Abstract
Acute myocardial infarction is an important cardiovascular disease worldwide. Although the mortality rate of myocardial infarction (MI) has improved dramatically in recent years due to timely treatment, adverse remodeling of the left ventricle continues to affect cardiac function. Various immune cells are involved in this process to induce inflammation and amplification. The infiltration of inflammatory cells in the infarcted myocardium is induced by various cytokines and chemokines, and the recruitment of leukocytes further amplifies the inflammatory response. As an increasing number of clinical anti-inflammatory therapies have achieved significant success in recent years, treating myocardial infarction by targeting inflammation may become a novel therapeutic option. In particular, successful clinical trials of canakinumab have demonstrated the important role of the inflammatory factor interleukin-1 (IL-1) in atherosclerosis. Targeted IL-1 therapy may decrease inflammation levels and improve cardiac function in patients after myocardial infarction. This article reviews the complex series of responses after myocardial infarction, including the involvement of inflammatory cells and the role of cytokines and chemokines, focusing on the progression of the IL-1 family in myocardial infarction as well as the performance of current targeted therapy drugs in experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myocardial infarction has the highest mortality rate in the world, and its pathogenesis mainly involves the formation of thrombi due to the rupture of unstable plaques. The thrombi, subsequently block the coronary arteries, leading to ischemia and hypoxia, and ultimately, myocardial infarction occurs (Hartikainen et al. 2020). However, immediate reperfusion therapy has largely solved clinical problems. Timely percutaneous coronary intervention (PCI) is beneficial for reducing acute mortality and the occurrence of left ventricular remodeling (Keeley et al. 2003). However, subsequent myocardial ischemia–reperfusion (IR) injury is still not negligible (Yellon and Hausenloy 2007). An ischemic incident causes cellular swelling and destruction, leading to the efflux of cellular contents. This efflux contributes to local and systemic inflammation both during the infarction phase of myocardial infarction and during the reperfusion phase (Toldo and Abbate 2018; Toldo et al. 2018).
NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome activation during acute myocardial infarction causes a strong inflammatory response and increases IL-1 activity. Elevated activity of IL-1, a classic proinflammatory cytokine, has been associated with a variety of inflammatory diseases and is now intensively studied in cardiovascular disease (CVD) (Dinarello 2011). It has been well established that IL-1 and its receptor are important initiators and promoters of the inflammatory process in CVD. There are two isoforms of IL-1, interleukin-1α (IL-1α) and interleukin-1β (IL-1β), both of which share a common receptor, interleukin-1 receptor type I (IL-1R1). IL-1α or IL-1β induces an inflammatory response in endothelial cells by binding to its receptor IL-1R1.The binding of both receptors activates a series of phosphorylation and ubiquitination responses and can activate downstream signaling pathways, including the NF-kB, JNK, and p38 MAPK pathways, among others (Libby 2017). IL-1β can induce an inflammatory response in endothelial cells by increasing the expression of adhesion factors and chemokines to promote the aggregation of inflammatory cells in the vasculature and endothelial invasion (Bevilacqua et al. 1985). In addition, IL-1α can serve as an alarm factor in the inflammatory response and initiate inflammatory cascades. Although IL-1α can work as an alarm factor for the inflammatory response and initiate an inflammatory cascade, it is an important danger signal at the onset of acute myocardial infarction (Buckley and Abbate 2018; Lugrin et al. 2015). The regulation of IL-1/IL-1R1 and its antagonists aid in the therapeutic modifications of excessive leukocyte production after myocardial infarction, reduce the inflammatory response and improve adverse remodeling (Abbate et al. 2011; Sager et al. 2015).
This article reviews the development of the inflammatory response after acute myocardial infarction, including that of inflammatory cells, inflammatory factors and focuses on the progression of the main targets of the IL-1 signaling pathway in the current study of acute myocardial infarction as well as the latest manifestations of the drugs targeting IL-1α and IL-1β, as well as their receptors and endogenous inhibitors.
Basic pathophysiology of acute myocardial infarction
Myocardial infarction leads to a strong inflammatory response, which is crucial for heart repair, however, this response is strongly associated with adverse cardiac remodeling and the development of heart failure (Frangogiannis 2014). Following cardiomyocyte infarction, inappropriate damage-associated molecular patterns are released extracellularly to cause a dramatic immune response and induce the generation of inflammasomes (Zuurbier et al. 2019). Activation of the NLRP3 inflammasome, with consequent increases in caspase-1, IL-1β, and interleukin-18 (IL-18) levels, will continue to amplify the inflammatory response (Toldo and Abbate 2018; Toldo et al. 2018). RNA silencing of the inflammasome or pharmacologic inhibition of the inflammasome can provide some protection to the cardiac system after myocardial infarction by reducing infarct size and decreasing myocardial fibrosis (Gao et al. 2019; Mezzaroma et al. 2011; Zhang et al. 2020). The effects of inflammation on myocardial infarction are varied and complex. As previously mentioned, damage-associated molecular patterns (DAMPs) released into the extracellular compartment activate the NLRP3 inflammasome; subsequently, caspase activation induces precursors to form mature IL-1β and IL-18, both of which are released extracellularly through the gasdermin-D (GSDMD) pore (Vande Walle and Lamkanfi 2016). This eventually leads to cell swelling and rupture, and cardiomyocytes undergo pyroptosis. In addition, the production of proinflammatory cytokines is an important marker of inflammation after MI. The release of cytokines such as tumor necrosis factor-α (TNF-α) and IL-1β promotes chemokine synthesis and favors leukocyte infiltration (Bujak et al. 2008; Frangogiannis et al. 1998; Sager et al. 2015). This increases the abundance of inflammatory cells in the infarcted area and increases the inflammatory response after MI (Ong et al. 2018). Rat MI models have been constructed in which persistently elevated levels of TNF-α, IL-1β, and interleukin-6 (IL-6) are proportional to the left ventricular end-diastolic diameter (Ono et al. 1998). This finding also confirms that the adverse inflammatory responses will affect the fibrotic function of the left ventricle. This ultimately leads to adverse remodeling of the heart and deterioration of cardiac function.
Cytokines and chemokines
The secretion of large amounts of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, after myocardial infarction is an important hallmark of the onset of the inflammatory response. The secretion of TNF-α promotes the synthesis and release of chemokines and adhesion factors, exacerbating the inflammatory response (Maekawa et al. 2002). Capturing TNF-α effectively inhibits TNF-α to alleviate inflammation and improve cardiac function (Zheng et al. 2023). Different tumor necrosis factor receptors play different roles in the pathophysiology of TNF-α. In mouse models of heart failure after myocardial infarction, TNF, TNFR1 and TNFR2 were found to be upregulated, and left ventricular remodeling was exacerbated in TNFR2−/− heart failure mice. These findings suggest that TNFR2 may have a protective role against heart failure. TNFR1 promotes deleterious remodeling, whereas TNFR2 is cardioprotective in terms of cardiorespiratory remodeling, systolic dysfunction and so on. Both have different effects on key downstream mediators, such as NF-κB, inflammatory signaling responses and apoptosis (Befekadu et al. 2022; Hamid et al. 2009). Extracellular vesicles in the plasma of patients with MI were found to eliminate TNF-α-induced cell death, thereby realizing their cardioprotective potential (Khandagale et al. 2022). IL-1 and IL-6 are also important proinflammatory factors after myocardial infarction. IL-1 will be described and reviewed in more detail later. IL-6 is an essential inflammatory factor in both myocardial infarction and atherosclerosis (Moriya 2019) and has recently been shown to contribute to weaving the inflammatory network during acute coronary syndromes (Nakao and Libby 2023).
IL-6 also plays an important role in cardiac and hepatic crosstalk. Moreover, the ischemic heart was found to deliver signals to the liver through the acute inflammatory IL-6/STAT3 pathway, and the liver reacts by downregulating the mineralocorticoid receptor to mitigate ischemic injury through a series of responses (Sun et al. 2023a). Monoclonal antibodies targeting IL-6 have also been tested in many clinical trials for the treatment of myocardial infarction with promising results. The use of tocilizumab significantly reduces patient neutrophil counts and improves inflammation (Huse et al. 2022; Woxholt et al. 2023).
Similarly, chemokines play a key role in adverse remodeling after MI. Chemokine ligand 2 (CCL2) and chemokine ligand 7 (CCL7) of the chemokine family have long been shown to mediate the recruitment of proinflammatory cells (Dewald et al. 2005; Kim and Luster 2013; Zouggari et al. 2013). Elevated chemokine ligand 16 (CXCL16) levels during acute cardiovascular events increase mortality. Anti-CXCL16 neutralizing antibody administration suppresses monocyte infiltration and enhances cardiac function after myocardial infarction (Zhang et al. 2023). Chemokine ligand 17 (CCL17) is also a proinflammatory mediator of CC chemokine receptor 2 (CCR2) macrophages and dendritic cells after myocardial infarction, indicating that inhibition of CCL17 may be an effective strategy to promote Treg recruitment, inhibit myocardial inflammation and diminish left ventricular remodeling (Feng et al. 2022). The CC chemokine ligand 5 (CCL5) likewise induces the migration of inflammatory cells to areas of inflammatory lesions, and inhibition of CCL5 expression and the NF-κB signaling pathway helps to attenuate adverse myocardial fibrosis after MI (Han et al. 2022). Moreover, timely administration of chemokines that act as anti-inflammatory agents might favor macrophage phenotype switching and promote ventricular repair (Wang et al. 2023a). One study used M2 macrophage‐derived small extracellular vesicles (M2EVs) to deliver miR-2b-2p to inhibit the metabolism of CCR2-expressing macrophages, thus affecting cardiac repair (Li et al. 2023a).
Macrophages
Monocytes affected by chemokines that accumulate in the region of myocardial infarction differentiate into macrophages that phagocytose cellular debris. Macrophage emergence after infarction helps wound recovery, but excessive inflammatory activation also leads to adverse cardiac remodeling (Davidson et al. 2019; Fan et al. 2019). In addition to monocytes from the blood, resident cardiac macrophages have received increased amounts of attention in recent years (Chen et al. 2023). Differential monocyte recruitment by resident CCR2− and CCR2 + cardiac macrophages after myocardial injury was revealed by single-cell sequencing (Bajpai et al. 2019). At late stages of cardiac repair, macrophages are converted to an anti-inflammatory phenotype that promotes the activation of myocardial fibroblasts and endothelial cells, which facilitates cardiac repair (Honold and Nahrendorf 2018). An increasing amount of studies related to the suppression of inflammation after myocardial infarction through macrophage polarization have been conducted. In particular, biomaterials, extracellular vesicles and others have applications in this area. An NF-κB signaling inhibitor was coassembled with interleukin-10 (IL-10) and an NF-κB inhibitor (SN50) to form a novel anti-inflammatory SN50/IL-10/NapFFY hydrogel with cardioprotective characteristics (Wang et al. 2023b). This novel material reduces cardiomyocyte apoptosis and increases angiogenesis in the border region by promoting M2 macrophage polarization. Labeling macrophages with AuNP-amphoteric glucose was used to assess the potential for macrophage-mediated delivery to infarcted hearts (Wang et al. 2023c); This approach targets the myocardial infarction area with the help of macrophages as delivery vehicles. Extracellular vesicles (EVs) of bone marrow mesenchymal stem cells (MSCs) pretreated with atorvastatin (ATV) are capable of superior cardiac repair of myocardial infarction by inhibiting M1 macrophage polarization and promoting M2 macrophage polarization (Ning et al. 2023). There is also emerging evidence that macrophage metabolism plays an important role in macrophage phenotypic polarization and that proinflammatory and anti-inflammatory macrophages have different routes of glucose metabolism, with mitochondrial oxidative phosphorylation and glycolysis, both ATP-producing pathways, being differentially upregulated or inhibited in the two macrophage types (Cai et al. 2023; Mills and O’Neill 2016; Mouton et al. 2018; Nassef et al. 2021). Mitochondrial dysfunction in macrophages and antioxidant strategies targeting mitochondria will be the next research focus.
Neutrophils
In the early inflammatory phase of myocardial infarction, neutrophils are the first leukocytes to reach the infarcted area of the myocardium, accompanied by a strong inflammatory response and immune cell infiltration (Epelman et al. 2015; Prabhu and Frangogiannis 2016). Following acute infarction, in addition to the phagocytosis of cellular debris, neutrophils cause concomitant cardiac injury through the release of reactive oxygen species (ROS), protein hydrolases, and inflammatory cytokines (TNF-α and IL-1β) (Carbone et al. 2013; Ma et al. 2013). NETosis is a mode of inflammatory cell death in neutrophils(Papayannopoulos 2018). Considerable research evidence suggests that Neutrophil Extracellular Traps (NETs) play a harmful role in CVD (Bonaventura et al. 2020). In myocardial infarction, NETs can form thrombi by promoting fibrin deposition (Fuchs et al. 2010). An increase in NETs also leads to activation of the NLRP3 inflammasome and inflammatory macrophages (Hu et al. 2019). A study revealed that weakening neutrophil activity during the acute inflammatory phase after myocardial infarction improves cardiac repair and function without affecting neutrophil development and differentiation, through the establishment of a neutrophil-specific dual specificity phosphatase 6 gene (Dusp6) knockout mouse model (Zhou et al. 2022). Interactions between neutrophils and platelets lead to clot formation and vascular obstruction after myocardial infarction (Stark and Massberg 2021). Platelets participate in neutrophil recruitment and activation by releasing chemokines and adhesion molecules (Duerschmied et al. 2013; Karshovska et al. 2013). A study showed that neutrophils control thrombopoiesis by forming intravascular megakaryocyte extensions, in turn accelerating their growth and release into the circulation (Petzold et al. 2022). In addition to macrophage polarization, postinfarction neutrophil polarization over time has received much attention. Neutrophils isolated from the left ventricle of infarcted mice highly expressed proinflammatory markers on Day 1 but highly expressed anti-inflammatory markers on Days 5 and 7 (Ma et al. 2016). Neutrophil polarization deserves more research attention. These findings provide new directions for future research on neutrophils in CVD.
Lymphocytes
Extensive animal studies have shown that lymphocytes also contribute significantly to myocardial infarction (Yan et al. 2013; Zouggari et al. 2013). B lymphocytes appear to play an adverse role in remodeling after myocardial infarction (Zouggari et al. 2013) and promote the aggregation and activation of monocytes. Cardiac function after acute injury can be improved by modulating cardiac B lymphocyte subsets (Adamo et al. 2018). In addition, the absence of splenic marginal zone B lymphocytes eliminates adverse ventricular remodeling induced by B cells after MI (Sun et al. 2022). Regulatory T cells are now in the spotlight, Tregs are highly enriched in mice with myocardial infarction and ischemia/reperfusion injury, and phenotypically and functionally distinct populations of cardiac Tregs with cardioprotective roles have been identified (Xia et al. 2020). Surface molecules on Treg cells have also been investigated. For example, CD69 overexpressing Treg cells have been found in patients with myocardial infarction, and studies in mouse models have shown that CD69 expression on Treg cells increases survival after left anterior descending coronary artery ligation. The transfer of CD69+ Tregs into myocardial infarcted Cd69−/− mice induces γδT cell apoptosis and reduces IL-17A production, increasing survival in mice (Blanco-Domínguez et al. 2022). Another study demonstrated the importance of ecto-5'-nucleotidase (CD73), a surface marker of Tregs, in the resolution of inflammation and cardiac healing after MI (Zhuang et al. 2022). Hypertension-induced dysfunctional Tregs play a pathogenic role in the progression of IR (Sun et al. 2023b). These studies strongly support the idea that Tregs may be therapeutic targets for cardiac repair after myocardial infarction.
IL-1 in myocardial infarction
IL-1 is a proinflammatory cytokine. Myocardial cells are damaged after myocardial infarction. These damaged cells releasing large amounts of cellular contents, including adenosine, extracellular RNA, and IL-1α, which stimulate innate immune signaling (Chen et al. 2014; Lugrin et al. 2015). These substances act as DAMPs and bind to Toll-like receptors (TLRs), cytosolic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and the cell surface receptor for advanced glycation end-products (RAGE) on the cell surface to activate nuclear factor kb (NF-κB) which induces inflammasome synthesis (Toldo et al. 2015). This also activates the transcription and synthesis of cytokines and chemokines (Zhang et al. 2015). As the sensing component of the inflammasome, NLRP3 leads to an intense inflammatory response and IL-1 activity. There are many members of the IL-1 family. Thus we will select a few of the most important and widely studied targets in CVD, including the IL-1 receptor, the two ligands of IL-1, IL-1α and IL-1β, and its natural inhibitor, IL-1Ra and summarize the recent findings regarding these targets.
IL-1R, IL-1Ra
The IL-1 receptor family contains ten different but related gene products. IL-1R1 and the accessory protein IL-1RAcP, play major roles in the inflammatory response. IL-1R2 does not send signals but plays a role in the isolation of IL-1β, called the “decoy receptor” (Boraschi and Tagliabue 2013). IL-1 mediated inflammation begins with the binding of IL-1α or IL-1β to IL-1R1, and subsequently IL-1 receptor accessory protein (IL-1RAcP) forms a trimer with these proteins (Casadio et al. 2001). In addition, the Toll/interleukin-1 receptor/resistance protein (TIR)-structural domains on the receptors IL-1R1 and IL-1RAcP exist as heterodimers (Thomas et al. 2012). Subsequently, oligo-TIR recruits myeloid differentiation primary response gene 88 (MyD88) to create a receptor complex with high binding affinity, for which it can recruit downstream signal transduction molecules (Brikos et al. 2007). The downstream signals generated can be transmitted via IL-1R kinase (IRAK) (Cao et al. 1996) or through NF-κB, JNK, or p38 MAPK after a series of phosphorylation reactions or ubiquitination, which further increases the transcription and translation of inflammatory mediator genes (Gilmore 1999; Zandi et al. 1998). IL-1R1 is important for the transduction of the IL-1 inflammatory signaling pathway, and cardiac fibroblast-specific IL-1R1 deficiency reduces adverse cardiac remodeling after myocardial infarction (Bageghni et al. 2019). As a natural endogenous antagonist of the IL-1 receptor, IL-1Ra limits excessive inflammation. The balance of IL-1/IL-1 Ra signaling at the IL-1R1 level regulates cardiac remodeling after MI in mice (Abbate et al. 2011). Interleukin-1Ra has also recently been demonstrated to prevent the progression of heart failure associated with acute pressure overload possibly related to decreased production of fibrosis mediators and cytokines (Javan et al. 2022). The use of transplanted autologous haematopoietic stem/progenitor cells (HSPCs) as a source of an IL-1 receptor antagonist (IL-1Ra) via a lentiviral (LV)-mediated gene transfer strategy for systemic delivery has been suggested to ensure stable IL-1Ra production, which has been shown to increase in a variety of models (Colantuoni et al. 2023). Alternatively, with the aid of biomaterial transportation, subcutaneous injection of agarose hydrogels containing genome-edited Ccl2-IL-1Ra iPSCs showed significant therapeutic effects in a model of inflammatory arthritis (Collins et al. 2023). Applying this technique to diseases such as coronary heart disease may lead to better therapeutic results. As a decoy receptor, IL-1R2 binds ligands but does not elicit an inflammatory response. It prevents the cleavage and activity of interleukin-1α and controls necrosis-induced sterile inflammation (Zheng et al. 2013). IL-1R2 lacks the characteristic intracellular TIR structural domain, so this receptor is unable to initiate downstream signaling upon interaction with its ligands (Colotta et al. 1993). IL-1R2 and IL-1R1 compete for binding to the ligand, thereby preventing the binding of the proinflammatory cytokines IL-1α and IL-1β to the receptor (Malinowsky et al. 1998; Re et al. 1996). The soluble receptor (sIL-1R2), which is released into the bloodstream, also binds pro-IL-1α and pro-IL-1β, preventing cleavage by the caspase-1 enzyme, and exhibits anti-inflammatory activity (Smith et al. 2003). Moreover, studies by scholars have shown that IL-1R2 protects cardiomyocytes from apoptosis during I/R injury by downregulating interlcukin-17 receptor A (IL-17RA)/STAT1 signaling and IL-1R2 was found to be significantly elevated in the plasma of MI patients. However, knockdown of IL-1R2 in infarcted mice increases neutrophil and macrophage infiltration into ischemic areas of the heart (Lin et al. 2022).
IL-1α
In addition to the receptor, two important ligands of the IL-1 signaling pathway, IL-1α and IL-1β, have received increasing amounts of attention. IL-1α is a “bifunctional cytokine” that not only binds to receptors on the cell surface but also translocates to the nucleus through nuclear localization sequences, thereby affecting gene transcription (Werman et al. 2004). The precursor of IL-1α is biologically active and is called membrane IL-1α, especially on monocytes and B lymphocytes (Kurt-Jones et al. 1985). IL-1α, which is passively released after cell necrosis acts as a proinflammatory DAMP (Dinarello 2018). Resident tissue macrophages respond to IL-1α and produce IL-1β. Necrotic cardiomyocytes release IL-1α, which can be abolished by IL-1 receptor antagonists and IL-1α-blocking antibodies. Specifically knocking down IL-1α in a model of myocardial ischemia–reperfusion significantly reduced cardiomyocyte inflammation (Lugrin et al. 2015). J. Lugrin’s study also noted that systemic defects in IL-1α in a myocardial infarction model decreased early myocardial inflammation and the expression of profibrotic genes, and ameliorated adverse left ventricular remodeling (Lugrin et al. 2023). Similarly, transgenic mice overexpressing the IL-1α gene exhibited cardiac hypertrophy and a 1.4- to 2.2-fold increase in the heart weight-to-body weight ratio (Isoda et al. 2001). Regarding the mechanism of action of IL-1α, intravital microscopy revealed that IL-1α mediated leukocyte-endothelial adhesion via IL-1R1 (Schunk et al. 2021). This finding also indicates why IL-1α can play an important role in the inflammatory response in MI and has become an important direction for immunotherapy. However, IL-1α may also have a positive effect on cardiac remodeling. After myocardial infarction, IL-1α in left ventricular fibroblasts may induce the expression of steroidogenic acute regulatory protein (StAR/STARD1) in border zone fibroblasts, which results in antiapoptotic effects. This cellular response that evolves in response to inflammation may favor improved cardiac function (Razin et al. 2021). Thus, the role of IL-1α after MI is complex and multifaceted. Thrombus formation due to abnormal coagulation can block coronary arteries leading to myocardial infarction. Recent studies have shown that IL-1α can also affect thrombosis by reducing the activation of thrombin inhibitors (Laura et al. 2023). This finding also revealed that IL-1α acts in both the coagulation and immune systems to influence CVD.
IL-1β
In addition to IL-1α, IL-1β is currently receiving increased amounts of attention in CVD. Moreover, the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) successfully demonstrated that targeting IL-1β effectively diminishes the inflammatory response in patients with coronary artery disease and reduces cardiovascular events (Ridker et al. 2017a). In a recent study, inhibition of IL-1β or clearance of macrophages was shown to be effective in improving cardiac function and lowering blood glucose in mice fed a high-fat diet, which was established as an animal model of diastolic heart failure (Liu et al. 2023). In contrast to IL-1α, IL-1β is a regulator of systemic inflammation, and the levels of IL-1β produced by infiltrating macrophages and circulating monocytes significantly affect systemic inflammation. The precursor of IL-1β must be processed through cysteine asparaginase-1 after activation of the NLRP3 inflammasome (Dinarello 2018). Thus, activation of the NLRP3 inflammasome and IL-1β has become an important target for inflammatory therapy in CVD. It has also been shown that inhibition of NLRP3 and IL-1β signaling after MI alleviates fibrosis and improves cardiac function in mouse models (Gao et al. 2021; Wei et al. 2023). The production of excess reactive oxygen species is closely related to IL-1β, and mitochondria, a major site of oxidative stress, have also received increased attention. Z-DNA binding protein 1 (ZBPN-1) is a pattern recognition receptor that regulates inflammation in response to mitochondrial DNA (mtDNA) in inflammatory cells and endothelial cells. ZBP1 has been shown to protect infarcted cardiomyocytes by reducing mtDNA-induced phosphorylation of inflammatory factors such as NF-κB, NLRP3, and IL-1β (Enzan et al. 2023). A reduction in postinfarction oxidative stress caused by the inhibition of IL-1β can prevent adverse cardiac remodeling (Emran et al. 2021). Precise targeting of mitochondria through the use of nanoparticles has also been proposed as a possible new therapeutic strategy for the treatment of myocardial IR injury (Ikeda et al. 2021).
Apart from IL-1β itself, the close relationship between noncoding RNAs and IL-1β has slowly been explored in cardiac infarctions. The lncRNA myocardial infarction-associated transcript (lncRNA MIAT) is a crucial lncRNA involved in the regulation of CVD. It inhibits the expression of IL-1β and TNF-ɑ, but it is also inhibited by the NLRP3 inflammasome (Wang et al. 2021). However, findings on the role of the lncRNA MIAT in atherosclerosis suggest that its upregulation may exacerbate this damage (Sun et al. 2019). MiR-135b overexpression in mice with heart infarction significantly preserved impaired cardiac function and attenuated the upregulation of the NLRP3/Caspase-1/IL-1β pathway (Li et al. 2020). In addition, miR-132 inhibited cardiomyocyte apoptosis, thereby improving myocardial remodeling in MI rats by downregulating IL-1β (Zhao et al. 2020). Furthermore, downregulation of miR-24-3p and upregulation of IL-1β were negatively correlated in blood samples from patients with acute myocardial infarction, and IL-1β promoted the proliferation of hypoxic human umbilical vein endothelial cells by downregulating miR-24-3p (Huang et al. 2022). These relevant studies revealed the relationship between noncoding RNAs and IL-1β in myocardial infarction, and therapeutic means targeting inflammatory factors through the perspective of noncoding RNAs could be a good option in future.
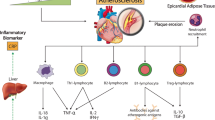
In addition to apoptosis, pyroptosis, which involves mainly NLRP3, caspase-1, IL-1β, and gasdermin-D, has received much attention in CVD research (Zhaolin et al. 2019). Pyroptosis also plays an important role in this process (Zhaolin et al. 2019). The relevant information is summarized in Fig. 1. Pyroptosis is characterized by the activation of NLRP3. Caspase-1 recognizes inactive IL-β and IL-18 precursors and converts them to mature inflammatory cytokines. These subsequently cleave GSDMD and mediate membrane pore formation, leading to rapid disruption of the plasma membrane followed by the release of cellular contents and proinflammatory mediators (Lacey et al. 2018; Wang et al. 2019c). This has resulted in the study of an increasing number of drugs targeting cellular pyroptosis in myocardial infarction (Ding et al. 2023; Li et al. 2023b; Peng et al. 2023). The inhibition of NLRP3 inflammasome activation is also emerging as an important means of inhibiting the occurrence of cellular pyroptosis after myocardial infarction, with some noncoding RNAs or herbs demonstrating better potential. For example, the lncRNA FAF attenuates hypoxia/ischemia-induced focal death via the miR-185-5p/PAK2 axis in cardiomyocytes, and Qishen granule and piperazine ferulate exert cardioprotective effects by inhibiting NLRP3 inflammatory vesicles and cellular focal death in myocardial infarction (Chen et al. 2022; Gu et al. 2022; Lei et al. 2022). In conclusion, the onset of pyroptosis is accompanied by an inflammatory response, and the activation of the NLRP3 inflammasome and the release of mature IL-1β amplifies the inflammatory cascade. Thus, how to target the IL-1β or NLRP3 inflammasome will become a new key point in the treatment of myocardial infarction. Sympathetic overactivation also plays an important role in cardiac remodeling after myocardial infarction, and the production of IL-1β and other inflammatory factors is also closely related to sympathetic activation (Lyu et al. 2020). Taurine diminished cardiac sympathetic innervation by modulating NLRP3 inflammasome/IL-1β-dependent pathways in a rat model of myocardial infarction (Lee et al. 2021). Cysteinyl asparaginase-1 acts as a shearing tool for IL-1β precursors, by inhibiting the activity of this enzyme, facilitating the reduction of IL-1β production, and upregulating intercellular linking proteins in cardiomyocytes to improve communication and thus protect the myocardium (Su et al. 2022). In addition, some observational clinical studies have demonstrated associations between the levels of NLRP3 and IL-1β and the risk of coronary heart disease (Guo et al. 2023; Mooney et al. 2022; Opstad et al. 2022; Pan et al. 2022).
Schematic representation of the main mechanisms of IL-1 action after myocardial infarction. After myocardial injury, cardiomyocytes release a large amount of cellular contents to act as DAMP, which will bind to the cell surface Toll-like receptor (TLR), cytoplasmic nucleotide-binding oligomerization structural domain-like receptor (NLR), and the cell surface receptor for the cell surface late glycosylation end product (RAGE), as well as IL-1R, in order to activate the synthesis of nuclear factor kb (NF-kB) that induces the synthesis of inflammatory vesicles, and the activated caspase-1 will shear pro-IL-1β and pro-IL-18. Inflammasome formation, activated caspase-1 will shear pro-IL-1β and pro-IL-18 to form mature bodies after release through the GSDMD pore to induce inflammatory pyroptosis, and released IL1β will also continue to induce downstream inflammatory responses. Adapted from “FullTemplateName”, by BioRender.com (CurrentYear). Retrieved from https://app.biorender.com/biorender-templates
Randomized clinical trials with IL-1 inhibitors
The role of the IL-1 family in CVD has been studied by a large number of researchers. Different kinds of drugs targeting IL-1, whether chemical drugs or monoclonal antibodies, have been studied in basic or clinical studies on CVD. These drugs inhibit IL-1 and its receptor, thus affecting the ability of the IL-1 signaling pathway to treat relevant diseases. The mechanism of action of drugs targeting IL-1 is shown in Fig. 1 (Table 1).
Anakinra
Anakinra is a recombinant human interleukin receptor antagonist that antagonizes both IL-1α and IL-1β (Aksentijevich and Kastner 2011). Anakinra is currently approved primarily for the treatment of rheumatic diseases (Ramírez and Cañete 2018). However, it has also been used in many clinical studies or basic experiments on CVD. In 2014, a phase II, double-blind, randomized, placebo-controlled study enrolled patients with non-ST-segment elevation myocardial infarction treated with daily subcutaneous injections of an IL-1 receptor antagonist or placebo for 14 days. At the end of treatment on day 14, the levels of high-sensitivity c-reactive protein (hsCRP) and IL-6 were significantly lower in the treatment group than in the placebo group. In contrast, 16 days after treatment was stopped (day 30), the treatment group’s high-sensitivity c-reactive protein levels were again elevated (Morton et al. 2015). After that, anakinra was also used in a clinical trial in patients with ST-segment elevation myocardial infarction. Patients treated with anakinra had significantly lower hsCRP areas under the curve, at month 12 had a significantly lower incidence of new heart failure or rates of heart failure death and hospitalization in the anakinra group but there was no difference in the incidence of serious infections between the anakinra and placebo groups (Abbate et al. 2020). There are also clinical trial demonstrating the ability of anakinra to reduce total white blood cell counts and neutrophil counts in patients with ST-segment elevation acute myocardial infarction (Del Buono et al. 2022b). These findings provide evidence for the anti-inflammatory clinical effects of anakinra. A recent study to determine whether the duration of treatment with PCI affects the efficacy of anakinra on the incidence of systemic inflammation and heart failure in patients with ST-segment elevation myocardial infarction (STEMI) was conducted. The results suggested that IL-1 blockade with anakinra reduced the acute systemic inflammatory response and prevented heart failure independent of treatment duration (Del Buono et al. 2023). In addition, whether the site of myocardial infarction influenced the effects of anakinra was also examined, and the results showed that anakinra was able to reduce the number of heart failure events regardless of anterior or nonanterior wall myocardial infarction (Del Buono et al. 2022a). The use of the IL-1 receptor antagonist anakinra also helps to combat postinfarction arrhythmias by inhibiting sympathetic sprouting after myocardial infarction, contributing to neural and cardiac remodeling (Yin et al. 2017). With IL-1 blocker medications, the risk of infection has been a key element of concern for clinicians. One meta-analysis that included three early randomized clinical trials with endpoints including all-cause mortality and new-onset heart failure also documented safety events involving injection-site reactions and serious infections, with injection-site reactions being significantly greater in patients treated with anakinra, and there was no significant difference in the incidence of serious infections (Abbate et al. 2022). This finding may provide strong evidence for the use of anakinra in the clinic, although more research is needed to support its use (Fig. 2).
The mechanism of action of drugs targeting IL-1. Binding of IL-1α or IL-1β to IL-1R1 can bind to IL-1RAcP to form a trimer thereby recruiting Myd88 and delivering downstream signals, for example, through the NF-kB signaling pathway. Anakinra can compete with IL-1 R1 for binding to IL-1α or IL-1β; Bermekimab can bind to IL-1α preventing IL-1α from binding to IL-1 R1; When Canakinumab binds to IL-1β, its Fab fragment overlaps with the D1 region of IL-1 R1, preventing IL-1β from binding to IL-1 R1; Gevokizumab reduces the complex’s ability to bind to IL-1 Racp and IL-1 R1 affinity. The two arms of rilonacept are formed by the extracellular regions of IL-1 R1 and IL-1 Racp thereby capturing IL-1. Adapted from “FullTemplateName”, by BioRender.com (CurrentYear). Retrieved from https://app.biorender.com/biorender-temp
Rilonacept
Rilonacept is a subcutaneously injectable soluble fusion protein consisting of the ligand-binding domain of the extracellular portion of IL-1 receptor 1 and IL-1 receptor accessory proteins, which acts as a receptor that binds to IL-1α and IL-1β to inhibit IL-1 signaling. The drug was previously approved for use in clinical trials for Cryptococcal Associated Periodic Syndrome (CAPS) and with chronic gouty arthritis, and was recently approved by the FDA for the treatment of recurrent pericarditis based on the results of a pivotal Phase 3 clinical trial. (Klein et al. 2021; Landmann and Walker 2017; Terkeltaub et al. 2009). Compared with placebo, rilonacept provided rapid alleviation of recurrent pericarditis episodes and a significant reduction in the risk of pericarditis recurrence. However, the most common adverse events associated with rilonacept injections were injection-site reactions and upper respiratory tract infections, but generally no deaths or serious adverse events occurred (Presti et al. 2021). Rilonacept is still mainly used for the treatment of other chronic inflammatory diseases such as rheumatic diseases, with less research related to coronary atherosclerotic heart disease. However, it should receive more attention from scholars because of its promising therapeutic effects in chronic inflammatory diseases. In addition, as an IL-1 receptor inhibitor, its effect in combination with anakinra should receive more interest.
Canakinumab
Canakinumab, a monoclonal antibody against IL-1β that blocks IL-1β signaling by binding to IL-1β, where the Fab fragment of the monoclonal antibody significantly overlaps with the D1 region of IL-1R1. Thus, prevents IL-1β from binding to the receptor again (Blech et al. 2013). In a randomized, double-blind trial involving 10,061 patients with prior myocardial infarction and high-sensitivity C-reactive protein levels of 2 mg/L or higher, different doses of canakinumab were administered subcutaneously, with the primary efficacy endpoints being nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. The results showed that a dose of canakinumab of 150 mg every 3 months resulted in a significant reduction in the recurrence of cardiovascular events, unrelated to a reduction in lipid levels. However, patients treated with canakinumab had a markedly higher number of deaths attributable to infection or sepsis than the placebo group, and those who died from infection tended to be older and more likely to have diabetes than patients who did not die from infection. Six confirmed cases of tuberculosis occurred throughout the trial period (Ridker et al. 2017a, 2018). This finding also provides strong evidence for the inflammation hypothesis in atherosclerosis, and the feasibility of targeting IL-1 in CVD has been supported. Surprisingly, in this study, it was found that canakinumab may also have some therapeutic effect on cancer, and that canakinumab treatment targeting the interleukin-1β innate immune pathway has the potential to significantly reduce lung cancer and lung cancer-mortality rates (Ridker et al. 2017b). Related possible mechanisms have also been summarized by scholars (Garon et al. 2020). In another study, 30 patients with previous myocardial infarction with high-sensitivity C-reactive protein ≥ 2 mg/l and a left ventricular ejection fraction (LVEF) < 50% were enrolled. The LVEF increased from 38% (33–43) to 44% (38–52) after 12 months of treatment with canakinumab. Moreover, the patients’ change in peak-oxygen consumption (VO2) also improved. This clinical trial also provides strong evidence that heart failure caused by coronary atherosclerotic heart disease (CHD) can be prevented by targeting IL-1β (Trankle et al. 2018). Some new mechanistic possibilities have also been identified in experimental animal studies through the use of analogs of canakinumab, where treatments targeting the NLRP3 inflammasome and IL-1β reduced leukocyte recruitment in the blood of atherosclerotic aortas, possibly by reducing the proliferation of bone marrow hematopoietic stem and progenitor cells (Hettwer et al. 2022). Combining statins with canakinumab for coadministration in the clinic achieved better outcomes, and dual therapy by lowering lipids and inflammation may provide additional benefits for patients with myocardial infarction and diabetes (Liberale et al. 2019). The success of the canakinumab trial provides promising evidence for targeting residual inflammatory risk in CVD, where IL-1β is an important inflammatory cytokine, and treatments for myocardial infarction will be effective.
Gevokizumab
Gevokizumab is another monoclonal antibody targeting IL-1β that works in a slightly different way than canakinumab, mainly by reducing the affinity of IL-1 for the signaling complex formed by IL-1R1and IL-1RAcP (Blech et al. 2013). Gevokizumab was first used in animal experiments to alleviate insulin resistance and treat diabetes mellitus (Handa et al. 2013; Owyang et al. 2010). These animal experimental results demonstrated that gevokizumab can effectively neutralize cytokine-mediated insulin resistance in adipose tissue. In addition, gevokizumab has also been subjected to a number of relevant animal experiment involving the establishment of a model of myocardial ischemia–reperfusion followed by the administration of gevokizumab, which was found to reduce the direct negative inotropic effects of IL-1β, oxidative stress in the left ventricle, as well as reduced inflammation and infarct size (Harouki et al. 2017). In addition, the use of gevokizumab reduces the levels of IL-1β and nerve growth factor in the paraventricular nucleus of animals with myocardial infarction. This attenuated sympathetic over-innervation after myocardial infarction and prevented arrhythmias (Wang et al. 2019a, b). Recently, more numerous clinical trials conducted with gevokizumab are centered on Behçet’s syndrome and cancer. However, clinical studies of antibodies in relation to myocardial infarction have not yet been reported, and most of them remain in animal experiments, with clinical effects to be further verified (Bettiol et al. 2019; Diwanji et al. 2023).
Bermekimab
Bermekimab is a monoclonal antibody targeting IL-1α that is currently being studied primarily for the treatment of pyogenic sweating infections. Despite a history of anti-TNF therapy, bermekimab has demonstrated good therapeutic effects (Gottlieb et al. 2020). Moreover, the use of this drug has been shown to reduce inflammation and pain. There is no current use of bermekimab in cardiac disease, but there is a randomized phase II trial to examine restenosis of the superficial femoral artery in patients after percutaneous revascularization. The administration of anti-IL-1α antibody immediately after revascularization tended to decrease the incidence of restenosis at the 3-month follow-up. However, there was no difference at 12 months, and adverse events did not differ between the two groups, some adverse events were observed in both groups (El Sayed et al. 2016). In addition, bermekimab has been studied for the treatment of cancer (Kurzrock et al. 2019) and could be considered for use in experiments involving myocardial infarction or atherosclerosis in future, where drugs targeting IL-1α have been studied for the treatment of CVD (Tissot et al. 2013).
Conclusion and perspective
Inflammatory mechanisms in CVD have been extensively studied, and therapies targeting inflammation have shown potential in the treatment of myocardial infarction in both animal and clinical trials. Various immune cells play important roles and are inextricably linked, and IL-1, an important innate immune factor, has an unique role in this regard. The IL-1 signaling pathway serves as an important initiator and facilitator of inflammation in CVD, and the two ligands, IL-1α and IL-1β, amplify a series of downstream inflammatory cascades through activation of the two ligands by binding to their receptor, IL-1R1. Relevant evidence is also supported by numerous clinical trials. Although targeting IL-1 for the treatment of myocardial infarction may have the undesirable consequence of increasing the risk of infection, the mechanism of action is not clear. Moreover, many clinical trials have demonstrated that IL-1 drugs can reduce the decrease in inflammatory markers such as CRP in the body. However, they also increase the reaction at the injection site and increase the risk of infection in the body. Effective resolution of this issue will expand the prospects for the use of IL-1-targeted drugs in CVD treatment and even in tumor immunotherapy.
The results of current clinical trials have provided more support for targeting IL-1 for the treatment of myocardial infarction. Both chemical drugs and monoclonal antibodies have certain effects on reducing cardiovascular events, so targeting IL-1 has a certain future for the treatment of myocardial infarction. The development of novel drugs, such as therapeutic vaccines, nanoparticles in combination with monoclonal antibodies, and hydrogels carrying chemical drugs via local injection and other methods to achieve local reactions, should be considered to avoid systemic adverse effects. These methods deserve more exploration in future and have broad prospects. The rational application and improvement of these drugs will provide a new direction for the anti-inflammatory treatment of myocardial infarction in future.
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Abbreviations
- MI:
-
Myocardial infarction
- IL-1:
-
Interleukin-1
- IR:
-
Ischemia–reperfusion
- NLRP3:
-
NOD-like receptor thermal protein domain associated protein 3
- CVD:
-
Cardiovascular disease
- IL-1α:
-
Interleukin-1α
- IL-1β:
-
Interleukin-1β
- IL-1R1:
-
Interleukin-1 receptor I
- IL-18:
-
Interleukin-18
- DAMPs:
-
Damage-associated molecular patterns
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- GSDMD:
-
Gasdermin
- CCL2:
-
Chemokine ligand 2
- CCL7:
-
Chemokine ligand 7
- CXCL16:
-
Chemokine ligand 16
- CCL17:
-
Chemokine ligand 17
- CCR2:
-
C–C chemokine receptor2
- CCL5:
-
Chemokine ligand 5
- M2EV:
-
M2 macrophage‐derived small extracellular vesicles
- IL-10:
-
Interleukin-10
- SN50:
-
NF-κB inhibitor
- EVs:
-
Extracellular vesicles
- MSCs:
-
Bone marrow mesenchymal stem cells
- ATV:
-
Atorvastatin
- ROS:
-
Reactive oxygen species
- NETs:
-
Neutrophil Extracellular Traps
- Dusp6:
-
Dual specificity phosphatase 6 Gene
- TLR:
-
Toll-like receptors
- NOD:
-
Cytosolic nucleotide-binding oligomerization domain
- NLR:
-
Cytosolic nucleotide-binding oligomerization domain-like receptors
- RAGE:
-
Cell-surface receptor for advanced glycation end-products
- IL-1RAcP:
-
IL-1 receptor accessory protein
- TIR:
-
Toll/interleukin-1 receptor/resistance protein
- Myd88:
-
Myeloid differentiation primary response gene 88
- HSPC:
-
Haematopoietic stem/progenitor cells
- IL-1Ra:
-
IL-1 receptor antagonists
- LV:
-
Lentiviral
- IL-17RA:
-
Interlcukin-17 receptor A
- CANTOS:
-
Canakinumab Anti-inflammatory Thrombosis Outcome Study
- ZBP1:
-
Z-DNA binding protein 1
- mtDNA:
-
Mitochondrial DNA
- lncRNA MIAT:
-
LncRNA myocardial infarction-associated transcript
- PCI:
-
Percutaneous coronary intervention
- STEMI:
-
ST-segment elevation myocardial infarction
- CAPS:
-
Crypto-cryrin-associated periodic syndromes
- LVEF:
-
Left ventricular ejection fraction
- ASC:
-
Apoptosis-associated speck-like protein containing a CARD
- pro-caspase-1:
-
Pro-cysteinyl aspartate specific proteinase-1
- hsCRP:
-
High-sensitivity C-reactive protein
- VO2:
-
Peak-oxygen consumption
- CHD:
-
Coronary atherosclerotic heart disease
References
Abbate A, Salloum FN, Van Tassell BW, Vecile E, Toldo S, Seropian I, Mezzaroma E, Dobrina A (2011) Alterations in the interleukin-1/interleukin-1 receptor antagonist balance modulate cardiac remodeling following myocardial infarction in the mouse. PLoS ONE 6:e27923
Abbate A, Trankle CR, Buckley LF, Lipinski MJ, Appleton D, Kadariya D, Canada JM, Carbone S, Roberts CS, Abouzaki N et al (2020) Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc 9:e014941
Abbate A, Wohlford GF, Del Buono MG, Chiabrando JG, Markley R, Turlington J, Kadariya D, Trankle CR, Biondi-Zoccai G, Lipinski MJ et al (2022) Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur Heart J Cardiovasc Pharmacother 8:503–510
Adamo L, Staloch LJ, Rocha-Resende C, Matkovich SJ, Jiang W, Bajpai G, Weinheimer CJ, Kovacs A, Schilling JD, Barger PM, et al (2018) Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight 3
Aksentijevich I, Kastner DL (2011) Genetics of monogenic autoinflammatory diseases: past successes, future challenges. Nat Rev Rheumatol 7:469–478
Bageghni SA, Hemmings KE, Yuldasheva NY, Maqbool A, Gamboa-Esteves FO, Humphreys NE, Jackson MS, Denton CP, Francis S, Porter KE, et al (2019) Fibroblast-specific deletion of interleukin-1 receptor-1 reduces adverse cardiac remodeling following myocardial infarction. JCI Insight 5
Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C et al (2019) Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 124:263–278
Befekadu R, Grenegård M, Larsson A, Christensen K, Ramström S (2022) Levels of soluble tumor necrosis factor receptor 1 and 2 are associated with survival after ST segment elevation myocardial infarction. Sci Rep 12:14762
Bettiol A, Silvestri E, Di Scala G, Amedei A, Becatti M, Fiorillo C, Lopalco G, Salvarani C, Cantarini L, Soriano A et al (2019) The right place of interleukin-1 inhibitors in the treatment of Behçet’s syndrome: a systematic review. Rheumatol Int 39:971–990
Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA Jr (1985) Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol 121:394–403
Blanco-Domínguez R, de la Fuente H, Rodríguez C, Martín-Aguado L, Sánchez-Díaz R, Jiménez-Alejandre R, Rodríguez-Arabaolaza I, Curtabbi A, García-Guimaraes MM, Vera A, et al (2022) CD69 expression on regulatory T cells protects from immune damage after myocardial infarction. J Clin Invest 132
Blech M, Peter D, Fischer P, Bauer MM, Hafner M, Zeeb M, Nar H (2013) One target-two different binding modes: structural insights into gevokizumab and canakinumab interactions to interleukin-1β. J Mol Biol 425:94–111
Bonaventura A, Vecchié A, Abbate A, Montecucco F (2020) Neutrophil extracellular traps and cardiovascular diseases: an update. Cells 9
Boraschi D, Tagliabue A (2013) The interleukin-1 receptor family. Semin Immunol 25:394–407
Brikos C, Wait R, Begum S, O’Neill LA, Saklatvala J (2007) Mass spectrometric analysis of the endogenous type I interleukin-1 (IL-1) receptor signaling complex formed after IL-1 binding identifies IL-1RAcP, MyD88, and IRAK-4 as the stable components. Mol Cell Proteom MCP 6:1551–1559
Buckley LF, Abbate A (2018) Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J 39:2063–2069
Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG (2008) Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol 173:57–67
Cai S, Zhao M, Zhou B, Yoshii A, Bugg D, Villet O, Sahu A, Olson GS, Davis J, Tian R (2023) Mitochondrial dysfunction in macrophages promotes inflammation and suppresses repair after myocardial infarction. J Clin Invest 133
Cao Z, Henzel WJ, Gao X (1996) IRAK: a kinase associated with the interleukin-1 receptor. Science (new York, NY) 271:1128–1131
Carbone F, Nencioni A, Mach F, Vuilleumier N, Montecucco F (2013) Pathophysiological role of neutrophils in acute myocardial infarction. Thromb Haemost 110:501–514
Casadio R, Frigimelica E, Bossù P, Neumann D, Martin MU, Tagliabue A, Boraschi D (2001) Model of interaction of the IL-1 receptor accessory protein IL-1RAcP with the IL-1beta/IL-1R(I) complex. FEBS Lett 499:65–68
Chen C, Feng Y, Zou L, Wang L, Chen HH, Cai JY, Xu JM, Sosnovik DE, Chao W (2014) Role of extracellular RNA and TLR3-Trif signaling in myocardial ischemia-reperfusion injury. J Am Heart Assoc 3:e000683
Chen X, Li Y, Li J, Liu T, Jiang Q, Hong Y, Wang Q, Li C, Guo D, Wang Y (2022) Qishen granule (QSG) exerts cardioprotective effects by inhibiting NLRP3 inflammasome and pyroptosis in myocardial infarction rats. J Ethnopharmacol 285:114841
Chen C, Wang J, Liu C, Hu J (2023) Cardiac resident macrophages: key regulatory mediators in the aftermath of myocardial infarction. Front Immunol 14:1207100
Colantuoni M, Jofra Hernandez R, Pettinato E, Basso-Ricci L, Magnani L, Andolfi G, Rigamonti C, Finardi A, Romeo V, Soldi M, et al (2023) Constitutive IL-1RA production by modified immune cells protects against IL-1-mediated inflammatory disorders. Sci Transl Med 15:eade3856
Collins KH, Pferdehirt L, Saleh LS, Savadipour A, Springer LE, Lenz KL, Thompson DM Jr, Oswald SJ, Pham CTN, Guilak F (2023) Hydrogel encapsulation of genome-engineered stem cells for long-term self-regulating anti-cytokine therapy. Gels (Basel, Switzerland) 9
Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A (1993) Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science (new York, NY) 261:472–475
Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ et al (2019) Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol 73:89–99
Del Buono MG, Damonte JI, Chiabrando JG, Markley R, Turlington J, Trankle CR, Kang L, Biondi-Zoccai G, Van Tassell BW, Abbate A (2022a) Effect of IL-1 blockade with Anakinra on heart failure outcomes in patients with anterior versus nonanterior ST elevation myocardial infarction. J Cardiovasc Pharmacol 79:774–780
Del Buono MG, Damonte JI, Trankle CR, Kadariya D, Carbone S, Thomas G, Turlington J, Markley R, Canada JM, Biondi-Zoccai GG et al (2022b) Effect of interleukin-1 blockade with anakinra on leukocyte count in patients with ST-segment elevation acute myocardial infarction. Sci Rep 12:1254
Del Buono MG, Damonte JI, Moroni F, Chiabrando JG, Markley R, Turlington J, Trankle CR, Kang L, Biondi-Zoccai G, Kontos MC et al (2023) Clinical and pharmacological implications of time to treatment with interleukin-1 blockade in ST-segment elevation myocardial infarction. J Pharmacol Exp Ther 386:156–163
Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG (2005) CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96:881–889
Dinarello CA (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117:3720–3732
Dinarello CA (2018) Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 281:8–27
Ding HS, Huang Y, Qu JF, Wang YJ, Huang ZY, Wang FY, Yi WJ, Liu XX (2023) Panaxynol ameliorates cardiac ischemia/reperfusion injury by suppressing NLRP3-induced pyroptosis and apoptosis via HMGB1/TLR4/NF-κB axis. Int Immunopharmacol 121:110222
Diwanji R, O’Brien NA, Choi JE, Nguyen B, Laszewski T, Grauel AL, Yan Z, Xu X, Wu J, Ruddy DA et al (2023) Targeting the IL1β pathway for cancer immunotherapy remodels the tumor microenvironment and enhances antitumor immune responses. Cancer Immunol Res 11:777–791
Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M et al (2013) Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 121:1008–1015
El Sayed H, Kerensky R, Stecher M, Mohanty P, Davies M (2016) A randomized phase II study of Xilonix, a targeted therapy against interleukin 1α, for the prevention of superficial femoral artery restenosis after percutaneous revascularization. J Vasc Surg 63:133-141.e131
Emran T, Chowdhury NI, Sarker M, Bepari AK, Hossain M, Rahman GMS, Reza HM (2021) L-carnitine protects cardiac damage by reducing oxidative stress and inflammatory response via inhibition of tumor necrosis factor-alpha and interleukin-1beta against isoproterenol-induced myocardial infarction. Biomed Pharmacother 143:112139
Enzan N, Matsushima S, Ikeda S, Okabe K, Ishikita A, Yamamoto T, Sada M, Miyake R, Tsutsui Y, Nishimura R et al (2023) ZBP1 protects against mtDNA-induced myocardial inflammation in failing hearts. Circ Res 132:1110–1126
Epelman S, Liu PP, Mann DL (2015) Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 15:117–129
Fan Q, Tao R, Zhang H, Xie H, Lu L, Wang T, Su M, Hu J, Zhang Q, Chen Q et al (2019) Dectin-1 contributes to myocardial ischemia/reperfusion injury by regulating macrophage polarization and neutrophil infiltration. Circulation 139:663–678
Feng G, Bajpai G, Ma P, Koenig A, Bredemeyer A, Lokshina I, Lai L, Förster I, Leuschner F, Kreisel D et al (2022) CCL17 Aggravates myocardial injury by suppressing recruitment of regulatory T cells. Circulation 145:765–782
Frangogiannis NG (2014) The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11:255–265
Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML (1998) Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 98:699–710
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD (2010) Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 107:15880–15885
Gao R, Shi H, Chang S, Gao Y, Li X, Lv C, Yang H, Xiang H, Yang J, Xu L et al (2019) The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol 74:105575
Gao RF, Li X, Xiang HY, Yang H, Lv CY, Sun XL, Chen HZ, Gao Y, Yang JS, Luo W et al (2021) The covalent NLRP3-inflammasome inhibitor Oridonin relieves myocardial infarction induced myocardial fibrosis and cardiac remodeling in mice. Int Immunopharmacol 90:107133
Garon EB, Chih-Hsin Yang J, Dubinett SM (2020) The role of interleukin 1β in the pathogenesis of lung cancer. JTO Clin Res Reports 1:100001
Gilmore TD (1999) The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene 18:6842–6844
Gottlieb A, Natsis NE, Kerdel F, Forman S, Gonzalez E, Jimenez G, Hernandez L, Kaffenberger J, Guido G, Lucas K et al (2020) A phase II open-label study of bermekimab in patients with hidradenitis suppurativa shows resolution of inflammatory lesions and pain. J Invest Dermatol 140:1538-1545.e1532
Gu J, Shi JZ, Wang YX, Liu L, Wang SB, Sun JT, Shan TK, Wang H, Wang QM, Wang LS (2022) LncRNA FAF attenuates hypoxia/ischaemia-induced pyroptosis via the miR-185-5p/PAK2 axis in cardiomyocytes. J Cell Mol Med 26:2895–2907
Guo J, Hu Z, Ren L, Zhao W, Zuo R, Guo S, Jia C, Gao W (2023) Circulating tumor necrosis factor-α, interleukin-1β, and interleukin-17A estimates increased major adverse cardiac event risk in acute myocardial infarction patients. J Clin Lab Anal 37:e24853
Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD (2009) Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation 119:1386–1397
Han X, Yang Y, Zhang M, Li L, Xue Y, Jia Q, Wang X, Guan S (2022) Liquiritin protects against cardiac fibrosis after myocardial infarction by inhibiting CCL5 expression and the NF-κB signaling pathway. Drug Des Dev Ther 16:4111–4125
Handa M, Vanegas S, Maddux BA, Mendoza N, Zhu S, Goldfine ID, Mirza AM (2013) XOMA 052, an anti-IL-1β monoclonal antibody, prevents IL-1β-mediated insulin resistance in 3T3-L1 adipocytes. Obesity (silver Spring, MD) 21:306–309
Harouki N, Nicol L, Remy-Jouet I, Henry JP, Dumesnil A, Lejeune A, Renet S, Golding F, Djerada Z, Wecker D et al (2017) The IL-1β antibody gevokizumab limits cardiac remodeling and coronary dysfunction in rats with heart failure. JACC Basic Transl Sci 2:418–430
Hartikainen TS, Sörensen NA, Haller PM, Goßling A, Lehmacher J, Zeller T, Blankenberg S, Westermann D, Neumann JT (2020) Clinical application of the 4th Universal Definition of Myocardial Infarction. Eur Heart J 41:2209–2216
Hettwer J, Hinterdobler J, Miritsch B, Deutsch MA, Li X, Mauersberger C, Moggio A, Braster Q, Gram H, Robertson AAB et al (2022) Interleukin-1β suppression dampens inflammatory leucocyte production and uptake in atherosclerosis. Cardiovasc Res 118:2778–2791
Honold L, Nahrendorf M (2018) Resident and monocyte-derived macrophages in cardiovascular disease. Circ Res 122:113–127
Hu Q, Shi H, Zeng T, Liu H, Su Y, Cheng X, Ye J, Yin Y, Liu M, Zheng H et al (2019) Increased neutrophil extracellular traps activate NLRP3 and inflammatory macrophages in adult-onset Still’s disease. Arthritis Res Ther 21:9
Huang J, Li Y, Jiang Z, Wu L, Liu Y, Ma S, Li L, Wang H (2022) IL-1β promotes hypoxic vascular endothelial cell proliferation through the miR-24–3p/NKAP/NF-κB axis. Biosci Reports 42
Huse C, Anstensrud AK, Michelsen AE, Ueland T, Broch K, Woxholt S, Yang K, Sharma K, Tøllefsen IM, Bendz B et al (2022) Interleukin-6 inhibition in ST-elevation myocardial infarction: Immune cell profile in the randomised ASSAIL-MI trial. EBioMedicine 80:104013
Ikeda G, Matoba T, Ishikita A, Nagaoka K, Nakano K, Koga JI, Tsutsui H, Egashira K (2021) Nanoparticle-mediated simultaneous targeting of mitochondrial injury and inflammation attenuates myocardial ischemia-reperfusion injury. J Am Heart Assoc 10:e019521
Isoda K, Kamezawa Y, Tada N, Sato M, Ohsuzu F (2001) Myocardial hypertrophy in transgenic mice overexpressing human interleukin 1alpha. J Cardiac Fail 7:355–364
Javan H, Li L, Schaaf CL, Lee YS, Salama ME, Dinarello CA, Selzman CH (2022) Interleukin-1 receptor antagonism abrogates acute pressure overload-induced murine heart failure. Ann Thorac Surg 114:98–107
Karshovska E, Weber C, von Hundelshausen P (2013) Platelet chemokines in health and disease. Thromb Haemost 110:894–902
Keeley EC, Boura JA, Grines CL (2003) Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet (london, England) 361:13–20
Khandagale A, Lindahl B, Lind SB, Shevchenko G, Siegbahn A, Christersson C (2022) Plasma-derived extracellular vesicles from myocardial infarction patients inhibits tumor necrosis factor-alpha induced cardiac cell death. Curr Res Transl Med 70:103323
Kim ND, Luster AD (2013) To B or not to B–that is the question for myocardial infarction. Nat Med 19:1208–1210
Klein AL, Imazio M, Cremer P, Brucato A, Abbate A, Fang F, Insalaco A, LeWinter M, Lewis BS, Lin D et al (2021) Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med 384:31–41
Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER (1985) Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci USA 82:1204–1208
Kurzrock R, Hickish T, Wyrwicz L, Saunders M, Wu Q, Stecher M, Mohanty P, Dinarello CA, Simard J (2019) Interleukin-1 receptor antagonist levels predict favorable outcome after bermekimab, a first-in-class true human interleukin-1α antibody, in a phase III randomized study of advanced colorectal cancer. Oncoimmunology 8:1551651
Lacey CA, Mitchell WJ, Dadelahi AS, Skyberg JA (2018) Caspase-1 and Caspase-11 mediate pyroptosis, inflammation, and control of brucella joint infection. Infect Immun 86
Landmann EC, Walker UA (2017) Pharmacological treatment options for cryopyrin-associated periodic syndromes. Expert Rev Clin Pharmacol 10:855–864
Laura BC, Morales-Maldonado MA, Rodgers A, Kitt LA, Humphry M, Figg N, Bennett MR, Clarke MCH (2023) Thrombin activated Interleukin-1α drives atherogenesis, but also promotes VSMC proliferation and collagen production. Cardiovasc Res
Lee CC, Chen WT, Chen SY, Lee TM (2021) Taurine alleviates sympathetic innervation by inhibiting NLRP3 inflammasome in postinfarcted rats. J Cardiovasc Pharmacol 77:745–755
Lei Z, Luan F, Zhang X, Peng L, Li B, Peng X, Liu Y, Liu R, Zeng N (2022) Piperazine ferulate protects against cardiac ischemia/reperfusion injury in rat via the suppression of NLRP3 inflammasome activation and pyroptosis. Eur J Pharmacol 920:174856
Li A, Yu Y, Ding X, Qin Y, Jiang Y, Wang X, Liu G, Chen X, Yue E, Sun X et al (2020) MiR-135b protects cardiomyocytes from infarction through restraining the NLRP3/caspase-1/IL-1β pathway. Int J Cardiol 307:137–145
Li Y, Tu Z, Chen F, Yang X, Deng R, Su F, Cheng Z, Li S, Ong SB, Wang D et al (2023b) Anti-inflammatory effect of Danhong injection through inhibition of GSDMD-mediated pyroptosis. Phytomed Int J Phytother Phytopharmacol 113:154743
Li L, Cao J, Li S, Cui T, Ni J, Zhang H, Zhu Y, Mao J, Gao X, Midgley AC, et al (2023a) M2 macrophage-derived sEV regulate pro-inflammatory CCR2(+) macrophage subpopulations to favor post-AMI cardiac repair. Adv Sci (Weinheim, Baden-Wurttemberg, Germany) 10:e2202964
Libby P (2017) Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol 70:2278–2289
Liberale L, Carbone F, Camici GG, Montecucco F (2019) IL-1β and statin treatment in patients with myocardial infarction and diabetic cardiomyopathy. J Clin Med 8
Lin J, Li Q, Jin T, Wang J, Gong Y, Lv Q, Wang M, Chen J, Shang M, Zhao Y et al (2022) Cardiomyocyte IL-1R2 protects heart from ischemia/reperfusion injury by attenuating IL-17RA-mediated cardiomyocyte apoptosis. Cell Death Dis 13:90
Liu H, Huang Y, Zhao Y, Kang GJ, Feng F, Wang X, Liu M, Shi G, Revelo X, Bernlohr D et al (2023) Inflammatory macrophage interleukin-1β mediates high-fat diet-induced heart failure with preserved ejection fraction. JACC Basic Transl Sci 8:174–185
Lugrin J, Parapanov R, Milano G, Cavin S, Debonneville A, Krueger T, Liaudet L (2023) The systemic deletion of interleukin-1α reduces myocardial inflammation and attenuates ventricular remodeling in murine myocardial infarction. Sci Rep 13:4006
Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, Müller O, Vergely C, Zeller M, Tardivel A, et al (2015) Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J Immunol (Baltimore, MD: 1950) 194:499–503
Lyu J, Wang M, Kang X, Xu H, Cao Z, Yu T, Huang K, Wu J, Wei X, Lei Q (2020) Macrophage-mediated regulation of catecholamines in sympathetic neural remodeling after myocardial infarction. Basic Res Cardiol 115:56
Ma Y, Yabluchanskiy A, Lindsey ML (2013) Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogene Tissue Repair 6:11
Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML (2016) Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 110:51–61
Maekawa N, Wada H, Kanda T, Niwa T, Yamada Y, Saito K, Fujiwara H, Sekikawa K, Seishima M (2002) Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. J Am Coll Cardiol 39:1229–1235
Malinowsky D, Lundkvist J, Layé S, Bartfai T (1998) Interleukin-1 receptor accessory protein interacts with the type II interleukin-1 receptor. FEBS Lett 429:299–302
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A (2011) The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA 108:19725–19730
Mills EL, O’Neill LA (2016) Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol 46:13–21
Mooney RE, Linden GJ, Winning L, Linden K, Kee F, McKeown PP, Woodside JV, Patterson CC, McKay GJ (2022) Association of TGFB1 rs1800469 and BCMO1 rs6564851 with coronary heart disease and IL1B rs16944 with all-cause mortality in men from the Northern Ireland PRIME study. PLoS ONE 17:e0273333
Moriya J (2019) Critical roles of inflammation in atherosclerosis. J Cardiol 73:22–27
Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W et al (2015) The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J 36:377–384
Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, Garrett MR, Ma Y, Harmancey R, Lindsey ML (2018) Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol 113:26
Nakao T, Libby P (2023) IL-6 helps weave the inflammatory web during acute coronary syndromes. J Clin Invest 133
Nassef MZ, Hanke JE, Hiller K (2021) Mitochondrial metabolism in macrophages. Am J Physiol Cell Physiol 321:C1070-c1081
Ning Y, Huang P, Chen G, Xiong Y, Gong Z, Wu C, Xu J, Jiang W, Li X, Tang R et al (2023) Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway. BMC Med 21:96
Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ (2018) Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther 186:73–87
Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S (1998) Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodeling. Circulation 98:149–156
Opstad TB, Nordeng J, Pettersen AR, Åkra S, Bratseth V, Zaidi H, Helseth R, Solheim S, Seljeflot I (2022) The NLRP3 genetic variant (rs10754555) reduces the risk of adverse outcome in middle-aged patients with chronic coronary syndrome. J Immunol Res 2022:2366695
Owyang AM, Maedler K, Gross L, Yin J, Esposito L, Shu L, Jadhav J, Domsgen E, Bergemann J, Lee S et al (2010) XOMA 052, an anti-IL-1{beta} monoclonal antibody, improves glucose control and {beta}-cell function in the diet-induced obesity mouse model. Endocrinology 151:2515–2527
Pan Q, Hui D, Hu C (2022) A variant of IL1B is associated with the risk and blood lipid levels of myocardial infarction in Eastern Chinese individuals. Immunol Invest 51:1162–1169
Papayannopoulos V (2018) Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18:134–147
Peng H, Chen L, Deng Y, Liao X, Yang Y (2023) Ginsenoside Rh2 mitigates myocardial damage in acute myocardial infarction by regulating pyroptosis of cardiomyocytes. Clin Exp Hypertens (New York, NY: 1993) 45:2229536
Petzold T, Zhang Z, Ballesteros I, Saleh I, Polzin A, Thienel M, Liu L, Ul Ain Q, Ehreiser V, Weber C et al (2022) Neutrophil “plucking” on megakaryocytes drives platelet production and boosts cardiovascular disease. Immunity 55:2285-2299.e2287
Prabhu SD, Frangogiannis NG (2016) The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119:91–112
Presti SL, Elajami TK, Reyaldeen R, Anthony C, Klein AL (2021) The role of rilonacept in recurrent pericarditis. Heart Int 15:20–25
Ramírez J, Cañete JD (2018) Anakinra for the treatment of rheumatoid arthritis: a safety evaluation. Expert Opin Drug Saf 17:727–732
Razin T, Melamed-Book N, Argaman J, Galin I, Lowy Y, Anuka E, Naftali-Shani N, Kandel-Kfir M, Garfinkel BP, Brielle S et al (2021) Interleukin-1α dependent survival of cardiac fibroblasts is associated with StAR/STARD1 expression and improved cardiac remodeling and function after myocardial infarction. J Mol Cell Cardiol 155:125–137
Re F, Sironi M, Muzio M, Matteucci C, Introna M, Orlando S, Penton-Rol G, Dower SK, Sims JE, Colotta F et al (1996) Inhibition of interleukin-1 responsiveness by type II receptor gene transfer: a surface “receptor” with anti-interleukin-1 function. J Exp Med 183:1841–1850
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD et al (2017a) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377:1119–1131
Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ (2017b) Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet (london, England) 390:1833–1842
Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ (2018) Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet (london, England) 391:319–328
Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y et al (2015) Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation 132:1880–1890
Schunk SJ, Triem S, Schmit D, Zewinger S, Sarakpi T, Becker E, Hütter G, Wrublewsky S, Küting F, Hohl M et al (2021) Interleukin-1α is a central regulator of leukocyte-endothelial adhesion in myocardial infarction and in chronic kidney disease. Circulation 144:893–908
Smith DE, Hanna R, Della F, Moore H, Chen H, Farese AM, MacVittie TJ, Virca GD, Sims JE (2003) The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity 18:87–96
Stark K, Massberg S (2021) Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol 18:666–682
Su XL, Wang SH, Komal S, Cui LG, Ni RC, Zhang LR, Han SN (2022) The caspase-1 inhibitor VX765 upregulates connexin 43 expression and improves cell-cell communication after myocardial infarction via suppressing the IL-1β/p38 MAPK pathway. Acta Pharmacol Sin 43:2289–2301
Sun G, Li Y, Ji Z (2019) Up-regulation of MIAT aggravates the atherosclerotic damage in atherosclerosis mice through the activation of PI3K/Akt signaling pathway. Drug Delivery 26:641–649
Sun Y, Pinto C, Camus S, Duval V, Alayrac P, Zlatanova I, Loyer X, Vilar J, Lemitre M, Levoye A et al (2022) Splenic marginal zone B lymphocytes regulate cardiac remodeling after acute myocardial infarction in mice. J Am Coll Cardiol 79:632–647
Sun JY, Du LJ, Shi XR, Zhang YY, Liu Y, Wang YL, Chen BY, Liu T, Zhu H, Liu Y, et al (2023a) An IL-6/STAT3/MR/FGF21 axis mediates heart-liver cross-talk after myocardial infarction. Sci Adv 9:eade4110
Sun X, Feng Y, Gong C, Bao X, Wei Z, Chang L, Chen H, Xu B (2023b) Hypertension-driven regulatory T-cell perturbations accelerate myocardial ischemia-reperfusion injury. Hypertension (Dallas, Tex: 1979) 80:2046–2058
Terkeltaub R, Sundy JS, Schumacher HR, Murphy F, Bookbinder S, Biedermann S, Wu R, Mellis S, Radin A (2009) The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis 68:1613–1617
Thomas C, Bazan JF, Garcia KC (2012) Structure of the activating IL-1 receptor signaling complex. Nat Struct Mol Biol 19:455–457
Tissot AC, Spohn G, Jennings GT, Shamshiev A, Kurrer MO, Windak R, Meier M, Viesti M, Hersberger M, Kündig TM et al (2013) A VLP-based vaccine against interleukin-1α protects mice from atherosclerosis. Eur J Immunol 43:716–722
Toldo S, Abbate A (2018) The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 15:203–214
Toldo S, Mezzaroma E, McGeough MD, Peña CA, Marchetti C, Sonnino C, Van Tassell BW, Salloum FN, Voelkel NF, Hoffman HM et al (2015) Independent roles of the priming and the triggering of the NLRP3 inflammasome in the heart. Cardiovasc Res 105:203–212
Toldo S, Mauro AG, Cutter Z, Abbate A (2018) Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 315:H1553-h1568
Trankle CR, Canada JM, Cei L, Abouzaki N, Oddi-Erdle C, Kadariya D, Christopher S, Viscusi M, Del Buono M, Kontos MC et al (2018) Usefulness of canakinumab to improve exercise capacity in patients with long-term systolic heart failure and elevated C-reactive protein. Am J Cardiol 122:1366–1370
Vande Walle L, Lamkanfi M (2016) Pyroptosis. Curr Biol CB 26:R568-r572
Wang Y, Tan J, Yin J, Hu H, Shi Y, Wang Y, Xue M, Li X, Liu J, Li Y et al (2019a) Targeting blockade of nuclear factor-κB in the hypothalamus paraventricular nucleus to prevent cardiac sympathetic hyperinnervation post myocardial infarction. Neurosci Lett 707:134319
Wang Y, Yin J, Wang C, Hu H, Li X, Xue M, Liu J, Cheng W, Wang Y, Li Y et al (2019b) Microglial Mincle receptor in the PVN contributes to sympathetic hyperactivity in acute myocardial infarction rat. J Cell Mol Med 23:112–125
Wang YY, Liu XL, Zhao R (2019c) Induction of pyroptosis and its implications in cancer management. Front Oncol 9:971
Wang Z, Kun Y, Lei Z, Dawei W, Lin P, Jibo W (2021) LncRNA MIAT downregulates IL-1β, TNF-ɑ to suppress macrophage inflammation but is suppressed by ATP-induced NLRP3 inflammasome activation. Cell Cycle (georgetown, Tex) 20:194–203
Wang C, Zhang M, Yan J, Wang R, Wang Z, Sun X, Dong S (2023a) Chemokine-like receptor 1 deficiency impedes macrophage phenotypic transformation and cardiac repair after myocardial infarction. Int J Cardiol 372:6–14
Wang D, Hu Y, Zhang L, Cai H, Wang Y, Zhang Y (2023b) Dual delivery of an NF-κB inhibitor and IL-10 through supramolecular hydrogels polarizes macrophages and promotes cardiac repair after myocardial infarction. Acta Biomater 164:111–123
Wang Q, Wang T, Lio C, Yu X, Chen X, Liu L, Wu Y, Huang H, Qing L, Luo P (2023c) Surface hydrolysis-designed AuNPs-zwitterionic-glucose as a novel tool for targeting macrophage visualization and delivery into infarcted hearts. J Controll Release 356:678–690
Wei X, Lv Y, Yang C, Gao R, Zou S, Xu Y (2023) Bufalin reduces myocardial infarction-induced myocardial fibrosis and improves cardiac function by inhibiting the NLRP3/IL-1β signalling pathway. Clin Exp Pharmacol Physiol 50:688–697
Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, Voronov E, Dinarello CA, Apte RN (2004) The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci USA 101:2434–2439
Woxholt S, Ueland T, Aukrust P, Anstensrud AK, Broch K, Tøllefsen IM, Ryan L, Bendz B, Hopp E, Kløw NE, et al (2023) Cytokine pattern in patients with ST-elevation myocardial infarction treated with the interleukin-6 receptor antagonist tocilizumab. Open Heart 10
Xia N, Lu Y, Gu M, Li N, Liu M, Jiao J, Zhu Z, Li J, Li D, Tang T et al (2020) A unique population of regulatory T cells in heart potentiates cardiac protection from myocardial infarction. Circulation 142:1956–1973
Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W et al (2013) Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 62:24–35
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357:1121–1135
Yin J, Wang Y, Hu H, Li X, Xue M, Cheng W, Wang Y, Li X, Yang N, Shi Y et al (2017) P2X(7) receptor inhibition attenuated sympathetic nerve sprouting after myocardial infarction via the NLRP3/IL-1β pathway. J Cell Mol Med 21:2695–2710
Zandi E, Chen Y, Karin M (1998) Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: discrimination between free and NF-kappaB-bound substrate. Science (new York, NY) 281:1360–1363
Zhang W, Lavine KJ, Epelman S, Evans SA, Weinheimer CJ, Barger PM, Mann DL (2015) Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J Am Heart Assoc 4:e001993
Zhang J, Huang L, Shi X, Yang L, Hua F, Ma J, Zhu W, Liu X, Xuan R, Shen Y et al (2020) Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging 12:24270–24287
Zhang J, Hao W, Zhang J, Li T, Ma Y, Wang Y, Li X, Cui W, Du J (2023) CXCL16 promotes Ly6Chigh monocyte infiltration and impairs heart function after acute myocardial infarction. J Immunol (Baltimore, MD: 1950) 210:820–831
Zhao Z, Du S, Shen S, Wang L (2020) microRNA-132 inhibits cardiomyocyte apoptosis and myocardial remodeling in myocardial infarction by targeting IL-1β. J Cell Physiol 235:2710–2721
Zhaolin Z, Guohua L, Shiyuan W, Zuo W (2019) Role of pyroptosis in cardiovascular disease. Cell Prolif 52:e12563
Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC (2013) Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1α, controlling necrosis-induced sterile inflammation. Immunity 38:285–295
Zheng W, Zhou T, Zhang Y, Ding J, Xie J, Wang S, Wang Z, Wang K, Shen L, Zhu Y et al (2023) Simplified α(2)-macroglobulin as a TNF-α inhibitor for inflammation alleviation in osteoarthritis and myocardial infarction therapy. Biomaterials 301:122247
Zhou X, Zhang C, Wu X, Hu X, Zhang Y, Wang X, Zheng L, Gao P, Du J, Zheng W et al (2022) Dusp6 deficiency attenuates neutrophil-mediated cardiac damage in the acute inflammatory phase of myocardial infarction. Nat Commun 13:6672
Zhuang R, Meng Q, Ma X, Shi S, Gong S, Liu J, Li M, Gu W, Li D, Zhang X et al (2022) CD4(+)FoxP3(+)CD73(+) regulatory T cell promotes cardiac healing post-myocardial infarction. Theranostics 12:2707–2721
Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guérin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L et al (2013) B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 19:1273–1280
Zuurbier CJ, Abbate A, Cabrera-Fuentes HA, Cohen MV, Collino M, De Kleijn DPV, Downey JM, Pagliaro P, Preissner KT, Takahashi M et al (2019) Innate immunity as a target for acute cardioprotection. Cardiovasc Res 115:1131–1142
Acknowledgements
This work was supported by the General Project of the National Natural Science Foundation of China (Nos.82070378, 82370352). The abstract was adapted from “FullTemplateName”, by BioRender.com (CurrentYear). Retrieved from https://app.biorender.com/biorender-templates.
Funding
National Natural Science Foundation of China, Nos. 82070378, Zihua Zhou, Nos. 82370352.
Author information
Authors and Affiliations
Contributions
JH and WK conceptualized the project and wrote the manuscript. ZZ supplied funds support. Co-first authors are ranked in the order in which they participated in the experiment. All authors reviewed the manuscript and discussed the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J., Kuang, W. & Zhou, Z. IL-1 signaling pathway, an important target for inflammation surrounding in myocardial infarction. Inflammopharmacol 32, 2235–2252 (2024). https://doi.org/10.1007/s10787-024-01481-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-024-01481-4