr, Keshgegian AA (1992) Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol 110:1757–1762
Mitchell P, Wang JJ, Smith W (1997) Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol 124:685–687
Shrum KR, Hattenhauer MG, Hodge D (2000) Cardiovascular and cerebro-vascular mortality associated with ocular pseudoexfoliation. Am J Ophthalmol 129:83–86
Atalar PT, Atalar E, Kilic H et al (2006) Impaired systemic endothelial function in patients with pseudoexfoliation syndrome. Int Heart J 47:77–84
Citirik M, Acaroglu G, Batman C, Yildiran L, Zilelioglu O (2007) A possible link between the pseudoexfoliation syndrome and coronary artery disease. Eye 21:11–15
French DD, Margo CE, Harman LE (2012) Ocular pseudoexfoliation and cardiovascular disease: a national cross-section comparison study. N Am J Med Sci 4:468–473
Tosun M, Simavli H, Önder HI, Erdurmus M (2014) Serum levels of omentin in pseudoexfoliation syndrome. J Glaucoma. doi:10.1097/IJG.0000000000000139
Türkyılmaz K, Öner V, Kırbas A, Sevim MS, Sekeryapan B, Özgür G, Durmus M (2013) Serum ykl-40 levels as a novel marker of inflammation and endothelial dysfunction in patients with pseudoexfoliation syndrome. Eye 27(7):854–859
Zhou JY, Chan L, Zhou SW (2014) Omentin: linking metabolic syndrome and cardiovascular disease. Curr Vasc Pharmacol 12:136–143
Topuz M, Celik A, Aslantas T, Demir AK, Aydin S, Aydin S (2013) Plasma adropin levels predict endothelial dysfunction like flow-mediated dilatation in patients with type 2 diabetes mellitus. J Investig Med 61(8):1161–1164
Kumar KG, Trevaskis JL, Lam DD et al (2008) Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 8:468–481
Celik A, Balin M, Kobat MA et al (2013) Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc Ther 31(3):174–178
Zhang C, Zhao L, Xu W, Li J, Wang B, Gu X, Chen J (2014) Correlation of serum adropin level with coronary artery disease. Zhonghua Yi Xue Za Zhi 94:1255–1257
Funding
No funding supported this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None declared.
Rights and permissions
About this article
Cite this article
Oğurel, T., Oğurel, R., Topuz, M. et al. Plasma adropin level in patients with pseudoexfoliation. Int Ophthalmol 36, 737–742 (2016). https://doi.org/10.1007/s10792-016-0185-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-016-0185-8