Abstract
Background
The neutrophil–lymphocyte ratio (NLR) and systemic immune-inflammation index (SII) are emerging inflammatory markers related to cardiovascular outcomes. This study investigated their relationships with cardiovascular disease (CVD) and mortality among individuals with prediabetes or diabetes and assessed their predictive roles.
Methods
A cohort of 6871 individuals with diabetes or prediabetes from the NHANES (2001–2018) was included. Weighted multivariate logistic regression models assessed NLR and SII associations with CVD risk, while survey-weighted Cox proportional hazards models evaluated their links to mortality. The predictive accuracy of the biomarkers for mortality was quantified by receiver-operating characteristic (ROC) curve analysis.
Results
Individuals in the higher NLR and SII groups exhibited a high incidence of CVD. A total of 1146 deaths occurred throughout an average follow-up duration of 191 months, of which 382 were caused by CVD. Participants with higher NLR markedly increased the risk of all-cause (HR = 1.82) and cardiovascular mortality (HR = 2.07). A similar result was observed in the higher SII group. RCS analysis identified a linear correlation between NLR and CVD risk and mortality (p > 0.05), while SII showed a nonlinear correlation (p < 0.05). ROC results demonstrated that NLR exhibited a higher predictive ability in mortality than SII.
Conclusions
Elevated levels of NLR and SII correlated with an increased risk of CVD and both all-cause and cardiovascular mortality in individuals with diabetes or prediabetes. The NLR appears to be particularly valuable for assessing risk and predicting outcomes in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) and its complications have emerged as a serious public health problem worldwide (Sun et al. 2022). As estimated by the International Diabetes Federation (IDF), diabetes affects 536.6 million (10.5%) globally in 2021, with projections indicating an increase to 783.2 million (12.2%) by 2045 (Sun et al. 2022). Prediabetes, also termed the intermediate stage between normal glucose regulation and diabetes, is far more prevalent than diabetes. Prediabetes affects approximately one-third of adults in the US and 720 million individuals worldwide (Echouffo-Tcheugui et al. 2023). Epidemiological evidence consistently links prediabetes to an elevated risk of diabetes-related complications, including cardiovascular disease (CVD), nephropathy, retinopathy, and neuropathy compared to those with normal glucose regulation (Brannick and Dagogo-Jack 2018; Echouffo-Tcheugui et al. 2023; Schlesinger et al. 2022). Moreover, individuals with diabetes or prediabetes also faced a remarkably heightened risk of both all-cause and cardiovascular mortality (Raghavan et al. 2019; Schlesinger et al. 2022). Therefore, identifying reliable predictors in individuals with diabetes or prediabetes is essential to decrease CVD risk and mortality and improve patient prognosis.
Chronic inflammatory processes are crucial in the pathogenesis of diabetic complications including nephropathy and vascular diseases (Yamamoto and Yamamoto 2013). The neutrophil–lymphocyte ratio (NLR) is an innovative biomarker of immunoinflammatory and is computed by neutrophil count/lymphocyte count (Zahorec 2021). The NLR integrates the contributions of innate (neutrophils) and adaptive (lymphocytes) immune responses (GARCíA-ESCOBAR A et al. 2023). Recently, accumulative evidence suggested that NLR is a strong predictor of CVD risk and related to the outcomes of CVD (Angkananard et al. 2018; JHUANG Y-H et al. 2019; Soylu et al. 2015). A prospective cohort study suggested that compared with lower NLR scores, higher NLR scores independently correlate with the severity of coronary artery disease (CAD) and elevated risk of CVD event (HR = 1.55) in patients undergoing angiography (Arbel et al. 2012). Studies have revealed that NLR is strongly related to higher glycemia and HbA1C levels and elevated risk of mortality in diabetic patients (Adane et al. 2023; Dong et al. 2023). The systemic immune-inflammation index (SII), proposed by Hu et al., reflects immunoinflammatory status through the calculation: platelet count × neutrophil count/lymphocyte count (Hu, et al. 2014). Previous research has demonstrated that SII has a superior prognostic value over other inflammation markers in cancer settings, including NLR and platelet–lymphocyte ratio (PLR) (Aziz et al. 2019; CHEN J-H et al. 2017; Jomrich et al. 2021). Emerging evidence suggested that SII was also a predictor of CVD risk and clinic prognostic (Cao et al. 2023; Xu et al. 2021; YANG Y-L et al. 2020; Ye et al. 2022). A nationwide cohort study demonstrated that SII was remarkably related to CVD risk even after accounting for multiple risk factors (Wang et al. 2023). A prospective cohort study indicated that elevated SII scores were linked to a 65% higher risk of major adverse cardiac events (MACEs) following percutaneous coronary intervention in patients with CAD compared with lower SII scores with SII proving to be a superior predictor of MACEs compared to traditional risk factors (YANG Y-L et al. 2020). Moreover, SII was positively correlated with both all-cause and CVD mortality in diabetic patients (Chen et al. 2023).
Despite these insights, investigations into the relationship between NLR and SII with CVD risk and mortality among prediabetic patients remain scarce. This study aimed to explore whether the NLR and SII have a predictive value for CVD risk, and mortality among patients with diabetes or prediabetes and to compare the predictive power for mortality.
Methods
Study design and populations
The National Health and Nutrition Examination Survey (NHANES) is a biennial cross-sectional research program aimed to evaluate the health and nutritional status of the U.S. population. The study was approved by the NCHS Institutional Review Board, and all participants provided informed consent; therefore, no extra ethical approval was required. Initially, 91,351 individuals were included in this study (NHANES 2001–2018). After excluding those with missing NLR and SII data (N = 16,559), without diabetes or prediabetes (N = 59,602), incomplete follow-up information (N = 1,199), and incomplete covariates (N = 7120), ultimately, 6871 subjects were included in the analysis (Supplementary Fig. 1).
Assessment of neutrophil–lymphocyte ratio and systemic immune-inflammation index
NLR and SII were used as exposure variables. NLR was determined by the ratio of neutrophil count to lymphocyte count. SII was computed by multiplying the platelet count and neutrophil count, then dividing by the lymphocyte count. Neutrophil, platelet, and lymphocyte counts (expressed as × 109/L) were measured using automated hematology analysis equipment.
Diagnosis of diabetes and prediabetes
Diabetes was diagnosed by fasting plasma glucose (FPG) ≥ 126 mg/dL, glycated hemoglobin A1c (HbA1c) value ≥ 6.5%, or 2-h blood glucose ≥ 200 mg/dL from an oral glucose tolerance test (OGTT), in addition to self-reported diagnosis or use of insulin or oral hypoglycemic medication (Zou et al. 2019). Prediabetes is defined by self-reported prediabetes status, FPG levels between 100 and 125 mg/dL, OGTT values ranging from 140 to 199 mg/dL, or HbA1c between 5.7 and 6.5% (Zou et al. 2019).
Diagnosis of cardiovascular disease
Diagnoses of CVD were determined by self-reported physician diagnoses derived from personal interviews. Participants were categorized with CVD if they affirmed having been diagnosed with congestive heart failure (CHF), coronary heart disease (CHD), angina pectoris, myocardial infarction (MI), or stroke by a healthcare professional (Zhang et al. 2023a). In addition, specific events related to these conditions were analyzed as secondary outcomes.
Ascertainment of mortality
Mortality status was determined via linkage to the National Death Index (NDI) records, with follow-up time durations computed from NHANES examination dates to death or December 31, 2019. Cardiovascular deaths were ascertained using ICD-10 codes I00–I09, I11, I13, I20–I51, and I60–I69.
Assessment of covariates
Structure questionnaires acquired information on sociodemographic characteristics, smoking, drinking, disease, and medication use. Education was categorized (less than high school, high school/equivalent, college, or above), income to poverty ratios (< 1, 1–3, > 3), and smoking status (never, former, current) (Li, et al. 1999). Drinking status (heavy, low-moderate drinkers, nondrinker) (Chang et al. 2017), BMI (normal, overweight, obese), hypertension (Yes/No) (Unger et al. 2020), lab analyses included triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum creatinine, serum uric acid, urinary albumin to creatinine ratio (ACR), and estimated-glomerular filtration rate (eGFR) calculated via CKD-EPI (Levey et al. 2009).
Statistical analysis
All statistical analyses considered NHANES’ complex survey design, integrating sample weights, clustering, and stratification. Continuous variables were reported as means ± standard deviation (SD), while categorical variables were presented as counts and weighted proportions. Group differences were assessed via weighted chi-square and Wilcoxon rank-sum tests. The optimal cut-off for NLR and SII was determined by maximally selected rank statistics (Zhang et al. 2023b). Multivariate logistic regression was employed to evaluate odds ratios (OR) with 95% confidence intervals (CI) for CVD risk in relation to NLR and SII. Survival outcomes were depicted using Kaplan–Meier curves, with between-group comparisons via log-rank tests. The survey-weighted Cox proportional hazards regression was employed to evaluate hazard ratios (HR) and 95% CI for mortality in connection with NLR and SII. The potential nonlinear relationships were explored via restricted cubic spline (RCS) analysis. Stratification and interaction analyses were carried out using variables including gender, age, BMI, smoking status, hypertension, and diabetes. Time-dependent receiver-operator characteristic curve (ROC) analysis was performed to assess the accuracy of the NLR and SII in predicting survival outcomes at multiple time points, utilizing the ‘timeROC’ package (Kamarudin et al. 2017). R software was used for all statistical analyses (version 4.2.1). A two-tailed p value < 0.05 was deemed statistically significant.
Results
Baseline characteristics of the study population
In this study, 6871 participants with diabetes or prediabetes were enrolled (Supplementary Fig. 1). Utilizing maximally selected rank statistics, we determined the optimal NLR threshold to be 3.42, dividing participants into a lower group (NLR ≤ 3.42, n = 6,214) and a higher group (NLR > 3.42, n = 747) (Fig. 1A). Similarly, the optimal SII threshold identified was 853.86 (Fig. 1B).
Baseline characteristics of the study participants were stratified according to NLR (Table 1). The mean (SD) age of participants was 51.3 (16.2) years, with 55.0% being male. The higher NLR group was predominantly older and of white ethnicity compared with the lower NLR group. They exhibited higher levels of TC, fasting glucose, serum creatinine, ACR, and neutrophil count, and suffered CVD, CHF, CHD, Angina, MI, stroke, hypertension, and diabetes. Furthermore, they also exhibited a lower proportion of never smokers and lower LDL, eGFR, and lymphocyte levels. The baseline characteristics stratified by SII are detailed in Supplementary Table 1.
Relationships between NLR and SII with the risk of CVD
The relationship between NLR and CVD risk was assessed by survey-weighted logistic regression. In the unadjusted model, individuals with higher NLR exhibited a heightened risk of CVD (OR = 2.36, p < 0.001). Following multivariate adjustment, the relationship persisted across Model 2 (OR = 1.48, p < 0.001) and Model 3 (OR = 1.29, p = 0.033) (Table 2). However, NLR as a continuous variable did not exhibit a significant correlation with CVD risk after full adjustment for covariates. For CHF, multivariate logistic regression analysis displayed that the risk of CHF notably elevated in the higher NLR group (OR = 1.80, p = 0.002) (Table 3) than in the lower NLR group.
After multivariate adjustment, SII, as a categorical variable, exhibited an association with increased risks of CVD (OR = 1.29, p = 0.034) and CHF (OR = 1.54, p = 0.035) (Table 2–3). Notably, this study did not find significant associations between NLR and SII with the risks of CHD, angina, heart attack, and stroke in this study (Supplementary Table 2–5).
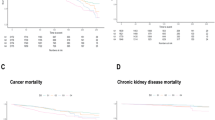
The potential nonlinear correlations between NLR and the risk of CVD and CHF were assessed using RCS. The results revealed a linear correlation between the NLR and the risk of CVD and CHF (both p for nonlinear > 0.05) (Fig. 2 A-B). Conversely, RCS results illustrated a U-shaped association between the SII and the risk of CVD and CHF (both p for nonlinear < 0.05) (Fig. 2 C-D).
Restricted cubic spline analysis between NLR and the risk of CVD (A) and CHF (B) in participants with prediabetes or diabetes. Restricted cubic spline analysis between SII and the risk of CVD (C) and CHF (D). Red lines represent references for odds ratios, and blue areas represent 95% confidence intervals. The model was adjusted for age, gender, race, education level, family income poverty ratio, smoking status, drinking status, BMI, TC TG, HDL, HbA1c, and hypertension
We also explored the association between NLR and the risk of CVD and CHF in different population subgroups based on age, gender, BMI, smoking status, hypertension, and diabetes. The CVD risk was elevated only in participants who were female, age (≥ 60), obese, and had hypertension (Fig. 3A). Regarding CHF, the correlation of NLR with CHF was observed in participants who were females, age (≥ 60), normal weight, overweight, never smokers, and individuals with hypertension and prediabetes (Fig. 3B). Interaction tests indicated no statistically significant interactions between NLR and the stratification variables (all interactions p > 0.05) (Fig. 3). Similarly, there were no significant interactions between SII and the stratification variables (all interactions p > 0.05) (Supplementary Fig. 2).
Associations of the NLR with all‑cause and cardiovascular mortality
Throughout a mean follow-up period of 191 months, a total of 1146 (16.7%) deaths occurred, including 382 (5.6%) caused by CVD. Kaplan–Meier survival curves suggested that higher NLR was related to increased all-cause mortality and cardiovascular mortality (both log-rank p < 0.001, Fig. 4). Weighted multivariable Cox regression analysis, following comprehensive adjustment for covariates, revealed that each unit increase in NLR was independently associated with a 12% increased risk of both all-cause mortality and cardiovascular mortality (both p < 0.001) (Table 4). Statistical significance persists when NLR is transformed from a continuous to a categorical variable. After adjusting for all covariates, higher NLR was linked to elevated risk of all-cause (HR = 1.82, p < 0.001) and cardiovascular mortality (HR = 2.07, p < 0.001) (Table 4). The core results remained consistent across subgroup analyses based on age, sex, BMI category, smoking status, hypertension, and diabetes (Fig. 5). Furthermore, age-stratified analyses demonstrated interactions between NLR and all-cause mortality, highlighting a particularly pronounced effect among older individuals (Fig. 5).
Additionally, RCS analysis illustrated a linear relationship between NLR and both all-cause and cardiovascular mortality (both p for nonlinear > 0.05) after full adjustment of covariates, specifically in participants with diabetes or prediabetes (Fig. 6).
Restricted cubic spline analysis between NLR and all-cause (A) and cardiovascular (B) mortality in participants with prediabetes or diabetes. Restricted cubic spline analysis between SII and the risk of all-cause (C) and cardiovascular (D) mortality. Red lines represent references for hazard ratio, and blue areas represent 95% confidence intervals. The model was adjusted for age, gender, race, education level, family income poverty ratio, smoking status, drinking status, BMI, TC TG, HDL, HbA1c, and hypertension
Associations of the SII with all‑cause and cardiovascular mortality
Regarding SII, Kaplan–Meier survival curves similarly indicated higher SII levels were linked to elevated risks of all-cause and cardiovascular mortality (Log-rank p < 0.001, Supplementary Fig. 3). Cox regression analysis showed that after full adjustment of covariates, each unit increase in log-SII score was associated with a 67% and 144% elevated risk of all-cause (HR = 1.67, p = 0.006) and cardiovascular mortality (HR = 2.44, p = 0.004) (Table 4), respectively. Statistical significance persists when SII as a categorical variable. After adjusting for all covariates, higher SII levels were found to be associated with a remarkably elevated risk of all-cause (HR = 1.64, p < 0.001) and cardiovascular mortality (HR = 1.98, p < 0.001) compared to lower SII levels (Table 4). (Table 4). Except for overweight individuals who showed SII was not associated with cardiovascular mortality, the correlations of SII with all-cause and cardiovascular mortality were unchanged across subgroups. No significant interaction between SII and the stratification variables was observed (Supplementary Fig. 4).
Interestingly, after full adjustment of covariates, the RCS curves displayed a U-shaped association between log-SII and all-cause mortality in individuals with diabetes or prediabetes (p nonlinear < 0.0001) (Fig. 6). As depicted in Fig. 6, when log-SII was less than 2.65, the risk of all-cause mortality tended to decline as log-SII continued to increase. However, after exceeding 2.65, all-cause mortality rose as log-SII grew. RCS curves exhibited a similar nonlinear connection between SII and cardiovascular mortality (p nonlinear = 0.006), as depicted in Fig. 6. These findings underscore the complex associations between systemic inflammation, as measured by SII, and mortality risks in individuals with diabetes or prediabetes.
The predictive ability of NLR and SII for mortality in patients with prediabetes or diabetes
Time-dependent ROC analysis displayed the area under the curve (AUC) values for NLR in predicting all-cause mortality in patients with prediabetes or diabetes, which were 0.674 at 3 years, 0.659 at 5 years, and 0.639 at 10 years (Fig. 7A). Similarly, NLR demonstrated AUC of 0.674, 0.665, and 0.657 for cardiovascular mortality over the corresponding periods (Fig. 7B). The AUC for log-SII were 0.612, 0.587, and 0.558 for all-cause mortality (Fig. 7C), and 0.612, 0.594, and 0.578 for cardiovascular mortality at 3-year, 5-year, and 10-year, respectively. (Fig. 7D). Additionally, we evaluated the predictive performance of neutrophils, lymphocytes, and platelets individually for all-cause and cardiovascular mortality (Supplementary Fig. 5). These findings underscored that NLR exhibited superior predictive ability over SII, as well as neutrophils, lymphocytes, and platelets, across both short-term and long-term mortality predictions.
Discussion
In this study, among the 6871 individuals diagnosed with diabetes or prediabetes from nine NHANES cycles (2001–2018), we found independent associations of higher NLR and SII with cardiovascular disease (CVD) and mortality. RCS analysis revealed a linear relationship between NLR and the risk of CVD and mortality, while SII showed a nonlinear correlation. Notably, time-dependent ROC analysis underscored the superior predictive performance of NLR over SII and other biomarkers in both short-term and long-term mortality assessments.
Chronic inflammation and immune system activation play pivotal roles in the pathogenesis of diabetes and its complications (Esser et al. 2014). Several key molecular mechanisms have been elucidated in recent years, revealing how inflammatory processes lead to vascular damage, plaque destabilization, and adverse cardiovascular events. Hyperglycemia leads to endothelial and smooth muscle dysfunction by increasing the expression of pro-inflammatory cytokines, leukocyte recruitment and activation, and subendothelial lipid accumulation, which are the main characteristics of diabetic macrovascular lesions (Petrie et al. 2018; SILVEIRA ROSSI J L et al. 2022). Chronic inflammation also leads to changes in platelet function and hemostatic components, such as fibrinogen, thrombin, and fibrin, promoting thrombosis and thereby increasing cardiovascular mortality in diabetic patients (Folsom et al. 2018; Lee et al. 2021). Additionally, neutrophil-derived neutrophil extracellular traps (NETs) have emerged as potent mediators of vascular inflammation, promoting thrombosis and exacerbating endothelial dysfunction through direct cytotoxic effects and activation of coagulation pathways. Several clinical and epidemiological studies have shown that plasma inflammatory markers, including C-reactive protein, interleukin1β, and interleukin-6, are frequently raised in diabetes patients, and correlate with prognosis (Kato et al. 2019; Lowe et al. 2014; Yang et al. 2021). In addition, clinical trials have shown that IL-1β inhibitor canakinumab reduces levels of inflammatory markers and the risk of cardiovascular events in patients with diabetes (Mikkelsen et al. 2022; Ridker et al. 2012, 2017; Rissanen et al. 2012). Hydroxychloroquine, an immunomodulatory drug, is primarily used for treating malaria and autoimmune disorders and has also been shown to reduce the risk of diabetes and CVD by decreasing the production of pro-inflammatory cytokines and blocking Toll-like receptor signaling (Pareek et al. 2014; Sharma et al. 2016; Wondafrash et al. 2020). This implies a potential cumulative effect between inflammation levels with hyperglycemia and CVD.
The white blood cell (WBC) count is a straightforward, cost-effective, and widely accessible marker of nonspecific inflammation. Multiple studies have shown that elevated levels of WBC are associated with both the onset and adverse outcomes of cardiovascular disease (CVD) (Jee et al. 2005; Kabat et al. 2017; Nilsson et al. 2014). For instance, a prospective study from Korea found that increased WBC count was significantly associated with both all-cause mortality and atherosclerotic cardiovascular death (Jee et al. 2005). Nilsson et al. further supported the clinical value of WBC count in predicting long-term survival, showing that higher WBC counts in the elderly were linked to poorer survival outcomes (Nilsson et al. 2014). However, WBC count does not differentiate between various types of white blood cells. The novel inflammatory markers NLR and SII, computed from the counts of neutrophils, lymphocytes, and platelets, better reflect the levels of systemic inflammatory response (Li et al. 2023; Marik and Stephenson 2020). NLR and SII have received attention as potential inflammation biomarkers for various diseases, including cancer, metabolic syndrome, neurodegenerative, and inflammation diseases (HASHEMI MOGHANJOUGHI P et al.. 2022; Sayed et al. 2020; Zhao et al. 2023). NLR and SII are also predictors of composite cardiovascular events (Angkananard et al. 2018; Ye et al. 2022). A large cohort study involving 13,929 middle-aged and older adults demonstrated a significant association between elevated SII and incident CVD among individuals without prior heart disease at baseline (Xu et al. 2021). Our results found that higher levels of NLR and SII were associated with CVD. Interestingly, the subgroup analyses showed that higher SII was strongly related to elevated CVD risk in participants with prediabetes than diabetes. This difference may be attributed to the use of diabetes medications, such as SGLT2 inhibitors and GLP-1R agonists, which have been proven to improve heart health (Packer et al. 2020; Palmer et al. 2021). In addition, contrary to previous studies that showed higher SII and NLR are associated with CHD risk (Ma and Li 2023; Wang et al. 2024), our results suggested no association between them. The possible reason is that smaller scales may blunt the link between NLR and SII with event outcomes.
Most studies have evaluated the association of hematologic parameters with the risk of all-cause and cardiovascular mortality only in diabetic patients. In a recent study, after full adjustment of covariates, neutrophils and platelets did not exhibit associations with either all-cause or cardiovascular mortality in individuals with diabetes, whereas lymphocyte was only related to all-cause mortality (Cardoso et al. 2021). Furthermore, this study showed a robust correlation between the NLR and both types of mortality (Cardoso et al. 2021). Consistent findings were observed in a national cohort study comprising 3,251 diabetes patients (Dong et al. 2023). Few studies assessed association between the NLR with mortality risk in prediabetic patients. Compared to normoglycemia, all-cause mortality also exhibited a higher risk in prediabetes. A meta-analysis demonstrated that the excess absolute risk in prediabetes at baseline for mortality rates was 7.36 per 10,000 person-years (Cai et al. 2020). In our investigation, we found that elevated NLR levels was linked to an elevated the risk of all-cause and cardiovascular mortality both in prediabetes or diabetes at baseline, and the core results were reliable in subgroup analyses. Consistent with the Chen et al.’s study in type 2 diabetes (Chen et al. 2023), we also found that higher SII correlated with increased the risk of all-cause and cardiovascular mortality, either in patients with diabetes or prediabetes. These results suggested that NLR and SII may also be a reliable predictor for managing in prediabetes patients. The RCS curve revealed a linear correlation between NLR with all-cause and cardiovascular mortality, while SII showed a nonlinear correlation. The U-shaped relationship between platelet count and mortality may be the underlying cause of this discrepancy. A cohort study enrolling 21,252 individuals suggested that platelet < 175 × 109/L or > 300 × 109/L was dramatically increased for mortality (Vinholt et al. 2016). Furthermore, Tsai M et al. found that both thrombocytopenia and increased platelet count were associated with cardiovascular mortality (TSAI M-T et al. 2015).
The predictive power of NLR and SII for all-cause and cardiovascular mortality was evaluated. SII, which integrates three common immune cells, may possess a higher potential clinical application. In the field of cancer, SII had a better predictive value for prognosis than NLR (Aziz et al. 2019; CHEN J-H et al. 2017; Jomrich et al. 2021). In contrast, in the present study, the time-dependent ROC results showed that NLR showed a higher predictive ability than SII in predicting mortality in individuals with diabetes or prediabetes.
This study possesses several notable advantages, and its substantial sample size and extended follow-up duration contribute to reliable results and strong statistical power. In addition, all data were collected in a standardized manner, thus avoiding selection bias. Second, the use of sampling weight enhances the applicability of our findings to the U.S. population. However, several limitations should be considered in this study. First, causality could not be established since this study was observational design. Second, NLR and SII was obtained from a single baseline blood sample and may not represent the average levels during the follow-up period, so the effect of changes in the NLR and SII on the risk of cardiovascular events and mortality across the follow-up period cannot be determined. Third, although we endeavor to account for potential confounding covariates, residual confounders cannot be entirely ruled out. The predominant composition of the study cohort from the United States may limit the generalizability of the findings globally.
Conclusion
This cohort study suggested that NLR and SII are valid predictors of the risk of CVD and both all-cause and cardiovascular mortality in populations diagnosed with diabetes or prediabetes, with a linear correlation between NLR and CVD and mortality, whereas there is a nonlinear correlation in SII. The predictive power of NLR was superior to SII. Therefore, measurement of the NLR may be useful in evaluating the risk and predicting the outcome of such patients. Further studies should examine whether interventions targeting NLR improve clinical outcomes and understand the underlying mechanisms.
Data availability
The data for this study were obtained from the public database of the National Center for Health Statistics https://www.cdc.gov/nchs/nhanes/.
References
Adane T, Melku M, Worku YB et al (2023) The Association between neutrophil-to-lymphocyte ratio and glycemic control in type 2 diabetes mellitus: a systematic review and meta-analysis [J]. J Diabetes Res 2023:3117396
Angkananard T, Anothaisintawee T, McEvoy M et al (2018) Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis [J]. Biomed Res Int 2018:2703518
Arbel Y, Finkelstein A, Halkin A et al (2012) Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography [J]. Atherosclerosis 225(2):456–460
Aziz MH, Sideras K, Aziz NA et al (2019) The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study [J]. Ann Surg 270(1):139–146
Brannick B, Dagogo-Jack S (2018) Prediabetes and cardiovascular disease: pathophysiology and interventions for prevention and risk reduction [J]. Endocrinol Metab Clin North Am 47(1):33–50
Cai X, Zhang Y, Li M et al (2020) Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis [J]. BMJ 370:m2297
Cao Y, Li P, Zhang Y et al (2023) Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: results from NHANES [J]. Front Immunol 14:1087345
Cardoso CRL, Leite NC, Salles GF (2021) Importance of hematological parameters for micro- and macrovascular outcomes in patients with type 2 diabetes: the Rio de Janeiro type 2 diabetes cohort study [J]. Cardiovasc Diabetol 20(1):133
Chang Y, Li Y, Guo X et al (2017) The Prevalence of hypertension accompanied by high homocysteine and its risk factors in a rural population: a cross-sectional study from Northeast China [J]. Int J Environ Res Public Health 14(4):376
Chen JH, Zhai ET, Yuan YJ et al (2017) Systemic immune-inflammation index for predicting prognosis of colorectal cancer [J]. World J Gastroenterol 23(34):6261–6272
Chen C, Chen Y, Gao Q, Wei Q (2023) Association of systemic immune inflammatory index with all-cause and cause-specific mortality among individuals with type 2 diabetes [J]. BMC Cardiovasc Disord 23(1):596
Dong G, Gan M, Xu S et al (2023) The neutrophil-lymphocyte ratio as a risk factor for all-cause and cardiovascular mortality among individuals with diabetes: evidence from the NHANES 2003–2016 [J]. Cardiovasc Diabetol 22(1):267
Echouffo-Tcheugui JB, Perreault L, Ji L, Dagogo-Jack S (2023) Diagnosis and management of prediabetes: a review [J]. JAMA 329(14):1206–1216
Esser N, Legrand-Poels S, Piette J et al (2014) Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes [J]. Diabetes Res Clin Pract 105(2):141–150
Folsom AR, Lutsey PL, Heckbert SR et al (2018) Longitudinal increases in blood biomarkers of inflammation or cardiovascular disease and the incidence of venous thromboembolism [J]. J Thromb Haemost 16(10):1964–1972
García-Escobar A, Vera-Vera S, Tébar-Márquez D et al (2023) Neutrophil-to-lymphocyte ratio an inflammatory biomarker, and prognostic marker in heart failure, cardiovascular disease and chronic inflammatory diseases: New insights for a potential predictor of anti-cytokine therapy responsiveness [J]. Microvasc Res 150:104598
Hu B, Yang XR, Xu Y et al (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma [J]. Clin Cancer Res 20(23):6212–6222
Jee SH, Park JY, Kim HS et al (2005) White blood cell count and risk for all-cause, cardiovascular, and cancer mortality in a cohort of Koreans [J]. Am J Epidemiol 162(11):1062–1069
Jhuang YH, Kao TW, Peng TC et al (2019) Neutrophil to lymphocyte ratio as predictor for incident hypertension: a 9-year cohort study in Taiwan [J]. Hypertens Res 42(8):1209–1214
Jomrich G, Paireder M, Kristo I et al (2021) High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma [J]. Ann Surg 273(3):532–541
Kabat GC, Kim MY, Manson JE et al (2017) White blood cell count and total and cause-specific mortality in the women’s health initiative [J]. Am J Epidemiol 186(1):63–72
Kamarudin AN, Cox T, Kolamunnage-Dona R (2017) Time-dependent ROC curve analysis in medical research: current methods and applications [J]. BMC Med Res Methodol 17(1):53
Kato K, Otsuka T, Saiki Y et al (2019) Association between elevated c-reactive protein levels and prediabetes in adults, particularly impaired glucose tolerance [J]. Can J Diabetes. https://doi.org/10.1016/j.jcjd.2018.03.007
Lee MKS, Kraakman MJ, Dragoljevic D et al (2021) Apoptotic ablation of platelets reduces atherosclerosis in mice with diabetes [J]. Arterioscler Thromb Vasc Biol 41(3):1167–1178
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate [J]. Ann Intern Med 150(9):604–612
Li Y, Xia PF, Geng TT et al (2023) Trends in self-reported adherence to healthy lifestyle behaviors among US adults, 1999 to march 2020 [J]. JAMA Netw Open 6(7):e2323584
Li W, Li S, Shang Y et al (2023) Associations between dietary and blood inflammatory indices and their effects on cognitive function in elderly Americans [J]. Front Neurosci 17:1117056
Lowe G, Woodward M, Hillis G et al (2014) Circulating inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes and cardiovascular disease or risk factors: the advance study [J]. Diabetes 63(3):1115–1123
Ma J, Li K (2023) Systemic immune-inflammation index is associated with coronary heart disease: a cross-sectional study of NHANES 2009–2018 [J]. Front Cardiovasc Med 10:1199433
Marik PE, Stephenson E (2020) The ability of procalcitonin, lactate, white blood cell count and neutrophil-lymphocyte count ratio to predict blood stream infection. Analysis of a large database [J]. J Crit Care 60:135–139
Mikkelsen RR, Hundahl MP, Torp CK et al (2022) Immunomodulatory and immunosuppressive therapies in cardiovascular disease and type 2 diabetes mellitus: a bedside-to-bench approach [J]. Eur J Pharmacol 925:174998
Moghanjoughi PH, Neshat S, Rezaei A, Heshmat-Ghahdarijani K (2022) Is the neutrophil-to-lymphocyte ratio an exceptional indicator for metabolic syndrome disease and outcomes? [J]. Endocr Pract 28(3):342–348
Nilsson G, Hedberg P, Ohrvik J (2014) White blood cell count in elderly is clinically useful in predicting long-term survival [J]. J Aging Res 2014:475093
Packer M, Anker SD, Butler J et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure [J]. N Engl J Med 383(15):1413–1424
Palmer SC, Tendal B, Mustafa RA et al (2021) Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials [J]. BMJ 372:m4573
Pareek A, Chandurkar N, Thomas N et al (2014) Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone [J]. Curr Med Res Opin 30(7):1257–1266
Petrie JR, Guzik TJ, Touyz RM (2018) Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms [J]. Can J Cardiol 34(5):575–584
Raghavan S, Vassy JL, Ho Y-L et al (2019) Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults [J]. J Am Heart Assoc 8(4):e011295
Ridker PM, Howard CP, Walter V et al (2012) Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial [J]. Circulation 126(23):2739–2748
Ridker PM, Everett BM, Thuren T et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease [J]. N Engl J Med 377(12):1119–1131
Rissanen A, Howard CP, Botha J, Thuren T (2012) Effect of anti-IL-1β antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial [J]. Diabetes Obes Metab 14(12):1088–1096
Sayed A, Bahbah EI, Kamel S et al (2020) The neutrophil-to-lymphocyte ratio in Alzheimer’s disease: current understanding and potential applications [J]. J Neuroimmunol 349:577398
Schlesinger S, Neuenschwander M, Barbaresko J et al (2022) Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies [J]. Diabetologia 65(2):275–285
Sharma TS, Wasko MCM, Tang X et al (2016) Hydroxychloroquine use is associated with decreased incident cardiovascular events in rheumatoid arthritis patients [J]. J Am Heart Assoc. https://doi.org/10.1161/JAHA.115.002867
Silveira Rossi JL, Barbalho SM, Reverete de Araujo R et al (2022) Metabolic syndrome and cardiovascular diseases: going beyond traditional risk factors [J]. Diabetes Metab Res Rev 38(3):e3502
Soylu K, Gedikli Ö, Dagasan G et al (2015) Neutrophil-to-lymphocyte ratio predicts coronary artery lesion complexity and mortality after non-ST-segment elevation acute coronary syndrome [J]. Rev Port Cardiol 34(7–8):465–471
Sun H, Saeedi P, Karuranga S et al (2022) IDF Diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045 [J]. Diabetes Res Clin Pract 183:109119
Tsai MT, Chen YT, Lin CH et al (2015) U-shaped mortality curve associated with platelet count among older people: a community-based cohort study [J]. Blood 126(13):1633–1635
Unger T, Borghi C, Charchar F et al (2020) International society of hypertension global hypertension practice guidelines [J]. J Hypertens. https://doi.org/10.1161/HYPERTENSIONAHA.120.1502
Vinholt PJ, Hvas AM, Frederiksen H et al (2016) Platelet count is associated with cardiovascular disease, cancer and mortality: a population-based cohort study [J]. Thromb Res 148:136–142
Wang H, Nie H, Bu G et al (2023) Systemic immune-inflammation index (SII) and the risk of all-cause, cardiovascular, and cardio-cerebrovascular mortality in the general population [J]. Eur J Med Res 28(1):575
Wang Y, Zhuang Y, Lin C et al (2024) The neutrophil-to-lymphocyte ratio is associated with coronary heart disease risk in adults: a population-based study [J]. PLoS ONE 19(2):e0296838
Wondafrash DZ, Desalegn TZ, Yimer EM et al (2020) Potential effect of hydroxychloroquine in diabetes mellitus: a systematic review on preclinical and clinical trial studies [J]. J Diabetes Res 2020:5214751
Xu M, Chen R, Liu L et al (2021) Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: the Dongfeng-Tongji cohort study [J]. Atherosclerosis 323:20–29
Yamamoto Y, Yamamoto H (2013) RAGE-mediated inflammation, type 2 diabetes, and diabetic vascular complication [J]. Front Endocrinol (lausanne) 4:105
Yang YL, Wu CH, Hsu PF et al (2020) Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease [J]. Eur J Clin Invest 50(5):e13230
Yang X, Tao S, Peng J et al (2021) High-sensitivity C-reactive protein and risk of type 2 diabetes: a nationwide cohort study and updated meta-analysis [J]. Diabetes Metab Res Rev 37(8):e3446
Ye Z, Hu T, Wang J et al (2022) Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis [J]. Front Cardiovasc Med 9:933913
Zahorec R (2021) Neutrophil-to-lymphocyte ratio, past, present and future perspectives [J]. Bratisl Lek Listy 122(7):474–488
Zhang Q, Xiao S, Jiao X, Shen Y (2023a) The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018 [J]. Cardiovasc Diabetol 22(1):279
Zhang L, Chen S, Wang W et al (2023b) Inflammatory and nutritional scoring system for predicting prognosis in patients with newly diagnosed multiple myeloma [J]. J Inflamm Res 16:7–17
Zhao Y, Shao W, Zhu Q et al (2023) Association between systemic immune-inflammation index and metabolic syndrome and its components: results from the national health and nutrition examination survey 2011–2016 [J]. J Transl Med 21(1):691
Zou X, Zhou X, Zhu Z, Ji L (2019) Novel subgroups of patients with adult-onset diabetes in Chinese and US populations [J]. Lancet Diabetes Endocrinol. https://doi.org/10.1016/S2213-8587(18)30316-4
Acknowledgements
The authors would like to thank the National Health and Nutrition Examination Survey participants and staff for their invaluable contributions.
Funding
This study was supported by the National Natural Science Foundation of China (No.8197021705) and the Hunan Provincial Natural Science Foundation of China (No.2022JJ30069).
Author information
Authors and Affiliations
Contributions
XL C conceived the research and the manuscript. XL C and AH L conducted data collection and analysis. XL C and QL M interpreted the results. XL C drafted the manuscript, which QL M then revised. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Ethics approval for this study was granted by the Committee on Human Research at the University of California, San Francisco. The study was approved by the NCHS Institutional Review Board.
Informed consent
All participants provided informed consent.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Li, A. & Ma, Q. Neutrophil–lymphocyte ratio and systemic immune-inflammation index as predictors of cardiovascular risk and mortality in prediabetes and diabetes: a population-based study. Inflammopharmacol 32, 3213–3227 (2024). https://doi.org/10.1007/s10787-024-01559-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-024-01559-z